Controlled Drugs Team
Controlled Drugs
Controlled Drugs Team (CDAO) – South East
Incident and concern reporting
CD Destructions and Authorised Witnesses
Privately prescribing or obtaining Schedule 2 and 3 controlled drugs
Community pharmacy private controlled drugs contractor code
Controlled Drugs Local Intelligence Networks (CD LIN)
Our statutory duty
NHS England has a statutory duty to ensure that safe systems are in place for the management and use of controlled drugs. This is to prevent harm to patients and staff from any misuse of controlled drugs.
Controlled drugs are drugs that are subject to high levels of regulation as a result of government decisions about those drugs that are especially addictive and harmful.
Controlled Drugs Team (CDAO) – South East
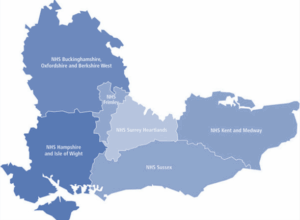
This team covers:
- Buckinghamshire, Oxfordshire and Berkshire West
- Frimley
- Hampshire and Isle of Wight
- Kent and Medway
- Surrey Heartlands
- Sussex
Julie McCann – Controlled Drugs Accountable Officer
Charlotte Vinter – Controlled Drugs Professional Manager
Carole Boarer – Controlled Drugs Professional Manager
Ami Luck – Controlled Drugs Programme Manager and Data Analyst
We can be contacted through our generic inbox england.southeastcdao@nhs.net
Incident and concern reporting
All incidents involving controlled drugs (including dispensing errors and discrepancies) should be reported to the Controlled Drugs Accountable Officer. This provides assurance that any risks have been mitigated and prompts any action to be taken. Reporting also allows for the identification of themes in reported incidents from which learning can take place.
We share the learning from incidents reported to us and update on relevant topics via our Local Intelligence networks and via newsletters.
Staff in organisations that do not have their own Controlled Drugs Accountable Officer (e.g. community pharmacies, GP practices, dental practices) should report their incidents to the Controlled Drugs Accountable Officer at NHS England via the CD Reporting Portal.
CD Destructions and Authorised Witnesses
All stocks of Schedule 2 controlled drugs must be witnessed by a person authorised by the Lead Controlled Drugs Accountable Officer for NHS England.
Any stocks of controlled drugs in Schedule 3, and 4 (Part 1) must be denatured before being disposed of by healthcare staff that are lawfully in possession of these.
Healthcare staff that are lawfully in possession of these can do so but should ensure they have a ‘T28 exemption’ from the Environment Agency that allows this on the relevant premises.
Once the application has been assessed and regulatory checks have been completed the CDAO will determine to issue an Authorised Witness Temporary License limited to 28 days to witness the destruction of controlled drugs with the quantities listed in the Authorised Witness application.
Please see below arrangements for each area.
If you have any questions, please contact us via england.southeastcdao@nhs.net
Arrangements for Buckinghamshire, Oxfordshire, Berkshire West, Frimley, Kent and Surrey Heartlands
We temporary authorise a witness within pharmacy or GP practice to witness the destruction of expired controlled drugs.
Please can you complete the ‘Apply to be a Temporary Authorised Witness’ application form via the CD Reporting Portal.
Arrangements for Hampshire and Isle of Wight
We pass all requests to the Hampshire & Isle of Wight Integrated Care Board Pharmacy Team to act as Authorised Witnesses.
We require a log of your request and what you have to destroy – please complete the ‘Apply to be a Temporary Authorised Witness’ application form on the CD Reporting Portal – you can leave ‘Details of Temporary Authorised Witness Applicant’ blank but please complete ‘Details of person undertaking the Controlled Drug destruction’.
They will be in touch with you directly to arrange a suitable day/time to visit.
Arrangements for Sussex
We pass all requests to the Controlled Drug Liaison Officers (CDLO) for Sussex Police.
We require a log of your request and what you have to destroy – please complete the ‘Apply to be a Temporary Authorised Witness’ application form on the CD Reporting Portal – you can leave ‘Details of Temporary Authorised Witness Applicant’ blank but please complete ‘Details of person undertaking the Controlled Drug destruction’.
They will be in touch with you directly to arrange a suitable day/time to visit.
Community pharmacies – multiples
NHS England anticipates that corporate bodies operating 5 or more pharmacy premises will seek to have suitably trained and competent employees designated as Authorised Witnesses by the appropriate NHS England CDAO. This is known as a Group Authority.
A corporate body operating 5 or more pharmacies must apply to the appropriate NHS England CDAO to designate suitably trained individual employees as Authorised Witnesses.
The appropriate NHS England CDAO is the one for the regional team where the corporate body’s registered office is located.
The appropriate NHS England CDAO may authorise suitably trained individual employees in a corporate body as Authorised Witnesses where the individuals described are not routinely involved in the management and use of CDs in the premises in which they are acting as an Authorised Witness.
The Superintendent Pharmacist of the corporate body is accountable to the appropriate NHS England CDAO for the governance arrangements around the witnessed destruction of CDs in their premises.
Please email for further information.
Patient returns of controlled drugs
Patient returned controlled drugs for Schedule 2, 3 and 4 (Part 1) can be destroyed without the presence of an Authorised Witness however the destruction should be witnessed by another colleague and logs kept of destructions e.g. a controlled drugs Patient Returns Book.
Pharmacies should ensure they have a ‘T28 exemption’ from the Environment Agency that allows this on the relevant premises.
Privately prescribing or obtaining Schedule 2 and 3 controlled drugs
Amendments to the Misuse of Drugs Regulations 2001 require private prescriptions or requisitions for medicines containing schedule 2 and 3 controlled drugs to be issued on controlled stationery bearing a unique prescriber identification number (PIN). Practitioners providing such treatment of disease, disorder or injury should ensure they are appropriately registered, indemnified, and where necessary registered with the Care Quality Commission (CQC).
You can apply for a new PIN or make changes to your PIN registered information (such as address, phone number) by contacting Charlotte Vinter c.vinter@nhs.net
If you have a PIN and need to order controlled stationery for prescribing you can obtain this from Primary Care Support England (PCSE) via their website.
If you wish to obtain stock CDs rather than prescribing and you already have a PIN you can download the mandatory FP10CDF form.
Please note that private CD prescribers can only hold ONE private CD prescriber code, regardless of the number of organisations they may work for. If you work for more than one organisation, please contact Charlotte Vinter to discuss where to register your current CD prescriber code.
Community pharmacy private controlled drugs contractor code
If you work at a community pharmacy in the South East region and need a private CD contractor code, please email england.southeastcdao@nhs.net
Controlled Drugs Local Intelligence Network (CDLIN)
The CD regulations require the NHS England Accountable Officer to facilitate a ‘Local Intelligence Network’ (LIN) to share information and intelligence on the safe use and management of controlled drugs.
Local meetings are currently held by ICB footprint twice yearly. Membership includes Designated Bodies, Responsible Bodies and other organisations where controlled drugs are involved e.g. substance misuse.
If you are a Designated Body and would like to update details of your membership or join our FutureNHS workspace, please email Charlotte Vinter c.vinter@nhs.net
Occurrence reporting
If an organisation has a CQC registered Controlled Drugs Accountable Officer, they are considered to be a Designated Body and should have their own internal process for recording and monitoring CD incidents. They are required by law to submit a quarterly report via the Occurrence Reporting module on the CD Reporting Portal.
Please note that a NIL-report must be submitted if no incidents/concerns have occurred in that quarter.
This information is required every quarter and can be submitted online via the CD Reporting Portal. Below is the schedule and deadlines for each quarter.
| Reporting Period | Date Covered | Deadline |
| Quarter 1 | 1 April to 30 June | 31 August |
| Quarter 2 | 1 July to 30 September | 30 November |
| Quarter 3 | 1 October to 31 December | 28 February |
| Quarter 4 | 1 January to 31 March | 31 May |
