Access to National Workforce Supply Routes for Community Pharmacy
Contents
- 1. Introduction
- 2. Workforce deployment model
- 3. National workforce supply routes
- 4. Onboarding and engagement
- 5. Indemnity and insurance
- Appendix 1: Workforce deployment model
- Appendix 2: Drawdown process
- Appendix 3: Health and safety guidance
Classification: Official
Publication approval reference: C1391
COVID-19 vaccination programme
Version 2, 18 August 2021
Updates since version 1 (published 27 January 2021) are highlighted in yellow.
This guidance is correct at the time of publishing. However, as it is subject to updates, please use the hyperlinks to confirm the information you are disseminating to the public is accurate.
If you have any queries about this workforce guidance, please contact PCNPO.WorkforceEscalation@nhs.net.
1. Introduction
Community pharmacy continues to have a significant role in the rollout of the COVID-19 vaccination programme, working with other providers in primary care to ensure coverage for the local population.
The national workforce supply routes have been established to provide additional capacity over and above local workforce teams. The routes allow community pharmacy to access both clinical and non-clinical staff and volunteers, via a designated lead provider in each integrated care system (ICS).
This guidance sets out detailed instructions which community pharmacy will need to access the national workforce supply routes, on the following key points:
- overall approach to workforce capacity planning for COVID-19 vaccination
- roles and responsibilities of organisations within the proposed workforce deployment model
- additional workforce that may be available from national workforce supply routes, including the proposed drawdown approach
- onboarding and engagement arrangements, including indemnity and insurance.
Note: Where lead providers are mentioned in this document, we are referring to the lead organisation regarding workforce supply within the ICS, which may be referred to locally as a lead employer, workforce bureau, or some other alternative.
2. Workforce deployment model
To support community pharmacy, this guidance provides additional information on the processes to access and onboard additional workforce.
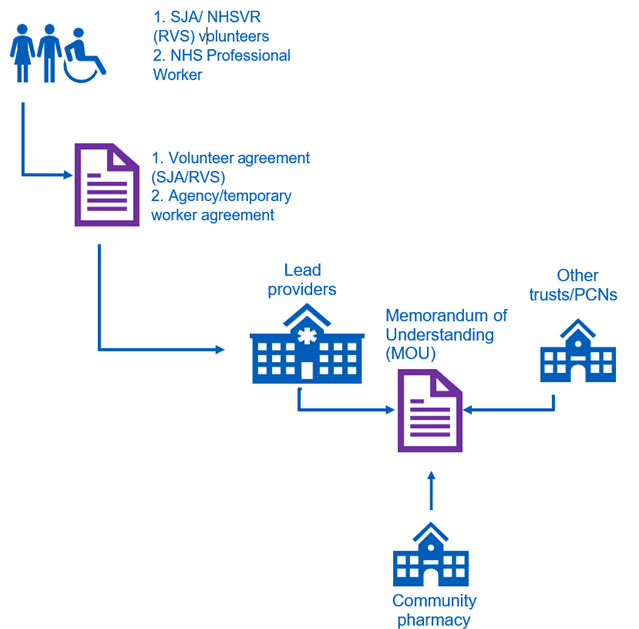
The workforce deployment model depends on a number of allocated responsibilities for community pharmacy, clinical commissioning groups (CCGs) and appointed lead providers in each ICS. A visual diagram of the workforce deployment model for the national workforce supply routes can be found in Appendix 1.
Responsibilities of commissioned community pharmacy
Community pharmacy should review this guidance in the planning for COVID-19 vaccination. Community pharmacy should continue to identify their staffing requirements and future workforce gaps, as well as put in place contingency arrangements for staff absence. Community pharmacy should contact the lead provider in their area for additional support and to access additional staff resource, via the national workforce supply routes.
It is important to note that within this document, it is not possible to take account of the complexity and variety of service delivery arrangements across commissioned sites. Therefore, it is not intended that this guidance should replace local decision-making within a site, from either the perspective of an employer or a provider. Moreover, arrangements between community pharmacy and lead providers regarding support will be agreed locally and the purpose of this guidance is to provide more information on the likely arrangements to be implemented locally.
Responsibilities of CCGs
It is expected that CCGs will provide local workforce mobilisation and support to community pharmacy, facilitating the utilisation of resources and expertise available locally.
Responsibilities of lead providers
Each ICS has a designated lead provider which will act as a workforce hub for the other providers, including community pharmacy.
The lead provider will work with all providers (including PCN groupings) to provide workforce support, such as:
- Communications with local providers within primary care.
- Completion of regional workforce reporting.
- System-level workforce planning and gap analysis.
- Liaison and drawdown of national workforce supply routes.
- Management of rostering systems (for volunteer or national workforce supply routes).
- Oversight and delivery of statutory and mandatory training.
The exact balance of responsibilities between lead providers and other providers (including community pharmacy) will be agreed locally in line with the needs and resources of the system.
The lead provider can also provide support to commissioned community pharmacy on local recruitment, and subject to a local agreement, such as a memorandum of understanding (MoU) being in place, may be able to employ additional staff on behalf of the commissioned site. An updated list of named contact details for the lead provider in each ICS has been shared with community pharmacy to enable planning and liaison to take place.
Finally, although outside the scope of this guidance, it is important to note that other healthcare providers, for example general practice or dental providers, may also be able to support with workforce supply, through a sub-contracting arrangement, which should be locally agreed and funded through the enhanced service.
3. National workforce supply routes
Community pharmacy will be supported to access the nationally sourced pools of staff and volunteers on a similar basis as other providers.
| Supplier | Overview of support to be provided | Roles provided by supplier | Contracting route |
|---|---|---|---|
| NHS Professionals (NHSP) | NHSP recruited a large number of staff, registered and non-registered and is responsible for the end-to-end process, from managing centralised demand to recruitment, virtual training and confirming employment hosting arrangements. More details are available here. |
|
Commissioned sites will be able to make requests for additional staff via their lead provider and put in place a collaborative agreement with them. The sites will be required to fund these posts locally through Agenda for Change pay rates. To access resource, LVS should follow the drawdown processes for the national workforce supply routes, listed in Appendix 2 |
| St John Ambulance (SJA) | SJA will manage and deploy volunteers to vaccination sites, including vaccinators. This is available across all seven regions. More details are available here. |
|
Commissioned sites can make requests for additional volunteers via their lead employer and put in place a collaborative agreement with them. Commissioned sites will not be required to fund these staff locally. To access resource, providers should follow the drawdown process for the national workforce supply routes, listed in Appendix 2 |
| Royal Voluntary Services (RVS) | The NHS Volunteer Responders programme, delivered by Royal Voluntary Service and the GoodSAM app, provide volunteers for non-clinical volunteering roles such as stewarding. The system lead provider identifies volunteers needed. |
|
Commissioned sites can make requests for additional volunteers via their lead employer and put in place a collaborative agreement with them. Commissioned sites will not be required to fund these staff locally. To access resource, providers should follow the drawdown process for the national workforce supply routes, listed in Appendix 2. |
| Occupational Health (OH) Providers | Multiple OH providers can provide additional registered OH professionals. |
|
An existing framework is in place with 23 suppliers for NHS organisations to access additional OH provider support. Commissioned sites can make requests for additional staff via their lead provider and put in place a collaborative agreement with the lead provider but will be required to fund these posts locally |
Commissioned Community Pharmacy will have to locally fund the cost of paid clinical and administrative staff sourced from national on the agreed Agenda for Change pay scales for the job role, but voluntary staff will be provided at no charge to commissioned sites. Role descriptions are available from lead providers for volunteers provided by St John Ambulance and NHS Volunteer Responders, or for temporary workers recruited by NHS Professionals. Where community pharmacy decide to undertake local recruitment themselves or subcontract from other providers, they are not required to use national job descriptions.
It is important to note that incorporation of these supply routes or roles as part of a site’s proposed vaccinating team is not mandatory. Local alternatives may be available to provide additional capacity, such as through the use of additional hours worked by existing staff, sub-contracting arrangements or working with other providers. As with the seasonal flu programme, commissioned sites are also encouraged to work with their CCG and local volunteer organisations. The local staffing model chosen must depend on local workforce characteristics and the need to maintain safe staffing and clinical supervision arrangements. The clinical lead providing the COVID-19 vaccination service is responsible for ensuring that appropriately trained and competent staff are in place to deliver the services.
4. Onboarding and engagement
Where commissioned sites identify a workforce need that cannot be filled locally, they can seek to secure workforce via the national workforce supply routes. This section sets out the responsibilities of both lead providers and community pharmacy under these arrangements.
A COVID-19 vaccination programme overview pack for staff and volunteers will be used to support onboarding and will be provided to volunteers from St. John Ambulance, NHS Volunteer Responder (provided by Royal Voluntary Service) as well as workers engaged by NHS Professionals.
Lead providers should:
- Provide the face to face training required and assure themselves of other training and programme induction provided, using appropriate competency assessment and training needs analysis.
- Undertake assurance of pre-employment checking and occupational health clearance.
- Ensure the volunteers and temporary workers have appropriate uniform for their assigned role.
- Put in place appropriate rostering to support the needs of the commissioned sites.
- Take lead responsibility for health and safety, but this is delegated to the commissioned site for site specific health and safety and onboarding.
- Follow normal engagement processes, including the completion of a volunteer agreement for volunteers or an honorary contract for staff.
- Put in place an MoU between the lead provider and the commissioned site to facilitate sharing of volunteers and staff among both primary care and other NHS providers. An example MoU is supplied for local providers to review and adapt, if necessary.
Commissioned community pharmacy should:
- Assure themselves of the competency and performance of staff and volunteers deployed to a designated site.
- Raise any volunteer or temporary workers performance issues with the lead provider.
- Ensure that NHS Volunteer Responders do not work with vulnerable persons or children, unless in a fully supervised capacity.
- Deliver an ‘on-site’ orientation and induction, in line with local procedures. This should normally include the following key information:
- site welcome
- satisfactory identity verification of deployed volunteer or worker
- access to IT and workstation assessment
- Explanation of site flow
- induction to operating procedures
- supply of site-specific personal protective equipment (PPE)
- confirmation of completion of all training requirements
- completion of legal documentation to enable administration of vaccine, eg signing of Patient Group Direction or National Protocol (where appropriate)
- confirmation of named site contact for any queries and the clinical supervisor contact for any concerns
- local health and safety instructions (eg fire safety); further detail on the local site responsibilities for health and safety are outlined in Appendix 3.
5. Indemnity and insurance
Where a community pharmacy has signed, and is operating under the LES, it will be the responsibility of that community pharmacy to source appropriate indemnity arrangements including for clinical negligence.
The Pharmacy Contractor must ensure that it has in place appropriate indemnity and/or insurance arrangements that provide adequate cover, including but not limited to clinical negligence cover, in relation to the delivery of the LES Agreement, and that the indemnity and/or insurance arrangements provide such cover for all clinical professionals and other staff working in connection with the delivery of the services pursuant to the LES Agreement.
Appendix 1: Workforce deployment model
Appendix 2: Drawdown process
Volunteers deployed from the national workforce supply routes
- Commissioned sites should contact the lead provider for their ICS to place a request for volunteers.
- The request must include the nature of the roles requested (eg clinical vaccinator/non-clinical volunteer), the time period requested, the details of the vaccination site, including a site contact.
- The lead provider will work with the national providers of volunteers and identify suitable candidates.
- The lead provider will assign these volunteers to the commissioned site and communicate where a request cannot be covered.
- Once the volunteer is assigned, the lead provider will ensure the training requirements of the role are fulfilled and confirm the shift with the volunteer.
- The lead provider will then pass the details of the volunteer to the commissioned site for local site onboarding. An MoU (or local equivalent) is required to be in place to facilitate the sharing of staff from the lead provider to other providers.
- The commissioned site will ensure that the volunteer is met on site at the start of their shift, so that local site onboarding can be completed.
- If there are any issues regarding the volunteer placement, the commissioned site should escalate these to the lead provider.
Temporary workers deployed from the national supply route
- Commissioned sites should contact the lead provider for their ICS to place a request for temporary workers from the national workforce supply.
- The request must include the nature of the roles requested (eg clinical vaccinator/non-clinical worker), the time period requested, the details of the vaccination site and a site contact.
- The commissioned site must undertake to pay the lead provider for the total cost of workers successfully placed with the commissioned site. This can be agreed by parties agreeing to an MoU (or local equivalent).
- The lead provider will work with the national provider of temporary workers to identify suitable candidates.
- The lead provider will assign temporary workers to the commissioned site and communicate where a request cannot be covered.
- Once the temporary staff member is selected, the lead provider will assure themselves that the training requirements of the role are fulfilled and confirm the shift with the volunteer.
- The lead provider will then pass the details of the assigned temporary staff to the commissioned site for local site onboarding. An MoU (or local equivalent) is required to be in place to facilitate the sharing of staff from the lead providers to other providers.
- The commissioned site will ensure that the temporary worker is met on site at the start of their shift so that onboarding can be completed.
- If there are any issues regarding the placement of temporary worker, the commissioned site should direct these to the lead provider.
- The lead provider will invoice the commissioned site, in line with the relevant local agreement or MoU.
Appendix 3: Health and safety guidance
The commissioned site, on behalf of the lead provider shall:
- Provide information in connection with any risks to health and safety involved in the assignment, including high-risk areas where exposure prone procedures take place, as well as the commissioned site policy for mitigating such risks.
- Be responsible for the health and safety, fire safety and security of volunteers or workers on the commissioned site which shall include, provision of fire safety instruction and ensuring fire safety equipment is in place.
- Provide volunteers or workers with all necessary personal protective equipment to undertake the tasks required.
- Undertake the necessary risk assessment where the proposed volunteer or worker is known to be at potential individual risk, including, without limitation, by being pregnant.
- Inform the lead provider of all outbreaks of infection, as declared by the community pharmacy.
- Inform the lead provider where a volunteer or worker is injured, infected, or attacked.
- In the event of a reportable incident under the Reporting of Injuries, Diseases and Dangerous Occurrences Regulations 2013 (RIDDOR), the commissioned site must inform the lead provider immediately, who will liaise work with the pharmacy to prepare an incident report.
- The commissioned site shall comply with all statutory obligations applicable to it in relation to volunteers or workers assignments, including without limitation those arising under the Health and Safety at Work Act 1974, the Employment Rights Act 1996, the Management of Health and Safety at Work Regulations 1999 and the Working Time Regulations 1998.
