Urgent cancer diagnostic services during COVID-19
Contents
- Equalities statement
- Introduction
- Background
- Adapting urgent cancer diagnostic pathways in the context of COVID-19
- Annex 1: Patient experience ‘I’ statements
- Annex 2: Tools and Resources
- Annex 3: Quality markers
- Acronyms
Classification: official
Publications approval reference: C0789
Urgent cancer diagnostic services during COVID-19
Version 1 January 2021
Equalities statement
Promoting equality and addressing health inequalities are at the heart of NHS England and NHS Improvement’s values. Throughout the development of the advice and processes cited in this document, we have:
- given due regard to the need to eliminate discrimination, harassment and victimisation, to advance equality of opportunity, and to foster good relations between people who share a relevant protected characteristic (as cited under the Equality Act 2010) and those who do not share it
- given regard to the need to reduce inequalities between patients in access to, and outcomes from, healthcare services and to ensure services are provided in an integrated way where this might reduce health inequalities.
This builds on established cancer services which already give consideration to reducing health inequalities.
Urgent cancer diagnostic referral will be open to any patient meeting the clinical referral criteria as per services already in place, based on clinical need and risk, and supported by clinical triage. Referral criteria to access the service do not pertain to any of the nine protected characteristics outlined in the Equality Act 2010. However, we recognise that during the COVID-19 pandemic, there may be deviation from previous standard practice which potentially negatively impacts on promoting equality and diversity. To protect against this, patients should be safety netted wherever possible.
With the expansion of virtual consultation and working, health services should consider how this may impact patient who face digital exclusion or poor IT-literacy and ensure that mitigation is in place.
1. Introduction
In their letter on 31 July 2020, Sir Simon Stevens (NHS Chief Executive) and Amanda Pritchard (NHS England and Improvement Chief Operating Officer) set out priorities for phase 3 of the COVID-19 response, including restoring full operation of all cancer services. The commitment to retain urgent cancer services as a priority was reiterated in a letter to the system from Dame Cally Palmer and Professor Peter Johnson on 30 November.
In response to this, there have been many enquiries from the system about how to manage cancer diagnoses safely alongside the COVID-19 pandemic. This document responds to those queries and provides a reference point for the system on how to manage urgent cancer diagnostics.
This document was originally produced and circulated to Cancer Alliances in a longer form including advice on maintaining COVID-secure facilities and expanding Rapid Diagnostic Centres in summer 2020. The version published here (January 2021) focuses only on rapidly deployable adjustments to cancer pathways in light of the current COVID-19 incident.
Please email the NHS Cancer Programme with any questions at england.cancerpolicy@nhs.net.
2. Background
This guide is based on engagement with Cancer Alliances, the cancer Clinical Advisory Group, clinical experts, patients, Royal Colleges and wider cancer stakeholders. It builds on the Rapid Diagnostic Centre Vision and 2019/20 Implementation Specification.
It sets out general principles and clinical considerations for adapting urgent cancer diagnostic pathways in the context of COVID-19 recovery. It is a reference document for primary care, secondary care and independent sector diagnostic services providers and should be read in conjunction with existing National Institute for Health and Care Excellence (NICE) guidelines on referrals and diagnostics; and the latest national/regional guidance related to COVID-19 and cancer services more broadly. It should be used to develop pathways which ensure patients are diagnosed and treated as quickly and as safely as possible.
The information should inform local decisions on how urgent cancer pathways are managed as part of COVID-19 recovery. In referring to this document, systems should consider the current advice on cancer treatment capacity and cancer treatment hubs, and ensure appropriate links are in place with these services.
The solutions set out are indicative and should be adapted to local circumstances, diagnostic capacity and workforce. These are not mandatory changes and individual clinicians, trusts and multidisciplinary teams (MDTs) should make the final decisions on the most appropriate action for individual patients and their local services.
We welcome feedback on the impact of this advice, how it is being implemented and if there are differing local approaches that are leading to greater capacity and faster diagnosis. Please send all feedback to england.cancerpolicy@nhs.net. We will update this document as we receive feedback.
3. Adapting urgent cancer diagnostic pathways in the context of COVID-19
Urgent cancer diagnostic pathways have been impacted by the COVID-19 pandemic. Pathways have been affected by infection control measures, social distancing, additional COVID-19 testing and self-isolation which may affect productivity, or the number of patients who can be seen at one time. Meanwhile, patients’ ability to easily access health services has also been affected. Anecdotally, some patients have found it difficult to arrange travel to healthcare facilities during the COVID-19 pandemic and access support networks. These challenges should be considered when co-ordinating patient care.
The following advice responds to queries on how urgent cancer diagnostic pathways should be organised to provide a diagnosis as quickly and as safely as possible for patients during the pandemic. It is not prescriptive and should be adapted by local systems based on their needs and capacity. A flow diagram for each suspected tumour pathway has been developed in consultation with the NHS Cancer Programme Clinical Advisory Group, national clinical advisers and other key stakeholders. Existing NICE guidelines on suspected cancer referrals and diagnostics should be considered alongside these pathway suggestions, however, flexibility in response to local circumstances is vital. Local timed or optimal pathway guidance should also be considered alongside these pathway adaptions.
3.1 NG12 as the basis for referrals
The NICE NG12 guidance should be used to initiate a cancer referral as is standard practice. Some NG12 symptom criteria overlap with symptoms for COVID-19, as outlined below. GPs should not rule out cancer because of this overlap and should consider these symptoms in combination with any other cancer symptoms, family history or other risk factors.
Typically, COVID-19 symptoms have features of an acute illness, whereas cancer symptoms can be more insidious. In differentiating between cancer symptoms and COVID-19 symptoms, GPs should consider how long patients have experienced symptoms. If a patient presents with a cough, for example, consider whether this is persistent (more than 3 weeks) and unexplained. GPs should also consider whether the patient presents with any other COVID-19 symptoms.
Red flag symptoms should not be ignored because they overlap with COVID-19 symptoms. If a patient presents with a cough or fever alone, they should not be ruled out for cancer as these may indicate malignancy. GPs should consider other presenting cancer indicators in their decision to refer. Patients should have a swab test for COVID-19 and, if appropriate, a chest-X-ray for respiratory symptoms.
Patients should receive clear communications about what the COVID-19 symptoms are and how they differ from or overlap with cancer symptoms. They should be given advice about when to talk to their GP/single point of contact if they develop symptoms or if symptoms progress. GPs should also discuss a patient’s risk of COVID-19 and provide reassurance about IPC measures.
Some patients may also present with suspected cancer and COVID-19 comorbidity. Where appropriate, patients with symptoms of COVID-19 should receive a COVID-19 swab test as part of their referral as outlined, even where these overlap with symptoms of cancer. Patients should follow national guidance on self-isolation before their cancer diagnostic appointment.
Please note the following symptoms overlap with NG12 criteria of cancer pathways:
Table 1: Overlapping COVID-19 and cancer symptoms
| NG12 cancer pathway | NG12 / COVID-19 symptom overlap | Additional NG12 cancer signs and symptoms to consider |
| Lung | Cough Fatigue Shortness of breath*consider whether these overlap with other COVID-19 or lung cancer symptoms *consider the rapidity of onset and persistence of cough |
Aged 40 and over Ever smoked Appetite/weight loss Chest pain Finger clubbing Chest signs Persistent/recurring chest infection*further detail on differentiating lung cancer and COVID-19 symptoms can be found here. |
| Mesothelioma | Cough Fatigue Shortness of breath*consider whether these overlap with other COVID-19 or cancer symptoms *consider the rapidity of onset and persistence of cough |
Aged 40 and over Ever smoked Asbestosis exposure Appetite/weight loss Chest pain Finger clubbing Chest signs Persistent/recurring chest infection |
| Leukaemia | Unexplained fever
*consider whether these overlap with other COVID-19 or cancer symptoms |
Pallor, Unexplained persistent or recurrent infection Generalised lymphadenopathy Unexplained bruising Unexplained bleeding Unexplained petechiae Hepatosplenomegaly |
| Lymphoma | Fever Shortness of breath*consider whether the patient has enlarged lymph nodes as wells as these symptoms Unexplained lymphadenopathy or splenomegaly |
Consider any associated symptoms, particularly fever, night sweats, shortness of breath, pruritus, weight loss, alcohol-related node pain |
| Myeloma | Weakness and fatigue Recurrent or persistent infections *consider whether these overlap with other COVID-19 or cancer symptoms |
Persistent pain (>4-6 weeks) especially back/bone pain or fracturesUnexplained anaemia Nose bleeds, abnormal bruising*NB additional symptoms described by Myeloma UK |
| Brain and central nervous system (CNS) | Loss of smell or taste
*NB. In COVID-19 positive cases, loss of taste or smell rarely occurs in the absence of other symptoms (persistent cough or fever) |
Progressive, sub-acute loss of central neurological function |
| Upper gastrointestinal (GI) (including hepato pancreato biliary) cancers | Diarrhoea (patients over 60 with weight loss)
*consider whether these overlap with other COVID-19 or cancer symptoms |
Unexplained weight loss Nausea VomitingHepato-pancreato-biliary Back pain Jaundice Abdominal pain Constipation New-onset diabetes Upper abdominal massOesophageal cancer: Dysphagia Upper abdominal pain Reflux Dyspepsia |
| Colorectal cancer | Change in bowel habit
*consider whether these overlap with other COVID-19 or cancer symptoms |
Unexplained weight loss Unexplained abdominal pain Unexplained rectal bleeding Iron-deficiency anaemia Occult blood in faeces Rectal bleeding |
| Non-specific symptoms
Symptoms are based on the Rapid Diagnostic Centre Vision and 2019/20 Implementation Specification |
Unexplained fatigue
*consider whether these overlap with other COVID-19 or cancer symptoms |
New unexplained and unintentional weight loss (either documented >5% in 3 months or with strong clinical suspicion)
New unexplained constitutional symptoms of four weeks or more New unexplained, unexpected or progressive pain, including bone pain, of 4 weeks or more |
3.2 Establishing patient confidence in the system
- Patients should receive communications on how their cancer pathway may be different to ensure their safety during the COVID-19 pandemic. Cancer Alliances can use the patient ‘I’ statements (Annex 1) to help develop this messaging.
- Patients should receive clear, timely communications if/when their diagnostics are rescheduled/delayed.
- Communications should highlight that there is a risk of COVID-19 in healthcare settings but provide confidence that every precaution will be taken to minimise this, and it is essential to receive a diagnosis as quickly as possible.
- Patients should be advised on how to minimise their risk of infection in healthcare settings and when travelling to healthcare settings (social distancing, self-isolating, hand hygiene, etc) in line with latest guidance.
- Care should be provided in line with personalised care principles, based on ‘what matters’ to people and helping to make them equal and active partners in their own health care.
- Patients and carers should experience a high level of care throughout the diagnostic pathway. Cancer Alliances can use quality markers (Annex 3) to ensure a positive patient experience of care.
- Patient navigators or similar roles would be ideally placed to provide clear, consistent messaging for patients. Local services should consider how the ‘single point of contact’ role outlined in the Rapid Diagnostic Centre Vision and 2019/20 Implementation Specification can be rolled out to support this function.
3.3 Ensuring safety and access for especially vulnerable groups
Cancer patients are often older and therefore at heightened risk of severe COVID-19. Black, Asian and minority ethnic (BAME) patients are also known to be at higher risk of severe COVID-19. Local systems should ensure there is robust management of PTLs across all providers so that patients continue to be tracked and treated in accordance with their clinical priority. Local providers should balance the clinical risk of cancer and need for rapid diagnosis with patients’ clinical risk of COVID-19, especially for patients in the ‘shielding group’.
Cancer investigations for those in the shielding group should take place in COVID-19-secure sites. Capacity in COVID-19-secure sites should be prioritised based on clinical need and patient vulnerability.
Local systems should apply safety-netting for those not immediately having investigations, with responsibility residing in provider trusts. Safety-netting processes/protocols should be robust and clinically appropriate.
National and local communications should reassure patients about the importance of coming forward for care. However, in some cases patients may decide not to attend their cancer diagnostic appointment due to concerns around COVID-19. If patients decline a diagnostic appointment, they should be offered a teleconsultation to discuss the importance of diagnosis and what they should do if their symptoms persist or progress. They should remain on PLTs if appropriate. Patients should be given a contact, through which they can rebook their appointment, should their symptoms progress or they decide to go ahead with their diagnostic tests. Cancer Research UK have developed safety netting messages which can be used as a reference.
Note: ‘COVID-19 assessment’ indicated in the adapted diagnostic pathways flowcharts below should cover patients’ suitability for assessment in COVID-19-secure sites: whether they fall into any COVID-19 vulnerable groups, exhibit COVID-19 symptoms or have recently tested positive for COVID-19. Any need for testing or self-isolation (in line with national guidelines) should be considered at this point.
3.4 Opportunities to increase diagnostic testing in primary care
To protect patients from COVID-19, it is important to reduce the number of attendances in acute care or hospital settings.
Increasing the capacity of primary care networks to perform initial diagnostic tests such as X-ray and ultrasound could reduce the need for secondary care attendances. This opportunity should be considered in local COVID-19 response plans, including any plans for mobile diagnostic capacity.
Clinical triage is essential to streamlining the referral process and to ensure patients receive the right tests at the right time. Where available and clinically appropriate, high sensitivity tests should be available by direct GP access pre-referral (eg ultrasound for suspected gynaecological cancers or sarcoma, CT or MRI for brain tumours). The implementation/piloting of rule/out tests should be evidence-based and locally evaluated where possible. In general, greater access to straight-to-test pathways should be made available to streamline services.
3.5 Adapting tumour specific pathways
The following flow diagrams have been developed to indicate how pathways could be optimised to ensure patients are diagnosed as quickly as possible in a safe and consistent way. They are based on previously developed timed pathway documents and have been adapted in consultation with clinical experts to the current COVID-19 pandemic context.
These are indicative and should be adapted to local circumstances and used in reference to any local optimal pathway guidance. This document cannot cover all clinical scenarios. These are not mandatory changes and individual clinicians, trusts and MDTs should always make the final decisions on the most appropriate action for individual patients and their local services.
These pathways should be considered in reference to recent guidance clarifications on the Cancer Waiting Times system.
Suspected lung cancer
Clinicians should refer to and follow the National Optimal Lung Cancer Pathway (NOLCP). The following adaptions have been suggested due to the fall in referrals experienced during the pandemic and the need for actions to prevent the spread of COVID-19. They should be read in combination with the NOLCP, NG12 and NG122 guidelines. As symptoms for lung cancer overlap with COVID-19 (cough, fatigue, shortness of breath), care should be taken to maintain COVID-19-secure sites.1

Footnotes
- Symptoms for lung cancer may overlap with those for COVID-19 (further advice on differentiation of COVID-19 and lung cancer symptoms can be found here). Extra measures may be considered to help ensure sites remain COVID-19-secure; for example local services may consider dedicated facilities for lung cancer patients or dedicated capacity.
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include investigation results, comorbidities, PS score, performance status, medications and COVID-19 symptoms/shielding status.
- Telephone or video consultation can be used to determine suitability for straight-to-test CT. Preparation for any tests, including any COVID-19 assessments, can be communicated to patients. Patients should be advised on how their hospital experience may be different during COVID-19 and how they can minimise the risk of transmission.
- Where clinically urgent (eg suspected small cell lung cancer) diagnostics should not wait for COVID-19 PCR results or self-isolation. Where a COVID-19-negative diagnosis can be confirmed, diagnostic tests can be performed in COVID-19-secure sites.
- Local systems may wish to consider use of mobile units or CXR in primary care to reduce hospital admissions.
- CT should be hot reported wherever possible. Where possible and clinically appropriate, tests should be spread across as few sites and appointments.
- Ensure all investigations that are necessary to plan treatment are completed with a minimum of visits to the hospital. Avoid investigations that have no influence on management, especially where no treatment is likely.
Suspected upper gastrointestinal cancers: oesophago-gastric
For patients with symptoms indicative of upper GI, endoscopy (OGD) remains the recommended standard testing. Where this is felt to impose a high risk for the patient/staff,1 radiological investigations such as barium swallow or contrast enhanced CT of chest and abdomen may be preferred as the initial test. This pathway follows the British Society of Gastroenterology (BSG) guidelines, including specific BSG guidance during COVID-19, and builds on the NHS Cancer Programme Timed Pathway work.

Footnotes
- Endoscopy is an aerosol generating procedure and can increase the chance of exposure to COVID-19.
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include investigation results, Covid19 symptoms/shielding status should also be included.
- Virtual clinical triage for all patients referred onto the pathway. Telephone consultation can be used to determine suitability for straight to test OGD and pre-assessment. Preparation for any tests can be communicated to patients. Patients should be advised on how their hospital experience may be different during Covid19 and how they can minimise the risk of transmission. PHE advice should be followed.
- Patients >55y old with new dyspepsia and unexplained weight loss should proceed to OGD urgently as lifting of COVID restrictions allow, as should patients >55y old with anaemia and / or new dyspepsia (< 6 months). For dyspeptic patients OGD should not be performed in the absence of alarm features – a policy of PPI and H. pylori testing should be undertaken as per NICE guidance.
Suspected upper gastrointestinal cancers: hepato-pancreatic-biliary
Patients with symptoms indicative of hepato-pancreatic-biliary cancers should be referred based on NICE NG12 and NG85 guidance. Use of telephone/video consultations should be maximised, with clear recording of fitness and comorbidity. This pathway follows the British Society of Gastroenterology (BSG) guidelines, adapted in response to COVID-19, and builds upon the NHS Cancer Programme Timed Pathway work.

Footnotes
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include investigation results, COVID-19 symptoms/shielding status should also be included.
- Virtual clinical triage for all patients referred onto the pathway. Telephone consultation can be used to determine suitability for tests. Preparation for any tests can be communicated to patients. Patients should be advised on how their hospital experience may be different during COVID-19 and how they can minimise the risk of transmission. PHE advice should be followed.
Suspected lower gastrointestinal cancers
For patients with lower GI symptoms, colonoscopy remains the recommended standard investigation. Where this is felt to impose a high risk for the patient, CT colon or plain CT may be used as alternative, definitive or initial tests retrospectively.

Footnotes
- Trusts should consider providing GPs with specialist telephone advice and guidance before formal referral. Referral information to allow effective virtual triage will be locally determined with commissioners but should include investigation results (full blood count, MCV ferritin, urea and electrolytes, eGFR. C-reactive protein FIT), medical images (where appropriate), frailty score and COVID-19 symptoms/shielding status should also be included. A Clinical guide for triaging patients with lower gastrointestinal symptoms has been produced.
- Telephone or video consultation can be used to determine suitability for straight to test upper and lower GI endoscopy, as well as to prioritise referrals for urgent colonoscopy or CT (CTC/plain CT).
- While many patients with a FIT <10uyg/gm may not require colonoscopy, patients should not be discharged from the 2WW pathway on the basis of a FIT test alone, except in existing FIT pioneer service evaluation sites that were piloting the use of FIT before the COVID-19 outbreak
- Local prioritisation arrangements should ensure that there is a single, clinician-managed route to urgent care for patients on both screening and symptomatic lists requiring this. Please refer to the ‘clinical guide for risk stratifying participants on the bowel cancer screening pathway’
- Where colon capsule endoscopy is used, robust data collection and follow-up processes must be in place.
- Patients placed on tracking lists should have a virtual consultation to provide information and reassurance. They should be given advice on what to do if their symptoms progress.
Suspected haematological cancers
All referrals for symptoms indicative of haematological cancers will follow the current NG12 guidelines. As some symptoms for haematological cancers overlap with COVID-19 (fever and fatigue), care should be taken to maintain the integrity of COVID-19-secure sites.1

Footnotes
- Symptoms for haematological cancers may overlap with those for COVID-19. To help ensure sites remain COVID-19-secure, patients should undergo COVID-19 assessment and follow guidelines. Local services may consider dedicated facilities for haematological cancer patients or dedicated capacity with deep clean prior to alternate use.
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include filter test results, GPs should indicate whether the patient is suitable for virtual clinical triage or whether a face-to-face assessment for lymphoma is required. COVID-19 symptoms/shielding status should also be included.
- All primary care tests should be undertaken in line with NG12 guidelines in a way to minimise the risk of the contraction and spreading of COVID-19 in line with guidance on COVID-19 infection prevention and control.
- Telephone or video consultation can be used to determine suitability for straight to test and pre-assessment. Preparation for any tests, including COVID-19 assessments, can be communicated to patients. Patients should be advised on how their hospital experience may be different during COVID-19 and how they can minimise the risk of transmission.
Suspected breast cancer
For patients with symptoms indicative of breast cancer, referrals will be managed using standardised assessment protocols stratified with by age. All referrals will follow the NG12, NG101 and CG81 guidelines with assessment at a one-stop diagnostic service by the breast diagnostic team comprising of surgeons, advanced nurse practitioners, breast physicians, radiologists / radiographers and pathologists.

Footnotes
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include information on any primary care filter tests, breast symptoms, comorbidities, COVID-19 symptoms/shielding status should also be included. GPs should indicate whether the patient is suitable for virtual clinical triage or whether a face-to-face assessment is required.
- GPs can refer to the Pan London Suspected Breast Cancer Referral Forms – Version 2 as a useful clinical tool.
- Telephone or video consultation and Association of Breast Surgeons (ABS) guidelines can be used to determine suitability for appropriate breast assessment. Preparation for any tests can be communicated to patients. Patients should be advised on how their hospital experience may be different during COVID-19 and how they can minimise the risk of transmission. PHE advice on IPC should be followed.
- Based on NG12, in the absence of associated red flag symptoms, ie lump or skin changes, breast pain alone is not a symptom of cancer and should not be automatically referred on an urgent cancer pathway. Digital information has been developed by ABS.
- Staff should refer to the Association of Breast Surgeons (ABS) Best practice diagnostic guideline for patients presenting with breast symptoms and ABS Covid19 Statements and allocate patients to appropriate diagnostic tests based on this guidance. Where possible diagnostics should be completed on one day.
Suspected gynaecological cancer
For most women, an appointment in primary or secondary care will usually be needed for pelvic examination.

Footnotes:
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include investigation results as appropriate (ultrasound, CA125, pelvic examination, germ cell tumour markers), COVID-19 symptoms/shielding status should also be included.
- Telephone or video consultation can be used to determine suitability for straight to test and pre-assessment. Preparation for any tests can be communicated to patients. Patients should be advised on how their hospital experience may be different during COVID-19 and how they can minimise the risk of transmission. PHE advice should be followed.
- Results should be hot reported wherever possible, and tests should be coordinated to into as few hospital visits as possible.
- In the context of the COVID-19 pandemic, direct access ultrasound should be considered for women aged 55 and over with unexplained symptoms of vaginal discharge who are presenting with these symptoms for the first time or have thrombocytosis or report haematuria. It should also be considered for patients with haematuria and low haemoglobin or thrombocytosis or high blood glucose levels. Local discussions should be taken on the feasibility of this adaption
Suspected skin cancer
For patients with suspected skin cancer, a skin biopsy/excision may be required upon remote examination of skin lesions.
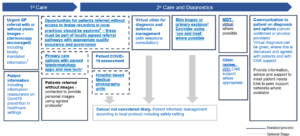
Footnotes:
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include initial investigation results, Photos should be included with or without dermoscopy. COVID-19 symptoms/shielding status should also be included.
- All 2ww referrals without photos need to be clinically assessed or have their photo taken by medical photography. Telephone consultation may be used to determine the patient’s appropriateness to be seen in clinic for assessment and/or sent direct to biopsy or excision. Pre-operative advice for any surgical procedure should be communicated and sent out to patients ahead of their appointment. Patients should be advised on how their hospital experience may be different during COVID-19 and how they can minimise the risk of transmission. PHE advice should be followed.
- Where evidence-based solutions are available, local systems should consider piloting/implementation
- Where a patient is seen in clinic consider ‘see and treat’ management if this can be implemented safely with the appropriate levels of consent obtained.
Suspected head and neck cancer
For patients with symptoms indicative of head and neck cancers referrals will follow the current NG12. Local systems should also refer to and follow NICE NG36 guidelines on the use of imaging for diagnosis of head and neck cancers.

Footnotes:
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include investigation results, clinical photographs, COVID-19 symptoms/shielding status should also be included.
- Existing risk stratification tools should be used to determine patients most at risk. This should consider factors including but not limited to: age, gender, smoking and alcohol histories, voice changes, difficult swallowing and presence of neck lumps.
- Telephone or video consultation can be used to determine suitability for straight to test and pre-assessment. The ENT tool supported by the British Head and Neck Oncologists can be used. Pre-habitation (virtual where possible) and preparation for any tests can be communicated to patients. Patients should be advised on how their hospital experience may be different during COVID-19 and how they can minimise the risk of transmission.
- During the COVID-19 pandemic the use of nasendoscopy should be minimised as much as possible as it is an aerosol generating procedure and causes patients to cough. Where nasendoscopy is required PHE Guidance on COVID-19 infection prevention and control should be followed.
Suspected eye cancer in adults
Optometrists should refer directly to an ophthalmologist, following NICE guidelines for suspected cancer and any protocols prepared by their local hospital. Ophthalmologists should refer patients with suspected eye cancer to a supraregional ocular oncology centre following the same principles. Any relevant images should be included in the referral for triage at a virtual clinic, possibly avoiding the need for the patient to attend a hospital clinic in person.

Footnotes:
- Referrals should include images of the tumour and made using the recipient department’s referral form, downloaded from the hospital website. Recipient hospital will request images from referring optometrist or ophthalmologist if these are not provided. Covid19 symptoms/shielding status should also be included. Clinicians should refer patients using any referral forms prepared by the recipient hospital, downloading the relevant form from the hospital website.
- Fast track ocular imaging at hospital eye department will be performed if adequate images are not provided by the referring optometrist or ophthalmologist. The patient should be advised not to expect a face-to-face consultation with an ophthalmologist unless the imaging suggests cancer, in which case the patient will be seen by the ophthalmologist on the same day if possible.
- Simple procedures (incisional or excision biopsy for diffuse and nodular conjunctival lesions respectively) can be performed at the patient’s local hospital, if this can be done safely after prior discussion with the surgeon and/or remote supervision of the procedure by the ocular oncologist by means of a secure video link
- Whenever possible, investigations for anaesthesia are undertaken at local hospital to reduce the number of visits to the ocular oncology centre.
Suspected retinoblastoma in children
Optometrist/health visitor/midwife should patients refer urgently via the GP to a paediatric ophthalmologist, following NICE guidelines for suspected cancer and any protocols prepared by their local hospital. Paediatric Ophthalmologists should refer children with suspected retinoblastoma to a supraregional Retinoblastoma Service following the same principles. Referring ophthalmologist should undertake dilated fundus examination of the parents to rule out retinoma, in cases with no family history of retinoblastoma.
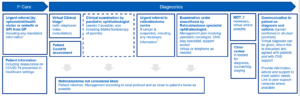
Footnotes
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include investigation results, clinical photographs, COVID-19 symptoms/shielding status should also be included.
- Telephone or video consultation can be used to determine need for clinical examination. Preparation for any tests can be communicated to carers. Carers should be advised on how hospital experiences may be different during COVID-19 and how they can minimise the risk of transmission. PHE advice should be followed.
- Necessary information from referring paediatric ophthalmologist includes: ophthalmic examination findings of child and dilated fundus examination of both parents if there is no family history of retinoblastoma.
Suspected brain and central nervous system (CNS) cancers
All referrals for symptoms indicative of neurological cancers should follow the current NICE NG12 and NG99 guidelines. As some symptoms for neurological cancers may overlap with COVID-19 (loss of smell or taste), care should be taken to maintain the integrity of COVID-19-secure sites.1

Footnotes:
- Symptoms for neurological cancers (loss of taste and smell) may overlap with those for COVID-19. However, in COVID-19 positive cases, loss of taste or smell rarely occurs in the absence of other COVID-19 symptoms (persistent cough or fever). Anosmia in isolation is also rare as a cancer symptom. Patients with these symptoms should be COVID-19 assessed prior to face-to-face appointment
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include investigation results, COVID-19 symptoms/shielding status should also be included.
- Telephone or video consultation can be used to determine suitability for straight to test and pre-assessment. Preparation for any tests can be communicated to patients. Patients should be advised on how their hospital experience may be different during COVID-19 and how they can minimise the risk of transmission. PHE advice should be followed
- Appointments should be spaced to comply with PHE infection and control advice. MRI should be reported within 72 hours of imaging.
- GP to provide MDT coordinator with any additional clinical information.
Suspected sarcoma
For suspected sarcoma pathways local systems should look to increase capacity in primary care for direct access x-ray and ultrasound, where possible.

Footnotes
- Consider a suspected cancer pathway referral (for an appointment within 2 weeks) for adults if an X-ray suggests the possibility of bone sarcoma.
- Consider a suspected cancer pathway referral (for an appointment within 2 weeks) for adults if they have ultrasound scan findings that are suggestive of soft tissue sarcoma or if ultrasound findings are uncertain and clinical concern persists. Where possible, ultrasound should be carried out/reviewed by an expert in musculoskeletal imaging. If results are suggestive of lipoma then share information back to GP with reassurance and rebook with follow-up if GP has any concern or send ultrasound report to sarcoma centres.
- Referral information to allow effective virtual triage will be locally determined with commissioners but should include investigation results, COVID-19 symptoms/shielding status should also be included.
- Telephone or video consultation can be used to determine suitability for straight to test and pre-assessment. Preparation for any tests can be communicated to patients. Patients should be advised on how their hospital experience may be different during COVID-19 and how they can minimise the risk of transmission. PHE advice should be followed.
- Ultrasound should be carried out/reviewed by an expert in musculoskeletal imaging, with access to same-day MRI where the findings indicate possible malignancy. Further investigation could also include subsequent surveillance CXR in coordination with the specialist sarcoma diagnostic service.
Suspected urological cancers: prostate cancer
All referrals for symptoms indicative of prostate cancers will follow the current NICE NG12 and NG131 guidelines. This flow diagram should be read in conjunction with the NHS England Clinical Expert Group for prostate cancer: guidance to support the optimal timed pathway for prostate cancer.

Footnotes
- GPs should follow PHE’s Prostate Cancer Risk Management Guidelines on the risks and benefits of PSA testing. This should only be used by GPs who are counselling asymptomatic men aged 50 and over who are proactively asking about PSA testing. It does not apply to their consultations with men at high risk or men with symptoms of any age. If PSA is outside the normal range, then a clinical assessment should be done followed by a scan and biopsy where needed.
- Locally mandated information is determined with commissioners but should include demographics, investigation results (PSA, U&E/ eGFR, urine dipstick (+ MSU result if dipstick positive), and DRE), performance status, weight and BMI, medication, anti-coagulant history, and MRI scanning exclusion criteria. A PSA of >3ng/ml should be used as referral rate for men aged 50-69. A rectal swab may also be required. COVID-19 symptoms/shielding status should also be included.
- In some circumstances (eg very high PSA and almost certain cancer on MpMRI) a decision may be made to proceed to treatment without biopsy.
- Prostate biopsy: This should only include transperineal targeted (visual-estimation or image-fusion) to improve detection rates and reduce the risk of sepsis and COVID-19 droplet generation.
- Consider re-biopsy, surveillance or discharge depending on mpMRI and histology findings. Likert or PIRADS 4/5 with no atrophy or inflammation might be a ‘miss’ so should consider re-biopsy/surveillance. Likert or PIRADS 1-3 can be discharged to GP with personalised PSA threshold for re-referral.
Suspected urological cancers: renal and bladder cancer
All referrals for symptoms indicative of renal and bladder cancers will follow the current NICE NG12 and NG2 guidelines.

Footnotes:
- Locally mandated information is determined with commissioners but should include demographics, investigation results, performance status, weight and BMI, medication. COVID-19 symptoms/shielding status should also be included.
- Telephone or video consultation can be used to determine suitability for straight to test and pre-assessment. Preparation for any tests can be communicated to patients. Patients should be advised on how their hospital experience may be different during COVID-19 and how they can minimise the risk of transmission. PHE advice should be followed.
- If the abdomino-pelvic CT scanning does not clarify the case of bleeding then an ultrasound scan, CT urogram or cytoscopy can be used as per NICE guidelines
Annex 1: Patient experience ‘I’ statements
| RDC component | Expected experience for patients |
| Early identification | I – or a caregiver – will recognise that something isn’t right and will know to call my GP to discuss my symptoms with them. I may be prompted to call my GP by other local services with whom I feel comfortable discussing my symptoms I may have used an app to check my symptoms or called 111 and been told to call my GP. |
| Timely referral | My GP will offer me a telephone, video call or face-to-face appointment and will ask me questions about my symptoms. They may also ask me questions about COVID-19 symptoms and whether I or anyone in my household has been self-isolating. My GP will assess my symptoms and will quickly refer me to find out what is wrong. I may need to have some initial tests such as blood tests to provide more information about my symptoms. I will be given information on the need for these tests and how I will be protected against COVID-19 during these. I expect to be given the right amount of support from my GP/ caregiver as part of the telephone/video call, to ensure that I am able to provide all relevant information on my symptoms. |
| Broad assessment of symptoms | Once I am referred, a range of information about me will be considered to determine which diagnostic tests I might choose to have and in which order. Where possible these will happen on the same day and a in a way which is safe The choice about which tests I will have will be a shared decision between me and the health professional I speak to over the phone, video call or face-to-face appointment. Health professionals may decide that I do not need any further tests, but that I should be followed up to see if there have been any changes to my symptoms. I will be given information about what I should do if I notice any changes to my health. If I need to come to a health centre for tests, I may be asked to self-isolate before coming in. |
| Co-ordinated testing | I will know which tests I will have, what to expect during each test, how to prepare, and when and where I will have the tests. I will know some actions I can take to protect myself from COVID-19 before and during my appointment. Wherever possible, all my tests will be done on the same day so that I don’t need to travel more than necessary. To protect me from COVID-19, my tests might not be done in my local hospital but in a private clinic or other location. Tests and staff will follow strict guidelines to help protect me from COVID-19. If my appointment needs to be cancelled or delayed, my single point of contact will contact me and explain why, and what will happen next. |
| Timely diagnosis | My test results may be discussed by a team of experts. I may be asked if I would like to be told my diagnosis virtually at home or if I prefer to come in person. If I choose to hear my diagnosis at home, I will be encouraged to have someone with me for support. My diagnosis will be explained to me by a health professional as soon as possible. I will have the opportunity to ask questions and discuss what will happen next based on my personal preferences. I will be given information in formats that best suit my needs. |
| Appropriate onward referral | If I receive a serious diagnosis, whether it is cancer or not, I will have an onward referral to the right specialist team for further investigation and/or treatment. Wherever possible, I will not need to repeat tests or to provide the same information again. Whatever diagnosis I receive, I will be supported to understand what the diagnosis means for me. I will receive information appropriate to my needs about sources of support, advice and information that could help me and my carers. I will be informed that I will be involved wherever possible in decisions about my future care and treatment. |
| Excellent patient co-ordination and support | I will have a single point of contact who can: (1) help me obtain further information from experts (2) provide clear and supportive conversations about the diagnosis process, what matters to me and what happens next and (3) know how to signpost me to sources of support, advice and information that are appropriate to my needs. My carers and I will be helped to understand how I am able to manage aspects of my own health and wellbeing, such as being supported to make choices about lifestyle changes, being helped to access financial and employment advice and being signposted to sources of emotional support. I should call my single point of contact if I test positive for COVID-19 or develop symptoms at any point. They may then reorganise my appointments to keep me and other patients safe. |
Annex 2: Tools and Resources
General
This document builds on the Rapid Diagnostic Centre Vision and 2019/20 Implementation Specification.
COVID-19 specific material
Key guidance in response to COVID-19
- NICE guidelines on arranging planned care in hospitals and diagnostic services during COVID-19
- Guidance on COVID-19 infection prevention and control
- Guidance on COVID-19 equipment hygiene
- Guidance on use of PPE during COVID-19
- Operating framework for urgent and planned services in hospital settings during COVID-19
- Clarification of Cancer Waiting Times guidance during COVID-19 pandemic
- UK Government advice on staying alert to stay safe
- UK government’s guidance (COVID-19) on management of staff and exposed patients or residents in health and social care settings.
Patient support
Key resources to support patients through diagnostic pathways
- The cancer wellbeing group
- Cancer Care Map
- Macmillan ‘In Your Area’
- Personalised care principles
- Centre for Perioperative Care (CPOC) Fitter, better, sooner toolkit
- CPOC guidance for patients having operations during COVID-19
- Macmillan emotional help
- University College London Hospitals – Managing your emotional wellbeing during the COVID-19 pandemic
- The Scottish NHS Inform Toolkit includes templates and patient materials on COVID-19 which can be adapted.
Staff support
- Staff can access freely available training on MECC principles.
- qcancer
- Gateway C Cancer Maps
- Cancer Research UK Risk Assessment Tool – risk assessment tool
- Cancer Research UK has developed advice on safety netting during COVID-19
- Guidance is available on video consulting in general practice.
Pathways
Timed pathways have been created for four cancer pathways; colorectal, lung, oesophago-gastric and prostate cancer. These have been published here.
Lung pathway
The Lung pathway has been adapted from the National Optimal Lung Cancer Pathway (NOLCP) to reflect adjustments needed to protect patients and staff from the risk of contracting and spreading COVID-19. The pathway should be considered in reference to NICE NG12 and NG122 guidelines.
Supporting materials:
[BROKEN LINK] Lung cancer and mesothelioma service guidance during the COVID-19 pandemic
Differentiation of the Cs in lung cancer: Cancer vs COVID
Upper GI Pathway
The Upper GI pathway follows the British Society of Gastroenterology (BSG) guidelines and has been adjusted in response to the COVID-19 pandemic.
Supporting materials:
Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management
Lower GI pathway
Supporting materials:
Clinical guide for triaging patients with lower gastrointestinal symptoms
Breast pathway
The Breast pathway follows the Association of Breast Surgeons (ABS) Best practice diagnostic guideline for patients presenting with breast symptoms and has been adjusted in response to the COVID-19 pandemic.
Prostate pathway
GPs can use Public Health England’s Prostate Cancer Risk Management Guidelines when testing PSA levels for suspected prostate cancer.
The NHS England and NHS Improvement Clinical Expert Group for prostate cancer: guidance to support the optimal timed pathway for prostate cancer.
Annex 3: Quality markers
Local systems should provide optimal experience of care along cancer diagnostic pathways.
The quality markers approach, developed with patients and carers, has been revised reflecting the pathway adaptions above. Three quality markers have been identified as particularly important to positive patient experience during COVID-19; Cancer Alliances should be able to evidence the implementation of these in a narrative format.
Cancer Alliances can share, learn and collaborate on quality markers via the Cancer Alliance Workspace.
Table 2: Quality marker considerations
| Number | Consideration | COVID-19 response focus |
| 1 | How does the service ensure that a patient’s ability to attend appointments is considered? (including how information is shared with them in a way that they understand: taking into account language, cultural, sensory, learning or other needs) | What issues could arise with the shielded population accessing appointments? Will people be able to attend appointments given public transport issues? What action will be taken to ensure that information is provided in a way that is accessible to all? |
| 2 | What actions are services taking to reduce anxiety for patients and carers – specifically with regards initial appointments? | What actions will be taken to reduce anxiety with regards virtual consultations? What actions will be taken to reduce anxiety around infection prevention and control? |
| 3 | How does the service make sure that patients and carers are told about the voluntary services that will best meet their support needs at every stage of the pathway? | How are patients & carers told of additional support available – through – for example the NHS Volunteer Responders. |
The following quality markers have also been highlighted as being of particular relevance to the current environment:
- How does the service ensure that all aspects of communication with patients and carers are presented in a way that they will understand, taking account of language, cultural, sensory, learning or other needs?
- How does the service ensure that at the point of discharge the patient is aware of what the next steps are?
Acronyms
2WW – 2 Week Wait (Referral)
ABS – Association of Breast Surgeons
BAME – Black Asian Minority Ethnic
CNS – Central Nervous System
CT – Computerised Tomography
CWT – Cancer Waiting Times
CXR – Chest X Ray
EMRAD – East Midland Radiology Consortium
GI – Gastrointestinal
MDT – Multi-Disciplinary Team
MECC – Making Every Contact Count
MIG – Medical Interoperability Gateway
NICE – National Institute for Health and Care Excellence
NOLCP – National Optimum Lung Cancer Pathway
OGD – Oesophago-Gastro-Duodenoscopy
PCR – Polymerase Chain Reaction
PHE – Public Health England
PPE – Personal Protective Equipment
PTL – Patient Tracking List
RDC – Rapid Diagnostic Centre
