Design note: COVID-19 ward for intubated patients
Contents
- Introduction
- Identifying a location
- Design considerations
- Engineering
- Appendix 1: Room layouts
- Appendix 2: Experience from the ExCel London surge Nightingale Hospital
Novel coronavirus (COVID-19) standard operating procedure
Design note: COVID-19 ward for intubated patients
1 April 2020 (version 1)
This guidance is correct at the time of publishing (22 March 2020). However, as it is subject to updates, please use the hyperlinks to confirm the information you are disseminating to the public is accurate.
1. Introduction
This document will to assist managers and estates teams in the rapid conversion of existing wards into facilities for intubating COVID-19 patients. It contains: bed layouts infrastructure prompts and oxygen advice.
For new builds from scratch and modular builds, please refer to existing Health Building Notes (HBNs) and Health Technical Memoranda (HTMs):
- Health Building Note 04-01: Adult inpatient facilities
- Health Building Note 04-01: Supplement 1 – Isolation facilities for infectious patients in acute settings.
- Health Building Note 04-02: Critical care units
- Health Technical Memorandum 03-01: Specialised ventilation for healthcare premises
For a full list of current estate guidance please refer to the complete list of GOV.UK publications related to NHS estates.
It has been updated with high level lessons leaned from the Excel London Nightingale Hospital – see Appendix 2.
2. Identifying a location
Current capabilities
Assess current facilities’ capabilities for creation of adequate isolation rooms or areas, identifying potential areas that could be converted effectively with minimum modifications.
This is best carried out through a desktop planning exercise using recent hospital plans. Focus on existing wards, theatres or catheterisation labs, including prep and scrub areas.
- Avoid any through routes for non-COVID-19 traffic.
- Consider logistical flows of clean and dirty waste.
- You must maintain fire evacuation routes.
Special environmental controls, such as negative pressure isolation rooms, are not necessary to prevent the transmission of COVID-19. However, in the early stages and in high-risk settings, patients with suspected or confirmed COVID-19 may be isolated in negative-pressure rooms.
Self-contained areas
Where possible, a designated self-contained area or wing of the healthcare facility should be used for the treatment and care of patients with COVID-19. This area should:
- include a reception area that is separate from the rest of the facility and, if feasible, have a separate entrance/exit from that for the rest of the building
- not be used as a thoroughfare by other patients, visitors or staff, including patients being transferred, staff going for meal breaks, and staff and visitors entering and exiting the building
- be separated from non-segregated areas by closed doors
- have signage displayed warning of the segregated area to control entry.
Use single rooms
Wherever possible, patients with suspected or confirmed COVID-19 should be placed in single rooms.
Organisation
Consider creating cohort areas which differentiate the level of care required. It may also be prudent to consider:
- the need for cohorting in single/mixed-sex wards/bays
- underlying patient condition (immunocompromised)
- age groups when cohorting children
- routine childhood vaccination status when cohorting children.
Thresholds and entrances
Once potential areas for treatment of COVID-19 patients have been identified, consider the support required. Hospitals should provide changing rooms/areas where staff can change into uniforms on arrival at work. These may already exist.
Those entering a COVID-19 area will require personal protective equipment (PPE).
Identify the location of the clean PPE store and space to change at the gateway/threshold of COVID-19 areas. A changing space should have scrub facilities and clean PPE storage.
All linen should be handled inside the patient room/cohort area. Clean linen should be stored adjacent to the entrance for supply.
Store all used/infectious linen in a designated, safe, lockable area while awaiting uplift. Also locate space for dirty PPE change and disposal.
Infection control
It is unlikely the Individual clinical wash basins are unlikely to be available for each bed. Make use of what is available.
All staff, patients and visitors should decontaminate their hands with alcohol-based hand rub (ABHR) when entering and leaving areas where suspected and confirmed COVID-19 patients are being cared for. Identify the location of the AHBR dispenser at each treatment/bed location.
Tissues, waste bins (lined and foot operated) and hand hygiene facilities should be available for patients, visitors and staff. Hands-free waste bins, with appropriate colour-coded waste bags, should be provided by each wash-hand basin.
Fire risk assessment
The fire risk assessment for the area being converted should be reviewed in view of a higher life safety sleeping risk cohort and the additional issue of more oxygen being in use. This may increase staffing levels or require modified PHE.
Refer to HTM 05-03 Part K and the relevant fire code sections as they apply:
- https://www.gov.uk/government/publications/managing-healthcare-fire-safety
- https://www.gov.uk/government/publications/guidance-in-support-of-functional-provisions-for-healthcare-premises
- https://www.gov.uk/government/publications/suite-of-guidance-on-fire-safety-throughout-healthcare-premises-parts-a-to-m
This review should be carried out by the trust fire safety advisor using their local knowledge of the site and training levels.
Ventilation
The density of ventilators may enrich the air with oxygen, increasing the combustion risk. Ensure there is good natural and mechanical ventilation.
3. Design considerations
Risk
In planning COVID-19 facilities, review the range of construction risks, including asbestos, vibration, dust and fungal disturbance and take action to mitigate them. In addition, consider:
- delineating an area for COVID-19 treatment so that the boundaries align with existing fire compartments
- invasive construction work should only start once the asbestos risk has been established
- where new or additional services are to be installed, suitable permanent or temporary fire stopping should be provided for any wall or floor penetrations, to maintain the fire integrity of the penetrated structure.
Activities
- Caring for a patient who is likely to require intubation and will need continuous medical and nursing care using piped medical gases; vacuum and life-support system using isolation procedures.
- Medical and nursing procedures requiring minimum three-sided access to patient while one to four staff use specialised equipment.
- Monitoring vital physiological signs.
- Clinical handwashing.
- Monitoring/diagnostic or therapeutic equipment will be used.
- Resuscitation trolley and associated equipment may be used.
- Patient will arrive on trolley.
- Mobile hoist may be used.
- A working supply of clean and sterile items is stored for immediate use.
Planning relationships
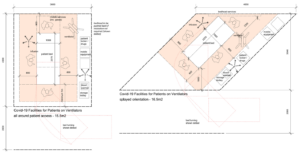
It is envisaged that this bed space will be required to supplement existing facilities in response to demand during the pandemic, and could be rapidly deployed by refurbishing/redesignating existing facilities or by providing a ‘quick-build’ solution using prefabricated units. Where a multiple bed space is used, a minimum space of 3.6 metres, bed centre – bed centre is recommended.
Space data: Area (m²)
Refer to loaded drawings.
Notes
Space is required for equipment that is used intermittently at the bed space. This may include:
- electroencephalogram (EEG) machine
- ultrasound/echocardiography
- endoscopy (fibre-optic light source)
- defibrillators
- haemodialysis
- haemo-filtration.
Separate data and voice outlets may be used where structure cabling solutions are not available.
Components
- 1 x BOARD, marker, whiteboard, dry-wipe, with pen holder, wall mounted, 600H 900W
- 2 x HOOK, single, large, wall mounted
- 8 x SOCKET outlet, switched, 13 Amp, twin
- 1 x CLEANER’S SOCKET outlet, switched, 13 Amp, single
- 1 x LUMINAIRE, examination, wall, adjustable, 1000 lux (mobile exam lamp is a project option)
- 1 x TRUNKING, medical services, length as drawn
- 1 x DISPENSER, barrier cream, disposable single cartridge, wall mounted
- 1 x DISPENSER, paper towel, wall mounted
- 1 x DISPENSER, medical hand sanitiser, lever action, wall mounted
- 1 x DISPENSER, disposable gloves (set of three) and disposable apron, wall mounted
- 1 x BED, CCU/ITU, radio-translucent rising backrest, two-way tilt, height adjustable (685–860), on castors
- 2 x HOLDER, sack, with foot-operated lid, medium, freestanding, 875H 430W 385D
- 1 x HOLDER, sharps box, up to 7 litre capacity, rail/trolley hang or wall mounted, 170H 125W 100D
- 3 x INFUSION volumetric pump, 188H 110W 60D
- 1 x INFUSION enteral feeding pump, 365H 178W 178D (project option)
- 1 x LOCKER, bedside, four compartment with lockable section/drawer, towel rail at rear, on castors, 902H 485W 485D.
- 1 x MONITOR, vital signs, multi-parameter, with accessories, 280H 360W 215D
- 1 x TROLLEY, modular storage, single open frame, including handle and worktop, with up to five sets of runners for 600 facing inserts, 850H 730W 450D
- 2 x STAND, infusion, twin hook, breaks, mobile
- 6 x SYRINGE pump, battery operated, 170H 35W 75D
- 1 x TRANSPORTER ventilator, 370H 270W 85D (compatible with medical supply unit)
- 1 x VENTILATOR, portable, adjustable minute volume, 460H 470W 310D.
Environmental data
Temperature and ventilation
- Risk of combustion: As there is likely to be enriched oxygen in the treatment area, the level of air changes through natural and mechanical ventilation must be maximised to lower the oxygen level and the risk of combustion.
- General notes: See Health Building Note 04-01 (Supplement 1).
Lighting
- Night general service illuminance: 10–20 Lux
- Local task illuminance: 1000 Lux, bed level (provided by examination lamp)
- Emergency escape route lighting required: In accordance with BS 5266 and Health Technical Memorandums.
Noise
- Noise intrusion 1 hour night: 35 dB.
- Noise intrusion f night: 45 dB.
Safety/fire
- Type of automatic fire detection: Smoke.
- Maximum surface temperature: 43oC.
- Domestic hot water discharge temperature: 41oC.
- Maximum cold water discharge temperature: <20oC.
General notes:
- Walls: Wall finishes to comply with Performance Requirements for Building Elements Used in Healthcare Facilities 8941:0.6 England.
- Wall finishes to be selected using the ‘Selection procedure for finishes’ included in Performance Requirements for Building Elements Used in Healthcare Facilities 8941:0.6 England.
- Floor and skirting: Floor finishes to comply with Performance Requirements for Building Elements Used in Healthcare Facilities 8941:0.6 England.
- Floor finishes to be selected using the Selection procedure for finishes’ included in Performance Requirements for Building Elements Used in Healthcare Facilities 8941:0.6 England.
- Ceiling: Ceiling finishes to comply with Performance Requirements for Building Elements Used in Healthcare Facilities 8941:0.6 England.
- Protection and shielding: Configuration, glazing, fire rating, security, etc to be determined by project team.
- Refer to HBN 00-04 (May 2007) for effective clear door widths. Two sets of doors:
- 1 x personnel, wheelchair and equipment access (1000 mm);
- 1 x personnel, bed, trolley, wheelchair and equipment access (1500 mm).
Notes
- All finishes to be selected using the ‘Selection procedure for finishes’ included in Performance Requirements for Building Elements Used in Healthcare Facilities 8941:0.6 England.
- All finishes selected must have an appropriate risk assessment to accompany the design decision.
- Infection control must be consulted on as described in Performance Requirements for Building Elements Used in Healthcare Facilities 8941:0.6 England.
4. Engineering
Systems affected
- Medical oxygen: liquid and gas cylinder supplies.
- Medical vacuum: with respect to recommendation not to provide piped vacuum in infectious diseases units (IDU).
Responsible parties
The following responsible parties should liaise to manage and treat:
- clinical managers
- clinical department heads
- infection control
- pathology
- pharmacy
- estates and facilities
- external medical gas suppliers.
Other parties who may be required to assist and advise:
- NHS England and NHS Improvement Estates
- medical gas authorising engineer
- medical gas contractors
- medical physics/electronic and biomedical engineering (EBME)
- medical device suppliers (oxygen concentrators).
Medical oxygen
The Department of Health and Social Care has stipulated the need to establish if existing medical oxygen systems can accommodate the supply of medical oxygen at a flow rate of 10 L/min, per bed. The main factors to consider are as follows:
The number and location of beds specifically selected to facilitate the anticipated COVID-19 infected patients. The purpose of asking this is to establish if existing departments were designed with hi-flow oxygen demand or if the department was designed for acute patient care. For example:
- within existing IDUs
- acute wards (single and multi-bedroom)
- critical care such as ITU/PICU/HDU.
The type of source of supply:
- Bulk liquid oxygen plant (VIE): note this could include:
- one compound comprising single vessels and gas manifolds
- one compound comprising duplex vessels
- two compounds each comprising duplex vessels feeding into separate parts of the mains distribution (diverse routes).
- Liquid mini-tank and gas manifolds.
- Liquid cylinders and gas manifold: specific to BOC.
- High pressure medical gas cylinders connected to an auto-change manifold, including manual emergency reserve manifold.
- Portable medical gas cylinders:
- J size cylinder and back feed kit for connection to AVSU NIST connectors
- CD, HX, etc size cylinders for individual patient administration
- oxygen concentrators for individual patient administration.
Limiting factors
To establish if your source supply and distribution systems can cope with increased, extensive demand, consider the following:
- Ask your liquid oxygen plant or manifold supplier/manufacture what the following are:
- the flowrate capability of each evaporator. This will determine the maximum flowrate the evaporators can maintain without detrimental effect on distribution pressure
- the flowrate capability of each pressure regulator. This will determine the maximum flowrate the regulators can supply without detrimental effect on distribution pressure.
Once it has been established in which departments the increased oxygen demand will be imposed:
-
- check mains distribution pipe sizes. This will determine the maximum flowrate at which the pipeline can maintain supply without detrimental effect on site-wide distribution pressure.
- Check departmental distribution pipe sizes. This will determine the maximum flowrate at which the pipeline can maintain supply without detrimental effect on local distribution pressure.
Action to be taken
The initial steps are:
- In conjunction with clinical managers, establish the number of bed spaces that can provide adequate isolation and treatment of COVID-19 infected patients. Ensure that the beds are provided with at least one medical oxygen supply terminal unit.
- Beds that must retain critical oxygen supplies, eg critical care, emergency department (ED) fesuscitation, and other departments/rooms in this category will retain the supply of oxygen.
- Based on the number of bed spaces identified for isolation and treatment of COVID-19 infected patients, calculate the expected total flow during simultaneous demand, eg 10 L/min (per patient) x 100 (number of beds) = 1,000 L/min (COVID-19 total flow).
Note: This should be offset against the original design and other departments that will still require the HTM 02-01 recommended diversified flow for areas such as: operating theatres, theatre recovery, critical care, ED resuscitation, etc. For example:
- Total offset diversified flow (TODF) (excluding COVID-19 total flow) + COVID-19 total flow (TF) = total COVID-19 enhanced flow. Example: Original total diversified flow = 3,000 L/min; COVID-19 TF = 1,000 L/min; TOFD = 2,750 L/min. Therefore, total COVID-19 enhanced flow 2,750 + 1,000 = 3,750 L/min.
As can be seen, the additional flow imposes a 25% increase on the original design flow. In this instance, the additional demand should be well within the capability of the existing liquid source supply.
- Review the medical oxygen source supply to establish that the storage capacity of the liquid and/or the oxygen cylinder source supplies are adequate and that the medical oxygen supplier (Air Products or BOC) can replenish for extended periods of time.
- Consider reducing the consumption of ‘piped’ medical oxygen for less critical departments, eg outpatient department (OPD), radiology, and use portable cylinders or oxygen concentrators.
- Where ventilators are to be used, the following will be considered:
- quantity of ventilators
- type of ventilator including manufacturer and model
- maximum and normal flow rates and pressures
- location where ventilators will be used.
- The purpose of this is to ensure that, where ventilators are to be used, they will not adversely impact on the normal operation of the plant.
Plant operation
Where oxygen consumption will increase, the following condition may be evident:
- Liquid oxygen plant mains pipeline pressure reduces to the low-level pressure setting of 3.85 bar. At this pressure the plant alarm will alert to pipeline pressure fault at the plant alarm panel(s).
Steps to remove this alarm are:
- Temporarily increase the regulator setting at the vacuum insulated evaporator (VIE) control panel; this should be no more than 4.8 bar (5.0 bar is the high pressure setting).
- Allow both primary evaporators (where provided) to supply to site.
- Estates to regularly monitor the pressures, adjust the regulators as required and ensure that the evaporators do not ice up excessively. Note: Consult your liquid oxygen supplier for recommended de-icing procedures.
- Ensure that the VIE compound and any approaches to the compound are not obstructed by vehicles, etc.
Where medical oxygen cylinder manifolds are used as the primary source supply:
- Temporarily increase the regulator setting at the manifold control panel; this should be no more than 4.8 bar (5.0 bar is the high pressure setting),
- Estates to regularly monitor the pressures and adjust the regulators as required. Estates and pharmacy to regularly monitor cylinder stock and ensure that there is adequate cylinder storage within the manifold room, or allocate a space nearby which can be used as a temporary cylinder storage facility. Note: Ensure that the cylinders are adequately secured and protected (if possible) from adverse conditions. Consult your medical oxygen cylinder supplier regarding (temporary) delivery drop-off point(s).
- Ensure that you have adequate cylinder handling trolleys and personnel suitably trained to move and replenish the cylinders.
- Ensure that the manifold room and any approaches to the area are not obstructed by vehicles, etc.
Where portable medical oxygen cylinders or oxygen concentrators are to be provided:
- Estates and pharmacy to regularly monitor cylinder stock and ensure that there is adequate cylinder storage within the central and local cylinder stores, or allocate a space nearby which can be used as a temporary cylinder storage facility. Note: Ensure that the cylinders are adequately secured and protected (if possible) from adverse conditions. Consult your medical oxygen cylinder supplier regarding (temporary) delivery drop-off point(s).
- Ensure that you have adequate cylinder handling trolleys and personnel suitably trained to move and replenish the cylinders.
- Clinical managers to ensure that clinical staff are suitably trained to handle and operate portable medical oxygen cylinders. Ensure that all the cylinder stores and any approaches to the designated areas are not obstructed.
Miscellaneous
Where medical oxygen consumption increases, steps must be taken to ensure the oxygen level (oxygen enrichment) is maintained below 23.5%. Failure to do so will increase the flammability of the room.
The following should be considered:
- Ensure that the rooms that could be subject to oxygen enrichment are adequately ventilated by mechanical and/or natural means.
- Where ventilation is deemed inadequate, consider using an alternative room.
- Regular environmental monitoring of potentially exposed rooms to ensure oxygen enrichment is controlled.
Medical air
Medical air is mainly supplied via the medical gas pipeline system and manufactured as required using compressors on the hospital site. In addition, medical air cylinders are normally used as the independent back-up supply to the pipeline system, or to supply air to patients where pipelines supplies are not available.
One of the main uses of medical air in the hospital is driving ventilators and incubators.
Medical vacuum
Although the medical vacuum system is not directly related to the use or consumption of medical oxygen, one factor which must be considered is HTM 02-01’s recommendation against the installation or use of piped medical vacuum in an ‘infectious diseases unit’. The recommendation is to use portable suction units which can be removed and sterilised.
Although this document’s purpose is to assist with implementing an ‘emergency’ action plan, and the rooms may only be allocated temporarily for COVID-19 infected patients, there may still be a risk of cross-contamination via an existing piped medical vacuum system.
Therefore, where a department/room is to be used for COVID-19 patients, the following should be considered by the estates and infection control manager/microbiologist:
- If the piped medical vacuum system is present, consider isolating the medical vacuum terminal unit and sealing it to prevent ingress of harmful bacteria.
- Where terminal units are individually fed from a dedicated medical vacuum AVSU, consider isolating the medical vacuum AVSU and inserting a black spade into the flange immediately from the pipe serving the terminal unit. By inserting the blank spade, you have effectively removed the effects of vacuum and normalised the pressure to room pressure. This should prevent harmful bacteria from entering the pipeline by way of natural pipeline and terminal unit leakage.
- Proprietary blanking plates that fit into the terminal unit or locally made covers should be installed to indicate the units are out of service and that portable units are being used. This said, it may well be an infection control and prevention (ICP) decision that the main system is used due to overall decontamination and room-loading issues.
Note: The hospital infection control procedures must be adhered to by all parties at all times.
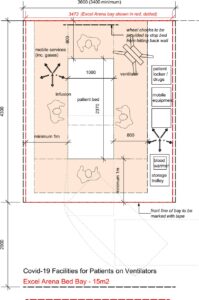
Appendix 1: Room layouts
Appendix 2: Experience from the ExCel London surge Nightingale Hospital
This Appendix sets out reflections on process and lessons learned during the planning and implementation of the surge Nightingale Hospital at ExCel London. An illustration of the bed spaces used at ExCel Nightingale Hospital and an ‘instruction manual’ produced by the architects for the site, BDP, can be found at the end of this Appendix.
Specification and requirements grew over the course of the installation. This means
that the planning team should anticipate a likely end point at the beginning and plan
accordingly, adapting as needs and resources change. In this case the project:
- started as a simple field hospital
- added gas and power
- then power and piped medical gases
- then lights
- then nurse call
- started with paper records then computer-based reporting; the facility
already had wireless capability throughout - now some dialysis bed spaces and new pipework are being added.
General considerations
This is temporary infrastructure with a limited design-life, which is likely to be several months. If in doubt, the planning team should refer to HBN and HTM requirements and in consultation with local clinical and infection control take pragmatic steps away from this to achieve what is deliverable in a very limited timeframe.
While a derogation schedule in the circumstance is unlikely to be possible, a record of the risk assessment and agreement from stakeholders should be kept. Pragmatic steps taken at ExCel arena have included construction of mock-ups and signed-off drawings by infection control and clinical leads.
Organisation and planning
Initial strategic planning decisions at ExCel:
- service runs dictated location and sizing of bays
- ceiling heights were 15m and 16m high, so unable to use traditional ceiling grids for services
- quite considerable space provided when taking into account corridor space and the benefits of opposite bed bays
- standalone liquid oxygen VIE plant set up and commissioned.Other principles evident from planning at ExCel are:
- separate entrances at opposite ends for staff and goods deliveries and for
patients and disposal - triage assessment areas for patients arriving
- support and administrative zone with logistical support and storage
- distinct COVID-19 patient zones with clean and dirty flows
- gateway zones between the support and COVID-19 zones, allowing the transfer of staff and materials in a controlled manner.
Capacity
Once the location and overall sizing of bays has been determined, they can be set out to the minimum bed space size, based on the organisation limits described below.
The available nursing ratio is likely to flex up and down based on acuity and the availability of support staff. A pair of nurse blocks is shown below:
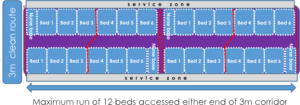
The capacity is determined by setting out the bed clusters on plan. A cluster is a
row of beds on each side of a circulation corridor.
Flows and support accommodation
There is a simple hierarchy of circulation:
- main routes: may be clean or dirty. 3.0m wide (2.4m minimum)
- cluster circulation: only for serving bed clusters 2.4m (2m minimum).
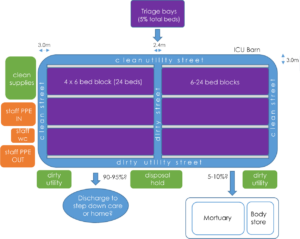
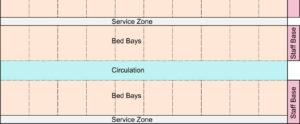
The support zone is the centre of operation. Its location will be determined by the availability of services and sanitary equipment. At ExCel the support zone contains the following:
- reception for staff
- operation and logistics centre
- toilet and shower for staff – inward-facing
- toilet facilities facing toward the COVID-19 area
- EBME medical equipment stores
- central sterile equipment stores
- central pharmacy
- central linen storage
- central consumables storage.
The gateway boundary between the support zone and COVID-19 area contains:
- lobbies to the COVID-19 patient zone with chambers for putting on PPE and for removal of PPE
- central scrub facilities
- distributed clinical, linen and clean stores supplied from the support zone.
Dirty flows at the perimeter should have direct access to:
- disposal holding positions for bagged waste including linen which may be in lockable 1,100 litre bins.
Bed cluster
Beds are clustered in multiples of 20. The maximum number of beds in a single cluster is 20, 10 each side. Behind each run of beds is an access gap for fitting and maintenance of structure and services.
At ExCel, clinical handwash facilities are planned for each bed cluster. A shortage of pre-manufactured IPS components has led to possible use of portable handwash facilities. This may not be an option.
Note that the circulation area requires separate delineation by floor marking to reduce cross infection risk.
Organisation and staffing assumptions
The available nurse-to-patient ratio is unclear and is likely to be variable. It is assumed that a team will be responsible for one or more bed clusters. The team will require good communication in an environment that may be noisy, confusing and stressful. Lines of sight and good lighting are important.
At ExCel, a staff call system is rigged at each patient bed with a single illuminated alert and reset at the end of each bank of 10 beds.
Movement of beds
Movement of beds must be considered early. The space occupied in movement of a large number of beds is substantial. There should be a large holding position adjacent to the point of patient entry/departure with facilities for cleaning.
Portering may be carried out by inexperienced staff. Temporary enclosures will be fragile and should be protected at corners with any available barrier. Within the bed bay, a conventional bed-head buffer would damage the fragile partitioning. Use a temporary bed width chock located to stop the bed wheels before the bed meets the end wall.
Nursing stations
The frequency of nursing stations will be based on the proportion of beds to staff. At ExCel, a station is planned at either end of each block of 10 beds. Where the end of a block has alternate clean or dirty flows, the nursing station should be at the clean end of a block of beds. The nursing station desktop must be a cleanable laminate type finish.
Assembly and access to services
Between each block of beds allow for a service, assembly access route to maintain the temporary assembly and manage any adjustment to the services.
Space around the bed
Design note: COVID-19 ward for intubated patients sets out the layout, equipment and space required for beds in an existing acute hospital setting. Where COVID-19 surge beds are being considered in a non-hospital setting, some pragmatic adjustment is acceptable.
Spacing, panel size and material
The most likely available partitioning systems will be based on modular exhibition panels. These are proprietary. Most have 1m or 0.5m horizontal dimensions and are approximately 2.4m high with a small gap at floor level. From this, a bay size of 3.5 x 4.3m may be used as the standard bed bay module with the flank panels extending 2m.
All bed spaces should be the same size for speed of assembly and standardisation. These can be used for intubation, and they can be flexed up or down.
Because the construction is temporary, supporting both electrical and piped oxygen, it is important that the bed bay construction is protected from damage. Rather than fixing a buffer to the partition, locate a bed-width chock on the floor to prevent beds striking the partition and damaging services.
Patient-facing partition material must have a cleanable wipe down surface. Metal partition stands breaking the smooth surface are acceptable. At ExCel Arena, OSB board has been applied to the rear of every other partition board to support stability and strength and provide a more robust fixing for gas and oxygen installation. A bracing framework over bed spaces was also needed to provide structural rigidity to modules and partitions.
Patient zone
Because the modular bed bay does not extend the full depth required, it is important that corridors and full 3.5 x 4.3m bed bays are marked to delineate the corridor movement zone. High visibility tape may be used but consider other strategies such as permanent markers.
Excel London bed layout
BDP NHS Nightingale instruction manual