Interim Clinical Commissioning Policy: Interim Clinical Commissioning Policy: Remdesivir for patients hospitalised due to COVID-19
Rapid policy statement
Publications approval reference: C1709
Publication date: 28 November 2022
Effective from: 28 November 2022
Contents
- Commissioning position
- Background and evidence
- Implementation
- Governance
- Surveillance and service evaluation
- Equality statement
- Definitions
- References
- Appendix 1
Commissioning position
Remdesivir is recommended to be available as a treatment option through routine commissioning for hospitalised adults and paediatric patients (at least 4 weeks of age and weighing at least 3 kg) with COVID-19 in accordance with the criteria set out in this document.
This policy covers the use of remdesivir for patients hospitalised due to symptoms of COVID-19 and to be treated with a 5-day course of remdesivir.
Separate policies cover the use of remdesivir in patients with hospital-onset COVID-19 and non-hospitalised patients with COVID-19.
Background and evidence
Remdesivir is an adenosine nucleotide prodrug that is metabolised intracellularly to form the pharmacologically active substrate remdesivir triphosphate. Remdesivir triphosphate inhibits SARS-CoV-2 RNA polymerase which perturbs viral replication.
Evidence
Current evidence shows that remdesivir improves clinical outcomes in both hospitalised and non-hospitalised patients with COVID-19.
The World Health Organization (WHO) updated its ‘Therapeutics and COVID-19: Living guideline’ on 16 September 2022 and the recommendations have been considered in the development of this policy (WHO, September 2022).
- Hospitalised patients: Evidence from the ACTT-1 trial showed that remdesivir improved time to recovery in patients hospitalised with COVID-19 by 5 days compared to placebo (Beigel et al, 2020). The World Health Organization (WHO) Solidarity trial indicated that remdesivir did not improve overall mortality, initiation of ventilation or duration of hospitalisation (Pan et al, 2020).
- Non-hospitalised patients: evidence from the PINETREE trial, which studied the use of remdesivir in non-hospitalised patients within 7 days of COVID-19 symptom onset and had risk factors for disease progression indicated that a 3-day course of remdesivir resulted in a relative risk reduction of 87% in hospitalisation or death at day 28 compared with placebo (Gottlieb et al, 2020).
Marketing authorisation
Remdesivir delivered intravenously has conditional marketing authorisation for use as a treatment for COVID-19 in Great Britain (under the Medicines and Healthcare Products Regulatory Authority (MHRA)) and a full marketing authorisation in Northern Ireland (under the European Medicines Agency (EMA)) for the following indications:
- treatment of COVID-19 in adults and paediatric patients (at least 4 weeks of age and weighing at least 3 kg) with pneumonia requiring supplemental oxygen (low- or high-flow oxygen or other non-invasive ventilation at start of treatment), for a treatment duration of
5-10 days. - treatment of COVID-19 in adults and paediatric patients (weighing at least 40 kg) who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19 within 7 days of symptom onset, for a treatment duration of 3 days.
Implementation
Eligibility criteria
Patients hospitalised due to symptoms of COVID-19
Patients are eligible for treatment under this policy if they fulfil the following eligibility criteria:
- SARS-CoV-2 infection is confirmed by polymerase chain reaction (PCR) test or where a multidisciplinary team (MDT) has a high level of confidence that the clinical and/or radiological features suggest that COVID-19 is the most likely diagnosis AND
- Hospitalised specifically for the management of COVID-19 symptoms AND
- Requiring low-flow* supplemental oxygen (see later section on ‘Immunocompromised patients’ for how this criterion applies to this group) AND
- Presented to hospital not more than 10 days since symptom onset AND
- Estimated glomerular filtration rate (eGFR) at least 30 ml/minute AND
- Alanine aminotransferase (ALT) below 5 times the upper limit of normal at baseline.
*Low-flow oxygen supplementation as defined in the NICE COVID-19 Rapid Guideline: Managing COVID-19.
Exemptions to the above eligibility criteria apply to the following patient groups:
- Patients with end-stage renal disease on haemodialysis are exempt from the eGFR treatment threshold above
- Significantly immunocompromised patients (see later section on ‘Immunocompromised patients’ for exemptions in this cohort).
Considerations during clinical decision-making should include the following (see also the clinical pathway in Appendix 1). (Clinical judgement in the initiation, review, escalation and de-escalation of patients receiving remdesivir treatment should be supported where possible by multidisciplinary team assessment.)
- Risk assessment
- Clinical judgement around treatment with remdesivir can be informed by a risk score. Those with a low 4C Mortality Score* (0 to 3) are highly likely to recover without treatment with remdesivir.
- Remdesivir should not be initiated in patients who present to hospital and are unlikely to survive (determined by clinical judgment). The 4C Mortality Score might be helpful in this assessment.
- Initiation of treatment
- The decision to initiate treatment with remdesivir should be made by the admitting care consultant.
- Remdesivir should not be initiated in patients who present to hospital more than 10 days after symptom onset (see exemption in immunocompromised patients below).
- Duration
- All patients treated under this policy must receive a maximum of 5 days of remdesivir in total (comprising a loading dose plus 4 further days of maintenance doses).
- Patients re-admitted for symptoms of COVID-19 (and meeting the eligibility criteria, with the exception of the requirement on the timing from symptom onset) are permitted a second course of up to 5 days upon readmission.
- Significantly immunocompromised patients (see below) are eligible for an extended course of remdesivir (up to 10 days), if agreed following multidisciplinary team assessment.
- Reassessment and review
- The use of remdesivir should be reassessed daily. Consider stopping remdesivir if:
- The patient clinically improves and no longer requires supplemental oxygen 72 hours after commencement of treatment; or
- The patient continues to deteriorate despite 48 hours of sustained mechanical ventilation.
- The use of remdesivir should be reassessed daily. Consider stopping remdesivir if:
- Immunocompromised patients
- For significantly immunocompromised patients** hospitalised for COVID-19 symptoms:
- A course of remdesivir can be extended to a maximum of 10 days
- The criterion on time between symptom onset and treatment initiation does not apply
- The criterion on the need for supplemental oxygen requirement does not apply.
- For significantly immunocompromised patients** hospitalised for COVID-19 symptoms:
*The 4C Mortality Score (available at https://isaric4c.net/risk/) is a validated risk stratification score, which can help inform clinical decision making for patients admitted to hospital with COVID-19 (Knight et al, 2020). Other clinical risk scores are available.
**Refers to patients with a significant impairment of humoral immune response (antibody production) and/or cellular immune competence.
Patients with hospital-onset COVID-19
Please refer to the UK Clinical Commissioning Policy for antivirals or nMABs for hospital-onset patients with COVID-19.
Non-hospitalised patients with COVID-19
Please refer to the UK Clinical Commissioning Policy for antivirals or nMABs for non- hospitalised patients with COVID-19.
Where possible, all patients being considered for treatment with remdesivir should have samples taken for serology (anti-S (spike) antibody) prior to treatment. However, SARS- CoV-2 antibody tests or results are not a requirement for treatment with remdesivir under the criteria specified in this policy.
Exclusion criteria and cautions
Patients who meet any of the following exclusion criteria are NOT eligible for treatment with remdesivir under this policy:
- Children aged less than 4 weeks of age and/or weighing less than 3kg
- Known hypersensitivity reaction to the active substances or to any of the excipients of remdesivir as listed in the Summary of Product Characteristics for Great Britain and Northern Ireland.
- Estimated glomerular filtration rate (eGFR) <30 mL/min (except in patients with end- stage renal disease on haemodialysis)
- Alanine transaminase (ALT) ≥ 5 times the upper limit of normal
Remdesivir should be discontinued in patients who develop any of the following:
- ALT ≥ 5 times the upper limit of normal during treatment with remdesivir (remdesivir may be restarted when ALT is < 5 times the upper limit of normal)
- ALT elevation accompanied by signs or symptoms of liver inflammation or increasing conjugated bilirubin, alkaline phosphatase, or international normalised ratio (INR)
An individual clinical decision should be made as to whether pre-treatment urea and electrolytes and liver function tests are required, based upon whether recent bloods are available or the patient is considered at risk of undiagnosed liver or kidney disease.
Hypersensitivity reactions including infusion-related and anaphylactic reactions have been observed during and following administration of remdesivir. Signs and symptoms may include hypotension, hypertension, tachycardia, bradycardia, hypoxia, fever, dyspnoea, wheezing, angioedema, rash, nausea, vomiting, diaphoresis, and shivering. Slower infusion rates, with a maximum infusion time of up to 120 minutes, can be considered to potentially prevent these signs and symptoms. Patients should be monitored for hypersensitivity reactions during and following administration of remdesivir as clinically appropriate. If signs and symptoms of a clinically significant hypersensitivity reaction occur, administration of remdesivir should be discontinued immediately and appropriate treatment initiated.
Dose
The recommended dosage for adults and paediatric patients (weighing at least 40kg) under this policy is a single loading dose of remdesivir 200 mg intravenously on day 1, followed by a once daily maintenance dose of remdesivir 100 mg for the remainder of the treatment course, which should not exceed five days (see exemption in immunocompromised patients above).
The recommended dosage for paediatric patients at least 4 weeks old (weighing at least 3kg but less than 40kg) under this policy is a single loading dose of remdesivir 5mg/kg intravenously on day 1, followed by a once daily maintenance dose of remdesivir 2.5mg/kg for the remainder of the treatment course, which should not exceed five days (see exemption in immunocompromised patients above).
Administration
200mg of remdesivir (day 1 loading dose) and 100mg of remdesivir (days 2-5 maintenance doses) should be diluted in either a 250ml or 100ml pre-filled bag of 0.9% sodium chloride solution and infused over a minimum of 30 minutes. Treatment should be initiated as soon as possible after admission to hospital and within 10 days of symptom onset.
Renal and liver function should be monitored carefully during treatment with remdesivir as clinically appropriate.
Genotyping and sequencing of samples
Sequencing is an important part of surveillance activities to monitor for the development of new variants and drug resistance. Therefore, in patients being considered for treatment with remdesivir, samples pre-treatment and where part of the clinical pathway, post-treatment, should be prioritised for sequencing. Genotype results do not form part of the eligibility criteria for treatment with remdesivir and treatment should not be delayed pending these results.
Pregnancy
There are no or limited amount of data from the use of remdesivir in pregnant women. Remdesivir should be avoided in pregnancy unless clinicians believe the benefits of treatment outweigh the risks to the individual (please see the relevant SmPC for further information). Women of child-bearing potential have to use effective contraception during treatment.
Co-administration
There is no interaction expected between remdesivir and other commissioned treatments for COVID-19. For further information please visit the University of Liverpool COVID-19 Drug Interactions website (https://www.covid19-druginteractions.org/checker).
Please refer to other published UK clinical commissioning policies setting out available COVID-19 treatments here.
Safety reporting
Any suspected adverse drug reactions (ADRs) for patients receiving remdesivir should be reported directly to the MHRA via the new dedicated COVID-19 Yellow Card reporting site at: https://coronavirus-yellowcard.mhra.gov.uk/
Governance
Data collection requirement
Provider organisations in England should register all patients using prior approval software (alternative arrangements in Scotland, Wales and Northern Ireland will be communicated) and ensure monitoring arrangements are in place to demonstrate compliance against the criteria as outlined.
Clinical outcome reporting
Hospitals managing COVID-19 patients are encouraged to submit data through the ISARIC 4C Clinical Characterisation Protocol (CCP) case report forms (CRFs), as coordinated by the COVID-19 Clinical Information Network (CO-CIN) (https://isaric4c.net/protocols/).
Effective from
This policy will be in effect from the date of publication.
Policy review date
This is an interim rapid clinical policy statement, which means that the full process of policy production has been abridged: public consultation has not been undertaken. This policy may need amendment and updating if, for instance, new trial data emerges, supply of the drug changes, or a new evidence review is required. A NICE Technology Appraisal or Scottish Medicines Consortium (SMC) Health Technology Assessment or All Wales Medicines Strategy Group (AWMSG) appraisal of remdesivir for COVID-19 would supersede this policy when completed.
Surveillance and service evaluation
There is an urgent need to generate more evidence and greater understanding around use of treatments in patients with COVID-19. Both surveillance and service evaluation are necessary to gain knowledge around the following: factors of relevance in determining neutralising monoclonal antibodies (nMABs) and antiviral treatment; the impact of nMAB and antiviral treatment in the community and hospital settings on the immune/virologic response and clinical recovery; and the public health sequelae of nMAB and antiviral use, such as generation of new mutations and/or variants.
Treating clinicians are asked to ensure that all PCR tests undertaken as an inpatient and/or in the community where any patient who is receiving ongoing PCR testing as part of secondary care (for example, through an outpatient clinic) should do this through the hospital laboratory where these samples should be retained for sequencing. Further serial sampling for specific patient groups may be requested as part of UKHSA genomic surveillance purposes, or country specific programmes.
Clinicians must ensure that any additional data collection requirements are met for the purpose of relevant surveillance, audit and evaluation around the use of nMABs and antivirals. It is expected that there will be ongoing monitoring (involving sample collection) of selected patients treated with nMABs and antivirals (led by UKHSA, for instance around the potential generation of new variants), as well as academic research to generate new knowledge around clinical effectiveness and other relevant aspects of public health.
Equality statement
Promoting equality and addressing health inequalities are at the heart of the four nations’ values. Throughout the development of the policies and processes cited in this document, we have:
- Given due regard to the need to eliminate discrimination, harassment and victimisation, to advance equality of opportunity, and to foster good relations between people who share a relevant protected characteristic (as cited under the Equality Act 2010 or equivalent equality legislation) and those who do not share it; and
- Given regard to the need to reduce inequalities between patients in access to and outcomes from healthcare services and to ensure services are provided in an integrated way where this might reduce health inequalities.
Definitions
| COVID-19 | Refers to the disease caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus. |
| Mechanical ventilation | A life support treatment which helps people breathe when they are not able to breathe enough on their own. |
| Extra Corporeal Membrane Oxygenation | A life support machine for people who have a severe and life-threatening illness that stops their heart or lungs from working properly. |
References
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 – Final Report. N Engl J Med. 2020;383(19):1813-1826. doi:10.1056/NEJMoa2007764
- Gottlieb RL, Vaca CE, Paredes R, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med. 2022;386(4):305-315. doi:10.1056/NEJMoa2116846
- WHO Solidarity Trial Consortium, Pan H, Peto R, et al. Repurposed Antiviral Drugs for Covid-19 – Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384(6):497-511. doi:10.1056/NEJMoa2023184
Appendix 1
Clinical pathway and criteria for the use of remdesivir in patients hospitalised with COVID-19 (adults and children 12 years and older)
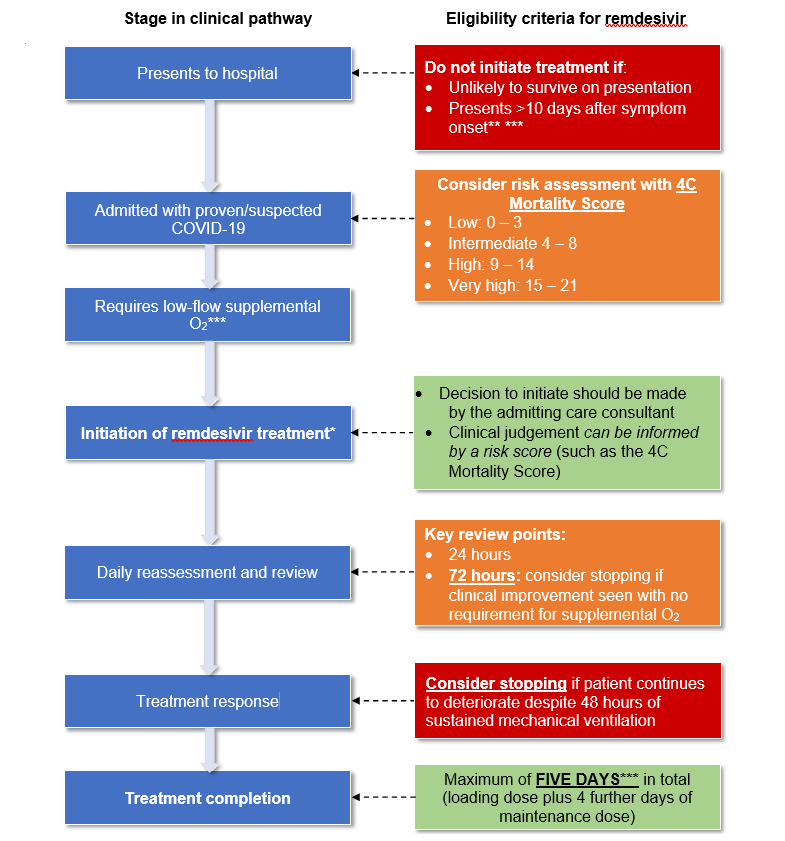
*There should be careful consideration before initiating remdesivir treatment
**Unless patient is readmitted with COVID-19 (see main policy document)
***See additional comments on immunocompromised patients
