Lateral flow antigen test FAQs
Classification: Official
Publications approval reference: C0913
Lateral flow antigen test FAQs
Second wave roll out trusts
Updated version 3, 28 January 2021 – Additional questions highlighted in yellow.
Frequently asked questions
These are the specific FAQs related to the lateral flow antigen tests. For all questions on HR processes following a positive test and related isolation questions, please refer to NHS Employers’ FAQs on asymptomatic staff testing.
Q. What type of test are we rolling out?
The Innova SARS-CoV-2 Antigen Rapid Qualitative Test uses a swab which has been in contact with the nostril of the person being tested. The swab is inserted into the extraction tube with the extraction fluid and then rotated and pressed to make sure that the sample from the swab is released into the extraction fluid (swab is then discarded at this point).
You then take the extraction tube with the nozzle cap and place 2 drops of extraction fluid into the sample well of the LFD testing device cartridge and wait for the results on the test device.
Q. What is the specificity and sensitivity of this particular test?
The government has published its latest research on these tests. This can be found here https://www.ox.ac.uk/news/2020-11-11-oxford-university-and-phe-confirm-high-sensitivity-lateral-flow-tests-following
Q. Is the test mandatory or voluntary?
Tests are voluntary, but staff should be encouraged to be involved in the testing to benefit their colleagues and patients.
Q. How frequently should staff be tested?
Staff should test themselves twice weekly every three to four days to fit with shift patterns and leave requirements; for example, Wednesday and Sunday, or Monday and Thursday.
Q. Should I continue testing after I’ve had the vaccine?
Yes, continue to test even though you have had the vaccine.
Q. If a staff member has a positive PCR COVID-19 test, when should they start the lateral flow antigen tests again?
A staff member who tested positive would recommence home testing 90 days after their positive test was taken. The staff member will need to liaise with their NHS organisations to track the date at which the retesting should start.
Q. What happens if staff get a positive result?
Staff should inform their manager of a positive result trust in the normal way. A confirmatory PCR test will be arranged. They and their household should isolate as set out in government guidance.
Q. What happens if my test is negative, but I have coronavirus symptoms?
If you have coronavirus (COVID-19) symptoms, please refer to NHS guidance online.
Q. Are we asking potentially positive staff to come to hospitals for a confirmatory PCR test?
Trusts should use their normal processes to access tests for staff members who have symptoms of COVID-19, whether that be through pillar 1 or 2. These processes assume that staff may be infected with COVID-19 and therefore suitable IPC and PPE will be in place. Staff should continue to isolate until they have the results of the PCR test.
Q. What should staff do with the used tests?
Staff can safely dispose of the test items in their normal household waste but should pour any residual buffer solution away first.
Q. What happens if the buffer solution is accidentally consumed?
As set out in the manufacturer’s safety instructions, the buffer solution is not hazardous; however, if accidentally ingested, a medical practitioner should be informed.
Q. What is the shelf life of the extraction (buffer) solution once opened?
The shelf life of extraction solution is 2 years, even after it is opened.
Q. How do I get additional bottles of extraction (buffer) solution as I don’t have any left (due to spillage etc.) but I still have kits left in my box of 25 kits?
Each region has identified one or more Trusts within the region who will hold a stock of extraction solution for onwards distribution.
For additional stock of extraction solution your Trust should contact their regional testing teams who will coordinate distribution.
Q. At what stage is Test and Trace informed of the result?
At the point the confirmatory PCR test result is known, and this is positive result, test results will, as normal, be referred to Test and Trace.
Q. If I am told to isolate by Test and Trace even though I have had the vaccine, do I need to do so?
Yes, continue to take advice and follow instructions given by Test and Trace.
Q. If staff are already regularly being tested through existing regimes – e.g. professionals visiting care homes, SIREN testing, PCR testing in Tier 3 areas, etc – should this be replaced by lateral flow tests?
If staff are already enrolled in another testing regime through their NHS organisation, this should not be replaced by the lateral flow tests unless agreed by your organisation.
If they are participating in research studies where the frequency of testing is not weekly (e.g. monthly) they should undertake twice-weekly LFD self-testing. For example, staff members participating in the SIREN study and having qRT PCR testing every two weeks should also be part of the twice-weekly LFD testing if they are a patient-facing member of staff.
Q. What about LAMP testing – I thought this was being used for asymptomatic staff testing?
LAMP testing has been piloted at a number of sites for consideration for asymptomatic staff testing. Further rollout will depend on the outcomes of the early adopter sites.
Q. How many tests will staff get?
The testing kits will arrive in boxes containing the following:
- 25 foil pouches containing the test cartridge and a desiccant
- two vials of 6 mls buffer solution
- 25 extraction tubes and 25 tube caps
- 25 sterilised swabs for sample collection
- The manufacturer’s instructions for use of the device (IFU). You will receive instructions for NHS staff separately from the box, and it is these NHS staff instructions that staff should follow.
Q. Can these tests be used for patients?
PCR tests should continue to be used for patients.
Q. Our trust would like to use these new faster tests to manage patient flow in the Emergency Department – are we okay to use in this way?
Yes. The tests can be used in accordance with the SOP at https://www.england.nhs.uk/coronavirus/publication/standard-operating-procedure-lateral-flow-device-testing-for-emergency-department-patient-pathways/.
Q. Should patients who have been in direct care of a staff member who tests positive with lateral flow be tested while the confirmatory PCR test result is pending?
Your organisation’s protocols for tracing contacts should be followed.
Q. Will this testing regime remove the need for staff who have been exposed to a positive Covid-19 case to self-isolate?
Government self-solation advice should be followed at all times. This test does not remove the need to self-isolate should you otherwise need to.
Q. Can 14-day isolation following contact tracing be shortened through use of this testing?
No. Fourteen-day isolation following notification that a staff member has been in close contact with a COVID-19 case without relevant PPE should be followed as per Test and Trace advice. Testing with lateral flow antigen tests are being used in pilot sites to verify whether daily testing might lessen the need to isolate, but this is not currently the advice and isolation should be followed as per instructed by Test and Trace.
Q. Is there any prioritisation of which staff this should be rolled out to first?
Sufficient volumes of the lateral flow devices will be sent to organisations to enable all staff to be given the test asap.
Q. Can staff use the tests for their symptomatic family members?
Staff and family members who have symptoms should access tests in the normal way.
Q. Can tests be used as a response to Covid-19 outbreaks?
Should an outbreak be declared in your organisation, testing regimes should be discussed in line with your normal organisational response.
Q. Why is the testing method different from that described in the manufacturer’s original instructions for use?
We are recommending the swab is used and the sample taken in a different way to the instructions for use, with more rotation of the swab at a lower level of penetration, to enable easier self-administration of the test. This is based on advice from experts. The manufacturer has been informed of the planned use of the tests for self-administered asymptomatic staff testing within the NHS and trusts have been asked to provide a local support package to include staff access to a helpline/further training and, if deemed necessary, on-site training arrangements. It is recommended that staff are observed by a trained healthcare colleague the first time they administer the test.
Q. You say that it is recommended that the first test is observed. This presents logistical issues, so can staff be trained to take the test but not observed?
We advise that any staff member who needs support undertaking the test is provided with appropriate support and training and observed on the first occasion. Trusts should use their discretion as which staff may require additional support. Observation of the first test is not mandatory for all staff.
Q. Is there advice on giving staff time back from undertaking the test at home?
The test should take no longer than 5 minutes to undertake, with a 30 minute wait for results.
Q. When deliveries arrive what size of space should be allocated for them?
Tests will arrive on pallets, there are usually 12 boxes on a pallet that contain 27 smaller boxes that contain 25 tests in each – 8,100 tests in total.
Q. Should the tests be kept in specific conditions; will they require security like Tamiflu did?
Tests can be stored in typical warehouse conditions; they do not need refrigeration but should be kept out of direct sunlight and not be exposed to heat. They are not expected to require any additional security than other items of NHS deliveries.
Q. Can hospitals procure their own supply of lateral flow tests?
Lateral flow tests are purchased and provided centrally, and trusts should not purchase them directly from suppliers.
Q. What are the financial arrangements in place to be able to support this roll out of testing?
Providers were reimbursed for the rolling out of lateral flow testing for staff with the agreed fixed payment value based on the number of patient facing staff.
This payment was made on the same day (15 December) as the provider’s month 6 retrospective top up payment.
Q. When will we receive our delivery of tests?
We are rolling out the second wave of deliveries week commencing 25th January; you will be notified two days in advance of any scheduled delivery of tests.
Q. For trusts with multiple sites can you confirm they can have tests delivered to multiple sites?
Due to the extent of the logistics required, we can only have a single delivery for each organisation. Trusts will be notified in advance of the delivery schedule.
Q. Can you give guidance on whether Community Interest Companies and Independent Sector Providers providing patient facing care will be included in the testing?
Yes, staff delivering NHS services in Community Interest Companies and Independent Sector Providers are within scope
Q. Are 111 and 999 call handler staff included in the testing?
While 111 and 999 call handler staff are not patient facing, they are considered a high risk group because of the way in which they work and the impact of staff absence on the service should an outbreak occur. We are therefore including them in asymptomatic testing.
Q. How frequently should a contractor/temporary worker be working in a trust to be included in testing?
Staff who are patient facing and are regularly working in your trust should be included in the testing. This should include those patient-facing staff who are directly contracted by your trust.
Q. Doctors on their foundation programme move hospitals, how should their testing programme work?
All foundation year doctors in your trust when you roll out staff testing should be included as they are patient facing. If they move to another trust while they are still testing and have a supply of tests, they should keep these tests but report the test results to the trust they are now working at. The same instruction should be given to any new starters, if they don’t already have tests from a previous employer, they should be provided with tests to start testing with you.
Q. Do we treat two positive lateral flow antigen test results as an outbreak?
Lateral flow antigen positive test results should be confirmed through PCR testing; if the confirmatory tests are also positive, then normal outbreak protocols would apply.
Q. Should staff continue swabbing during annual leave?
Staff may continue to swab whilst on annual leave of longer than a week, but it is not a requirement.
Q. Do I have to share my result if I am going into a care home?
Yes, please share your result. If you are unable to do this you may be asked to undertake an LFD before entering the care home.
Q. Is confirmatory PCR testing accessible through pillar 2, and if yes what field should be filled to avoid symptomatic questions?
You should use whatever PCR route is in use by your organisation. If this is through pillar 2, tick the box that indicates you are a key worker but not part of a pilot, you will then see an option to say ‘I’ve been told to take a Coronavirus test’ on the form.
Q. Can you confirm the reporting requirements?
Trusts are asked to collate and report to PHE the positive and negative lateral flow results at least once a week. This fulfils the statutory reporting requirements for COVID-19 testing. Trusts are also required to report back on an NHSEI daily sitrep the number of tests distributed and number of staff who are absent from work having tested positive on a lateral flow device.
Q. How should organisations collate the positive and negative results from staff?
We are aware that trusts have developed a number of efficient solutions for capturing the data e.g. using webforms or apps. Examples of these will be shared with regional testing leads. NHS Digital is also developing a digital solution which will be shared when available.
Q. How do I submit the testing returns to Public Health England (PHE)?
All NHS organisations will be required to collate and submit returns to Public Health England via a data upload to its Point of Care Test (POCT) portal at least once a week. This is a statutory requirement as COVID-19 is a notifiable disease.
Organisations which do not currently have access to this portal should send an email to POCT.Contact@phe.gov.uk. Users will then receive an email with a registration link. Once registered they can use the web app to upload the reporting template spreadsheet, which is available for download on the portal.
It is essential to use the reporting template provided by PHE to upload your Trust results.
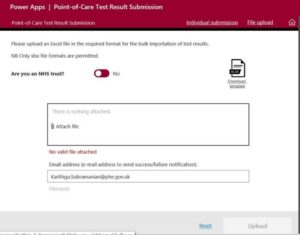
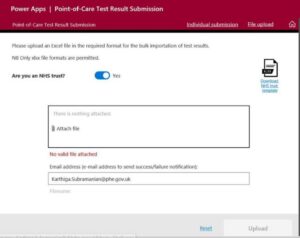
On the portal the ‘Requesting Organisation Type’ field must be populated with the dropdown “Healthcare Worker Testing”. This is essential to ensure that test and trace activity is not triggered as a result of lateral flow testing.
Trusts must use the drop down in ‘Requesting Organisation’ to identify their organisation. This ensures that a consistent trust name is used so that reports are correctly linked.
The default is to have the toggle as ‘no’ for “Are you an NHS trust?” (see below). You will need to change this to “yes”. You may need to leave blank the email address section as this is defaulted to the person logged-in.
Q. Can we get the lateral flow guidance leaflet in other languages?
There are no plans to translate the lateral flow guidance leaflet.
Q. What about staff that form part of the local authority team e.g. school nurses, or those in care homes or hospices?
The DHSC are responsible for rolling out lateral flow antigen tests to local authorities and staff delivering care outside the NHS. Contact LFDincidents@dhsc.gov.uk for more information.
Q. Where should I direct any enquiries?
Email questions to england.covid-LFD@nhs.net.
NHS Testing Programme
NHS England and NHS Improvement
Skipton House
80 London Road
London
SE1 6LH
This publication can be made available in a number of other formats on request.
Publication approval reference: C0913
