Standard operating procedure: Ordering inventory for COVID-19 vaccinations in NHS hospital hubs
Contents
Official
Publication approval reference: C0940
Novel coronavirus (COVID-19) standard operating procedure
Ordering inventory for COVID-19 vaccinations in NHS hospital hubs
This guidance is correct at the time of publishing. However, as it is subject to updates, please use the hyperlinks to confirm the information you are disseminating to the public is accurate.
1. Purpose
This standard operating procedure (SOP) describes the process for ordering inventory needed for vaccinating in NHS trust settings (‘hospital hubs’).
2. Scope
This procedure covers all aspects of ordering centrally procured National COVID-19 vaccination programme items – such as COVID-19 Vaccines, personal protective equipment (PPE), consumables, equipment – and should be used in conjunction with local procedures for the ordering of medicines, and the ‘Vaccine Ordering’ SOP set by the programme.
3. Responsibility
The relevant Provider Trust Chief Pharmacist or CCG Lead Pharmacist shall have oversight of the supply chain and oversight of management of stock on all vaccination sites operating within or under the jurisdiction of their NHS hospital hub.
Anyone that is trained and authorised to order supplies on the COVID-19 vaccination programme should use this SOP.
4. Definitions
Table 1: Terms and definitions
| Term | Definition |
| Hospital hub | Hospital that has been validated by the programme to receive and administer the COVID-19 vaccine(s) |
| PHE | Public Health England |
| PPE | Personal protective equipment |
| 3PL | Third party logistics organisation, providing warehouse and transportation |
| Foundry | The workflow management and data tool used |
| SIL | Supply items list |
| JCVI | Joint Committee on Vaccine Immunisation |
| Programme Control Tower | Central programme team overseeing logistics activities |
| ULT | Ultra-low temperature |
| NPC | National product code (recognised purchasing reference number on the NHS catalogue) |
5. Procedure
Hospital hubs are responsible for ensuring that they have all the necessary equipment, vaccines and consumables (including PPE) required to carry out COVID-19 vaccinations.
We (NHS England and NHS Improvement) have worked with clinicians and NHS procurement experts to develop inventories for each COVID vaccination delivery model with recommended items by NPC codes and estimates of quantities required.
There are three types of products that a site requires:
-
- Equipment: The equipment needed to setup a vaccination session from office equipment to furniture to IT equipment, as identified on the SIL.
- Vaccine: MHRA approved vaccines for COVID-19.
- Consumables (including PPE): All consumables required for vaccination sessions including medical equipment, clinical consumables and PPE as identified on the SIL.
When a new Hospital hub readiness assessment has been completed and assured, an order for a pre-defined set of Equipment agreed by the clinical team in the programme will be automatically triggered, pushing the relevant items to the site.
A specific ‘bundle’ of items linked to the type of vaccination site has been pre-determined by the programme.
The logistics organisation (3PL) responsible for delivering the bundle must set up the new hospital hub in their systems and complete their logistics route planning before delivering, which can take up to four days.
How many sites are being set up concurrently may delay this process by an extra day or two; timelines will be communicated to the site. Once the site is set up and the 3PL then receives an order from the programme, the equipment will arrive within three days.
The regional team will inform the hospital hub of the estimated delivery date.
If an item delivered is not needed; or if there are items required that are missing or damaged; or if a new item not on the ‘SIL’ is required crucial to being able to manage a vaccination session; then the person responsible for ordering stock should call the ‘Service Now’ helpline to raise a ticket which will be routed to the appropriate department.
The ‘Service Now’ helpdesk for the COVID-19 vaccination program is 0300 200 1000. The registered site address and the name of the person responsible for ordering (raising the ticket) will need to be provided for each call (see Appendix C).
The Helpdesk will pursue a resolution to the issue providing any information such as delivery estimates for the replacement equipment.
5.2 Allocation and ordering vaccine
The prioritisation of vaccine delivery to population cohorts has been recommended by the JCVI, and the programme will allocate vaccine in accordance with this prioritisation according to an agreed set of principles and in line with operational feasibility.
The process for managing the allocation of vaccines to these cohorts takes place centrally on a weekly cycle, assessing the latest supply quantity, the routing of priority cohorts through delivery models, the sites approved as able to administer vaccine, and the volume of patient throughput these sites can achieve. This is balanced to achieve equity across regions.
Operational metrics (eg residual stock, wastage, reported demand and capacity) will additionally inform the allocation of vaccine to sites.
On a weekly basis, an Allocation Committee will approve allocations, and the activation date of these allocations. Approved allocations will be communicated to PHE, and sites will be informed of their allocation.
The activation date is the date on which ordering will be made live via Immform.
On instruction from the Allocation Committee, PHE will ringfence the stock for that site.
Once the activation date is reached, PHE will activate the ability to order the stock via Immform, and the account holder will receive a notification that the site can order their stock.
When placing an order for vaccine, a site should consider the residual amount of vaccine left in storage, how much additional storage space is available in the appropriate storage conditions and ensure that they will be able to meet their target throughput. Packs should only be ordered where there is certainty that vaccine can be safely stored or used within the expiry window in order to minimise wastage.
The person ordering must ensure that adequate PPE and a well-ventilated room is available for receiving and unpacking dry ice shipments prior to ordering. All recommendations of the dry ice risk assessment conducted must be followed. The person responsible for ordering should also plan to be present at the site upon delivery and plan the transition of stock from the dry ice shipping box to the appropriate advised storage conditions.
A number of consumables deemed critical to the administration of the vaccine such as needles, syringes and saline have been linked to the vaccine and will be delivered along with the vaccine unless specifically advised.
SOP: VH1 – Ordering Pfizer-BioNTech COVID-19 Vaccine from Public Health England (PHE) should be used for ordering Vaccine. This SOP can be accessed at https://www.sps.nhs.uk/articles/pharmacy-institutional-readiness-for-management-of-pfizer-biontech-covid-19-vaccine-guidance-for-chief-pharmacists
The operational hours for ordering are in Appendix A.
The ‘Service Now’ helpline should be used to log tickets regarding any issues with vaccine ordering or supply, including emergencies (see Appendix C).
5.3 Ordering consumables and PPE
Consumables (including PPE) are automatically sent to a site based on their vaccine allocation.
A directly proportional amount of consumables (including PPE) is packed into a ‘bundle’ by the 3PL and sent to the Hospital hub that corresponds to the quantity of vaccine allocated to that site. This ensures that the consumables (including PPE) arrive at the Hospital hub at least three days before the vaccine.
It is important for a site to report residual stock periodically so that bundles can be adjusted by regions.
If a site has too many or too few of a particular item included in the bundle, the person responsible for ordering should raise a ticket on the ‘Service Now’ helpline, which will be routed to the relevant department to ensure that stock is adjusted or additional stock sent out.
Non-standard items such as ‘obese syringes’ (for morbidly obese patients), or extra small/large gloves can be ordered; the person responsible for ordering should raise a ticket on the ‘Service Now’ helpline which will be rooted to the relevant department.
6. Document history
| Version | Prepared by | Revision history | Date |
| V1.0 | AK | 1st Draft AK. Updates from the EECL team, SPS, and Clinical Governance team | 04 DEC 2020 |
7. References
SOP VH1 – Ordering COVID-19 mRNA Vaccine BNT162b2Vaccine Vaccine from Public Health England (PHE)
8. List of appendices
Appendix A
PHE hours of operation:
The following ordering times are in place for PHE:
- Orders placed by authorised accounts before 11:55am Monday to Friday will receive next day delivery (Tuesday to Saturday respectively).
- Orders placed after 11:55 Friday and before 11:55 Saturday will be delivered Monday.
- Orders placed after 11:55 Saturday and before 11:55 Monday will be delivered Tuesday.
- A separate delivery schedule will be developed for the Christmas holiday period.
- It should be noted that each hubs delivery frequency must be aligned to the rate of vaccine consumption so that waste is minimised. It is anticipated that most vaccine hubs will not receive more than two deliveries per week.
Appendix B
Figure 1: Ordering process diagram
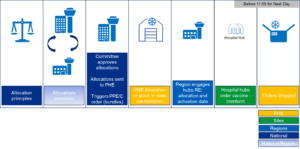
Appendix C
ServiceNow helpdesk hours of operation: 0600 – 2200 daily.
To raise a ticket, will need to know:
- Who the caller is
- What the issue relates to
- What site they are calling about
- Their contact details.
