How are we doing?
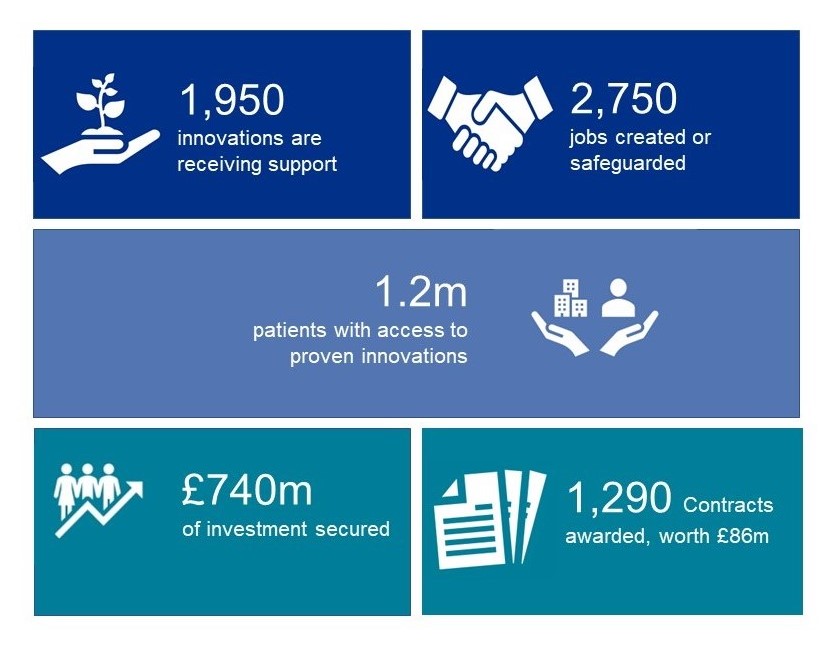
In 2022/23, we provided over 1.2 million patients access to proven innovations.
In the fourth year of our expanded role, following the pandemic and against the backdrop of continuing pressures on the NHS, we have delivered some incredible achievements across the health service.
Further information
The NHS Accelerated Access Collaborative (AAC) information portal is used to monitor the impact of the AAC. It includes a range of measures that can be substantially and directly affected by AAC programmes and a complimentary view of the broader innovation landscape. If you would like to find out more about the AAC information portal, please email england.irlsanalytics@nhs.net
