Community Pharmacy Phase 3 Site Designation Process
Contents
- Equalities and health inequalities statement
- 1. Introduction
- 2. Designation process
- 3. Designation Process Chart
- 4. Timescales
- Appendices
COVID-19 Vaccination programme: phase 3 2021/22
Classification: Official
Publication approval reference: C1350
14 July 2021, Version 1
Equalities and health inequalities statement
“Promoting equality and addressing health inequalities are at the heart of NHS England’s values. Throughout the development of the policies and processes cited in this document, we have:
- given due regard to the need to eliminate discrimination, harassment and victimisation, to advance equality of opportunity, and to foster good relations between people who share a relevant protected characteristic (as cited under the Equality Act 2010) and those who do not share it;
- given regard to the need to reduce inequalities between patients in access to, and outcomes from, healthcare services and in securing that services are provided in an integrated way where this might reduce health inequalities.”
If you have any queries about the designation process, please send these to england.pccovidvaccine@nhs.net.
If you have specific questions about your Expression of Interest, then these should be directed to your local NHS England regional team. Contact details can be found at https://www.england.nhs.uk/primary-care/pharmacy/pharmacy-contract-teams/.
1. Introduction
1.1. This document describes the process and timeline for Community Pharmacy Contractors (as defined in this document) to express an interest in delivering services under the Community Pharmacy Local Enhanced Service COVID-19 Vaccination Programme: Phase 3 2021/22 (the “LES”). The LES agreement is available here. This document also sets out the process and assurance criteria for sites to be designated in phase 3. It should be read by Pharmacy Contractors, CCGs, ICSs/STPs and NHS England regional teams.
1.2. The LES is commissioned by the Commissioner (as defined in this document) pursuant to the Pharmaceutical Services (Advanced and Enhanced Services) (England) Directions 2013 having engaged with Local Pharmaceutical Committees. Under direction 14, NHS England is only authorised to arrange for the provision of enhanced services with Pharmacy Contractors and so only Pharmacy Contractors are eligible to submit an Expression of Interest in accordance with this document.
1.3. In Phase 1 and Phase 2, around 700 pharmacies were approved to deliver Phase 1 and 2 COVID-19 vaccinations to fill gaps in geographical coverage and reduce health inequalities.
1.4. All community Pharmacy Contractors are invited to express an interest in delivering services under the LES regardless of whether they have participated in Phase 1 and/or 2 of the COVID-19 vaccination programme.
1.5. This document sets out the Designation Process which has been established to ensure that the commissioning of COVID-19 vaccination services is equitable and transparent and provides assurance to the Commissioner that all pharmacy-led sites administering COVID-19 vaccination under the LES meet the required criteria, including that of value for money. The Designation Process must be followed by all community Pharmacy Contractors who wish to be commissioned to deliver the LES and by the Commissioner representatives involved in the commissioning of the LES.
1.6. Pharmacy Contractors who have been commissioned to provide vaccinations in Phase 1 and/or Phase 2 vaccinations under the Local Enhanced Service: COVID-19 vaccination programme 2020/21 (“the LES 2020/21 Phase 1 and 2”) must follow the Designation Process described in this document if they wish to provide vaccinations in Phase 3 and therefore participate in the LES.
1.7. Aspects of the Phase 3 COVID-19 vaccination programme can only be finalised when JCVI has provided additional guidance. The LES Agreement has been provided in draft to give sufficient information to commence planning but some requirements and timescales may be subject to change. A final LES Agreement will be issued as soon as details are clear.
1.8. This document is aimed at:
- Regional teams of the Commissioner who have reviewed, in collaboration with Integrated Care Systems, population need, taking into account overall vaccination site coverage and capacity for Phase 3 across all delivery models and have established that designated vaccination sites are required to deliver system capacity and/or provide equitable access for its local population and wish to commission the LES from community Pharmacy Contractors.
- Pharmacy Contractors who believe that they can provide community pharmacy vaccination sites to deliver system capacity and wish to nominate a site to be designated for delivering Phase 3 COVID-19 vaccinations under the LES.
Commonly used terms
- Commissioner – the NHS Commissioning Board, responsible for commissioning of pharmaceutical services, and operating under the name NHS England
- Designation Process – the process set out in this document whereby Pharmacy Contractors can be commissioned to deliver the LES
- Designated Site – a site nominated by a Pharmacy Contractor and selected and approved by the Commissioner as the premises from which the vaccination will be administered to Patients (unless otherwise approved by the Commissioner) and as further described in the LES
- GPhC – the General Pharmaceutical Council
- JCVI – the Joint Committee on Vaccination and Immunisation
- JCVI Cohorts – the cohorts of Patients referenced following JCVI advice
- LES – has the meaning set out in paragraph 1.1 on page 1 of this document
- LES 2020/21 (Phase 1 and 2) – the Local Enhanced Service: COVID-19 vaccination programme 2020/21
- MHRA – the Medicines and Healthcare products Regulatory Agency
- Ministerial Decision – a decision issued by the Secretary of State for Health and Social Care
- National Booking Service (NBS) – service linked to the call/recall services where a Patient can book appointments for their COVID-19 vaccinations
- Patient – those eligible to receive the vaccination are Patients in JCVI Cohorts which have been announced and authorised by the Commissioner as eligible for the vaccination by the Pharmacy Contractor
- Pharmaceutical List – a pharmaceutical list prepared, maintained and published by NHS England pursuant to regulation 10(2)(a) of the Pharmacy Regulations
- Pharmacuetical Services – has the meaning set out in the Pharmacy Regulations
- Pharmacy Contractor – a person included in a list prepared under regulation 10(2)(a) of the Pharmacy Regulations
- Pharmacy Regulations – the National Health Service (Pharmaceutical and Local Pharmaceutical Services) Regulations 2013 as amended
- PHE – Public Health England or its successor(s) in title
- Point of Care System – a clinical system that has been assured by NHS Digital to record COVID-19 vaccination events
- PPE – personal protective equipment
- Terms of Service – the terms of service that the Pharmacy Contractor is required to adhere to as set out in the Pharmacy Regulations
2. Designation process
2.1. The Commissioner’s regional teams must work with local providers, local authorities and integrated care systems to prepare for phase 3. The mixed model of general practice, vaccination centres, hospital hubs and community pharmacies has played a critical role in reaching underserved communities. To build on the strengths of phase 1 and 2, systems must deploy a delivery model to spread capacity across various delivery models, considering the best delivery access for population requirements.
2.2. COVID-19 vaccination services will be commissioned to meet local population need and this may be from Pharmacy Contractors where they can meet the key designation requirements and where the Commissioner considers a particular Pharmacy Contractor best placed to meet that need. The Commissioner’s regional teams should, where possible, engage a lay member or patient representative in the process, to reflect patient involvement duties. When considering criteria relating to accessibility and equality of access, the Commissioner’s regional team should take account of the needs of the local population including specific health inclusion groups, and availability of alternate pharmaceutical services.
2.3. The Commissioner’s regional teams will engage with the Local Pharmaceutical Committee on commissioning the LES and should, where possible, continue to involve them in the process to represent local pharmacy contractors as a group.
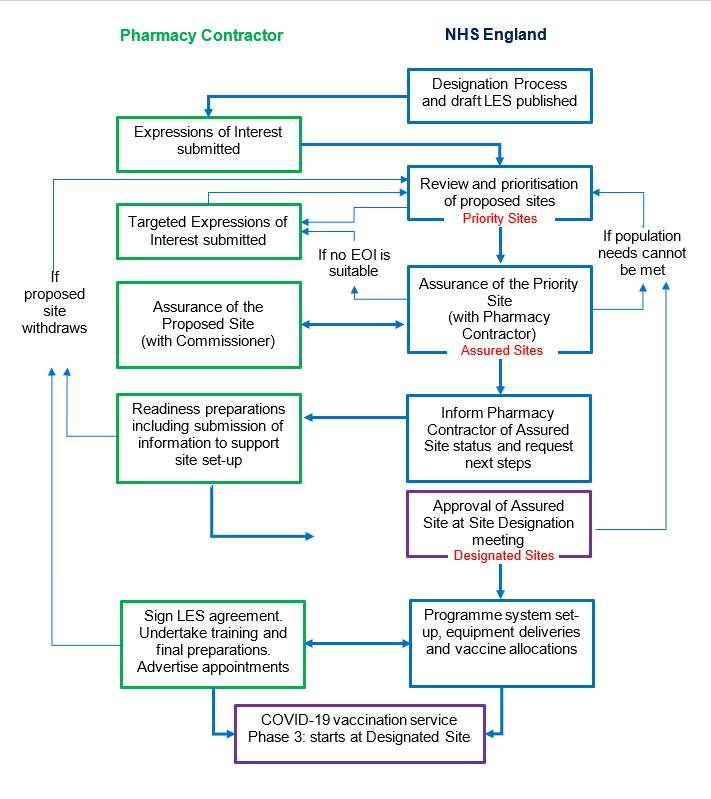
2.4. The Designation Process to establish community pharmacy-led vaccination sites will consist of 4 stages:
- 2.4.1. Expressions of Interest
- 2.4.2. Review and agreement of proposed sites
- 2.4.3. Assurance of the proposed site
- 2.4.4. Readiness preparation.
The Commissioner will hold scheduled Site Designation meetings to review preparedness of sites and approve sites as ready to proceed.
2.5. Stage 1: Expressions of Interest
2.5.1. Pharmacy Contractors should read this document carefully before submitting an Expression of Interest at https://cv19pharmacyeoi.necsu.nhs.uk
2.5.2. The form can be completed in parts, saved, then completed at a later date but must be submitted by 17:00 Wednesday 28th July 2021. Note that although this digital system has been extensively tested, it is wise to complete the form earlier than the deadline in case of connectivity problems. It is not possible to submit this form in another way. It is also not possible to extend the deadline for individual Pharmacy Contractors.
2.5.3. The following sections include information to support Pharmacy Contractors in completing their Expression of Interest form. All sections of this document and of the LES Agreement should be read and understood before submitting an Expression of Interest.
2.5.4. Expressions of Interest will be prioritised based on:
Accessibility and ability to improve health inequalities
2.5.4.1. When considering criteria relating to accessibility and equality of access, the Commissioner will take account of the needs of the local population including specific health inclusion groups and will assure themselves that potential impacts on health inequalities / access have been considered and a local EHIA undertaken.
2.5.4.2. The Pharmacy Contractor should demonstrate that they understand the population they will be supporting and have detailed their thoughts on how they can support high vaccination uptake and how they will reach underserved communities.
2.5.4.3. Pharmacy Contractors may wish to highlight the availability or proximity of parking, bus routes and/or areas of high population density as well as local road networks for proposed high volume vaccination sites.
Proposed site
2.5.4.4. In Phase 3, Integrated Care Systems should plan to maximise use of NHS estate, including community pharmacy premises. Pharmacy Contractors may use existing premises where they can accommodate the expected throughput of patients and maintain access to pharmaceutical services or use other NHS, public sector or community estates to meet population requirements. The table in the Appendix to this document indicates the requirement or preference in terms of use of NHS premises for arrangements with different levels of capacity. Commercial premises should be proposed by exception with clear costing included. Where Pharmacy Contractors nominate alternative premises they must be able to meet General Pharmaceutical Council standards.
2.5.4.5. If the proposed Designated Site is on the community pharmacy premises, Pharmacy Contractors may wish to indicate whether they intend to apply to the Commissioner’s regional teams to extend their opening hours whilst limiting the provision of other pharmaceutical services from their premises for the purposes of administering COVID-19 vaccines.
2.5.4.6. The Pharmacy Contractor may choose to propose that the proposed Designated Site is the community pharmacy premises but also offer vaccination services on a regular or occasional basis at associated premises. This model must be with the permission of the Commissioner and following guidance identified in the LES Agreement. Details of such a proposal should be included in the submitted Expression of Interest. The Pharmacy Contractor should demonstrate through their submitted Expression of Interest how their proposed site:
- Will provide value for money for the NHS; and
- Is suitable for delivering services under the LES and would not prevent the Pharmacy Contractor from meeting the terms of the LES; and
- Is of sufficient size that the proposed volume of Patients can maintain social distancing before and after the vaccination has taken place if required by individual Patients and/or current guidance. This must include appropriate post-vaccination observation and area(s) to respond should the Patient suffer an adverse reaction to the vaccination. The current post-observation period as at the date of this document for some vaccines currently in use is 15 minutes; and
- Will facilitate safe working conditions for staff and for vaccine handling. There must be sufficient space for appropriate equipment, storage and for preparing and drawing up vaccine. It is likely that Pharmacy Contractors will need to use up to three COVID-19 vaccine types and so the ability to separate vaccines in space or time must be demonstrated.
2.5.4.7. Pharmacy Contractors should not enter into any agreement with landlords or property custodians until the proposed site is fully approved.
Governance and clinical
2.5.4.8. The Pharmacy Contractor should demonstrate a track record of good governance or learning from previous irregularities.
2.5.4.9. The Commissioner may review the records it holds on a Pharmacy Contractor to exclude that Pharmacy Contractor where there are outstanding concerns about the ability to satisfy their Terms of Service, and to consider past history, including remedial actions taken.
Relevant experience in providing vaccinations in high volumes
2.5.4.10. The Expression of Interest must demonstrate to the Commissioner that the Pharmacy Contractor understands the complex nature of the service and would be able to deliver against the requirements of the LES, in particular clinical requirements, operational flexibility and management of footfall.
2.5.4.11. Equal consideration will be given to all Pharmacy Contractors, whether they have previously been commissioned to provide Phase 1 or Phase 2 COVID-19 vaccinations or not.
2.5.4.12. Pharmacy Contractors who have not previously been commissioned to deliver Phase 1 or Phase 2 COVID-19 vaccinations should provide the Commissioner’s regional team with information about other relevant experience of supporting other vaccination models, delivering different vaccination or aseptic services or otherwise demonstrate that they have an appreciation for the requirements of the service.
Ability to provide the desired volume of vaccines (“capacity”)
2.5.4.13. Each ICS and population will have different requirements as to the ideal vaccination capacity from a community pharmacy site.
2.5.4.14. In Phase 1 and Phase 2 smaller volume pharmacies have played an invaluable role in vaccinating specific populations, and medium and high-volume sites taking pressure off other healthcare providers by operating at scale. Both are valuable and needed in a mixed delivery model.
2.5.4.15. The intention is that there will be three types of community pharmacy sites participating in the vaccination programme. Low volume sites will be able to administer at least 100 vaccinations per week, medium volume sites will be able to administer 350 vaccinations or more per week, and large volume sites will be prepared to administer at least 1000 vaccinations per week. Pharmacy Contractors can express an interest at any/all of these volume levels. Please refer to the Appendix to this document for more information on the three levels of sites.
2.5.4.16. The Pharmacy Contractor must be realistic about the number of vaccinations that they can safely administer and demonstrate their ability to do this throughout the Designation Process. This should include consideration of any flu vaccinations which the Pharmacy Contractor plans to deliver from the same location as the proposed site.
2.5.4.17. Pharmacy Contractors may be rejected from the Designation Process if the Commissioner considers that they are proposing to administer too many vaccinations for the size of the proposed site and not allowing space for social distancing and/or post-vaccination observation.
2.5.4.18. The Pharmacy Contractor should demonstrate that they have planned for business continuity, including ensuring that appropriate availability of trained staff are in place so that the Pharmacy Contractor can continue to meet agreed vaccine commitments while ensuring that safe staffing levels are maintained. The Commissioner may review whether the Pharmacy Contractor has been able to maintain adequate staffing in their registered premises, for example by reviewing unplanned closure notices.
Value for money
2.5.4.19. The Pharmacy Contractor is required to demonstrate that the proposed site is good value for money.
2.5.4.20. The Pharmacy Contractor should give best estimates of the cost of set up and any ongoing costs for the proposed site.
2.5.4.21. The Commissioner should appreciate that such costs are approximate and so subject to change, but review an approximate cost per vaccine that the Commissioner believes will be adminstered at the proposed site.
2.5.4.22. Pharmacy Contractors should note that all financial claims must be authorised by the Commissioner before cost is incurred. If actual costs significantly exceed the estimates given in a Pharmacy Contractor’s Expression of Interest then the Commissioner may reject the Pharmacy Contractor’s proposal and work with other Pharmacy Contractors who have submitted an Expression of Interest and reject the initially preferred proposed site.
Engagement with local systems
2.5.4.23. Pharmacy Contractors should outline how they will link with other local heathcare vaccination providers and systems to ensure the vaccination service is co-ordinated and offers the best access for Patients.
2.6. Stage 2: Review and prioritisation of proposed sites
2.6.1. A commissioner regional team representative will undertake a process to review Expressions of Interest against the criteria set out in section 2.5 of this document. The representative will record their view as to whether each of the specified criteria has been met.
2.6.2. Where the Pharmacy Contractor has failed to demonstrate that they would be able to meet the requirements of the service the Expression of Interest will be rejected.
2.6.3. Pharmacy Contractors should note that even if a proposed site is assessed by the Commissioner’s regional team as having met all of the criteria, this does not automatically mean that it will be a Designated Site (and a LES agreement awarded) as further local factors such as capacity required, equity of access and geographical coverage need to be considered alongside operational considerations such as tech and data capacity, and supply chain for vaccines with complex handling requirements.
2.6.4. The Commissioner will therefore liaise with Integrated Care Systems (ICS) to prioritise proposed sites that meet the criteria set out in Section 2.5 for each locality according to locality specific criteria including: how well the proposed site would meet population need, capacity required, improved access and/or geographical coverage. They may also need to limit the number of prioritised sites to fit within the COVID-19 vaccination programme limitations relating to numbers of sites that can be supplied with vaccines and linked with data and technology tools.
2.6.5. The choice of which proposed site(s) in a locality should progress to the next stage will be undertaken remotely. The Commissioner should convene a virtual panel to discuss such decisions and ensure that they are consistent and transparent. This panel should include representatives of the local health system, the Commissioner, and where possible, may also include lay members and/or a representative of the Local Pharmaceutical Committee.
2.6.6. Proposed sites that have been chosen to progress to the next stage will be known as Priority Sites.
2.6.7. If, after prioritisation of proposed sites, the Commissioner determines that there are no applications that sufficiently meet the locality specific criteria, the Commissioner may invite further Expressions of Interest for that locality. The invitation to submit these Expressions of Interest will provide better visibility and more detail of the requirements in that locality or population. Invitations to submit these Expressions of Interest will be communicated via LPCs and publicised on the Expressions of Interest website.
2.7. Stage 3: Assurance of the Priority Sites
2.7.1. The Commissioner will undertake additional assurance to confirm that Priority Sites should be commissioned to provide COVID-19 vaccinations.
2.7.2. The assurance should be proportionate to the risk of the Pharmacy Contractor being unable to deliver the LES or to understand the appropriate requirements.
2.7.3. The Commissioner should draw on experience from other parts of the healthcare system, and may, if deemed appropriate by the Commissioner use peer review to support the assurance process.
2.7.4. The Commissioner may choose to assure a Priority Site through reviewing responses to questions posed by the Commissioner, to conduct a virtual interview or review of the Priority Site, to use peer networks or others with an experience in delivering similar programmes, or to visit the Priority Site as is deemed appropriate to the level of information provided in the expression of interest and any need to verify the information provided in the Expression of Interest.
2.7.5. More detailed value for money assessments should be completed as part of the assurance process and any additional reasonable expenses agreed with the Commissioner’s regional team.
2.8. Where the Commissioner’s regional team is assured that the Priority Site would be appropriate to be commissioned and provide Phase 3 COVID-19 vaccination, the Commissioner will inform the Pharmacy Contractor and invite them to take readiness preparation steps working towards an agreed deadline for a Site Designation meeting.
2.9. The Commissioner will only invite the Pharmacy Contractor to take readiness preparation steps where the Commissioner is confident that the Pharmacy Contractor should be commissioned to provide the LES, that the details of the Priority Site are known and unlikely to change before the Priority Site goes live, and that it fits within the agreed capacity limitations for the COVID-19 vaccination programme. This invitation will include authorisation for any additional reasonable costs of the Pharmacy Contractor, either ongoing or for set-up. At this point, the Priority Site shall be referred to as an Assured Site.
2.10. The Commissioner shall inform the national COVID-19 vaccination programme of the Assured Sites that they expect to have completed readiness preparations in advance of each Site Designation meeting.
2.11. Stage 4: Readiness preparation
2.12. In preparation for the Site Designation meeting, Pharmacy Contractors will be asked to complete a number of activities to confirm final assurance from the Commissioner’s regional team and to allow the COVID-19 vaccination supply chain and data and technology workstreams to ensure that they are able to recommend designation of the Assured Site.
2.13. These activities will include but are not limited to:
- 2.13.1. Final costings in advance of spend
- 2.13.2. Submission of information about the Assured Site, any equipment that is required from the national supply chain, details of relevant personnel who will be used to set up access to relevant systems:
- 2.13.2.1. Confirmation of Assured Site address if not premises that are already listed on the Pharmaceutical List. Note that this must be a postal address recognised by Royal Mail.
- 2.13.2.2. Contact details for proposed Foundry system access (to review information held about the Assured Site, site reports and supply chain information).
- 2.13.2.3. Contact details for users of the NHS Future Collaboration platform (to review guidance and other relevant information about the COVID-19 vaccination programme).
- 2.13.2.4. National Booking Service (NBS) administrators (to set up other NBS users and schedule appointments).
- 2.13.2.5. Preferred point of care system and administrators if relevant. (to record vaccinations).
- 2.13.2.6. Equipment needed for Assured Site set-up (as appropriate).
2.14. If activities have not been completed or required information has not been accurately submitted, then the Commissioner will not be able to approve the Assured Site at the Site Designation meeting.
2.15. Site Designation meetings
2.16. During the Site Designation meetings, the Commissioner will further consider whether the total number and geographical distribution of Assured Sites supports fair and equitable access for patients and can be supported by COVID-19 vaccination operational support functions in the necessary timeframe. Where the number of recommended Assured Sites exceeds the number that can be accommodated, the Commissioner’s regional teams will prioritise the list of Assured Sites according to capacity and access need. The Commissioner will then take a decision as to whether an Assured Site should be approved.
2.17. Assurance and site readiness will also be reviewed at the Site Designation meetings. Where the Pharmacy Contractor has not submitted the information required then the Assured Site will not be approved. The Commissioner, with input from the local ICS, may choose to set a new deadline and put the Assured Site forward for a new Site Designation meeting where capacity for a subsequent meeting can be found.
2.18. Assured Sites that have been approved at a Site Designation meeting will be known as Designated Sites.
2.19. Designated Sites will be informed that they have been approved, will be invited to sign the LES Agreement (when finalised) and given instructions to prepare for their Designated Site to go live. Equipment will be delivered if appropriate, and Data and Technology processes will be completed.
2.20. The names of Designated Sites will be published on the NHS England website.
2.21. Once approved, all Designated Sites must continue to meet the designation criteria against which they were assessed for the duration of the LES Agreement. The Commissioner should be informed immediately if for any reason a Designated Site ceases to meet the criteria.
Further opportunities to participate
2.22. As system requirements change over time, the Commissioner may choose to commission this service from additional sites. If this occurs during 2021 the Commissioner will consider again Expressions of Interest that have already been submitted and apply the prioritisation criteria from Stage 2 onwards.
3. Designation Process Chart
4. Timescales
The designation process for the initial nominated sites will be undertaken according to the following timescales:
| Date | Activity |
|---|---|
| 14 July 2021 | Designation Process and draft LES Agreement published |
| 14 July 2021 | Expressions of Interest from Pharmacy Contractors opens |
| 17:00 28 July 2021 | Deadline for Pharmacy Contractors to advise the Commissioner’s regional teams of proposed site for designation (closure of Expressions of Interest process). |
| 28 July to 4 August | Prioritisation of Proposed Sites by the Commissioner based on Expressions of Interest |
| 5 August 2021 and additional dates if required | Further Expressions of Interest from Pharmacy Contractors as required if Integrated Care System needs are not met. |
| Rolling through August | Assurance of proposed site to confirm that proposed site meets the ICS needs and should be commissioned. |
| Rolling through August – Assurance + 1 week | Readiness preparation before Site Designation meeting. |
| Rolling through August | Site Designation meeting to approve Assured Sites who have completed readiness assessments. |
| Group 1 sites – w/c 23 August 2021 Group 2 sites w/c 31 August 2021 |
Designated Sites are allocated vaccine, schedule clinics and make vaccination appointments live |
| September | Designated Sites go live |
Appendices
Annex A: Phase 3 COVID-19 vaccination programme – proposed community pharmacy site types by capacity
| Site capacity | |||
|---|---|---|---|
| Low | Medium | High | |
| Minimum vaccination events per week (subject to vaccine supply) | 100 | 350 | 1000+ |
| Location | Must be on NHS premises | Preference for NHS premises | Preference for NHS premises |
| Equipment provision | Minimal | As appropriate to site capacity | As appropriate to site capacity |
| Consumables | Sites to source | Sites to source | Sites to source |
| National booking service | Required | Required | Required |
| Point of care system | Required – site choice as to system provider where more than one is available | ||
| Clinical negligence indemnity | Provider responsibility | ||
| Support for IT hardware provision and connectivity | Minimal | As per current LES process – dependent on-site location (NHS v non-NHS site) | As per current LES process – dependent on-site location (NHS v non-NHS site) |
| Vaccine supply | Fortnightly | Weekly | Weekly |
