Standard operating procedure: Phases 1, 2 and 3 including vaccination of eligible children and young people
Contents
- Scope
- COVID-19 vaccines
- Operating model
- Preparation for local vaccination services
- Children and Young People
Classification: Official
Publication approval reference: C1433
Version 4
8 October 2021
This guidance is correct at the time of publishing. However, as it is subject to updates, please use the hyperlinks to confirm the information you are disseminating to colleagues and the public is accurate.
Any changes since v3.5 (June 2021) are highlighted in yellow.
1. Scope
This Standard Operating Procedure (SOP) applies to all providers who have been contracted to provide Local Vaccination Services (LVS) at designated sites.
The document covers Phases 1, 2 and 3 of the COVID-19 vaccination programme.
Some aspects of this document may only be appropriate to certain types of provider, which is indicated. This separate SOP is for providers delivering vaccination through roving models, for example care homes.
1.1 General guidance and advice
This SOP describes the operating model and design requirements for safe delivery of COVID-19 vaccines in the community and must be read in conjunction with the documents below and those which may be produced from time to time:
- Phase 1 and 2 enhanced service specification: COVID-19 vaccination programme (GP-led vaccinations).
- Phase 3 enhanced service specification: COVID-19 vaccination programme (GP-led vaccinations).
- Phase 1 and 2 local enhanced service: (community pharmacy-led vaccinations), as provided by the regional team.
- Phase 3 local enhanced service (community pharmacy-led vaccinations) COVID-19 vaccination programme.
- Phase 3 booster vaccinations. Letter of 15 September 2021 here.
- Vaccination of healthy children aged 12-15. Letter of 15 September here.
- Vaccination of 16-17 year olds. Letter of 5 August here.
- System guidance on vaccinating immunosuppressed individuals with a third primary dose, set out in the letter of 2 September here, and further guidance for general practice and PCN-led vaccination sites on 30 September here. We also wrote to trusts on 30 September here.
- COVID-19: vaccination programme guidance for healthcare practitioners (PHE guidance).
- The Green Book, particularly chapter 14a: COVID-19 – SARS-Cov-2
- UK Health Security Agency COVID-19 vaccination programme webpage.
NHS England’s guidance is on our website and on the FutureNHS workspace. New users can join FutureNHS by emailing P_C_N-manager@future.nhs.uk from an NHS email address (or similar work email address of eligible users).
Guidance, support and resources to help teams to ensure equality of access to the vaccine are available on the COVID-19 Vaccine Equalities Connect and Exchange Hub on FutureNHS.
2. COVID-19 vaccines
For vaccine, consumables and Supply Inventory List (SIL) supply, ordering and delivery support, please contact CS@nhsvaccinesupport.com or 0800 678 1650 – 7am-7pm, seven days a week.
Each vaccine will be deployed with accompanying information and will include advice for health professionals on administration, handling, transporting stock, preparation of dose, disposal and dealing with spillages. These documents are on the Specialist Pharmacy Service website.
Regulatory approval information specific to each vaccine is available:
Although a Conditional Marketing Authorisation has been granted for these vaccines, there may be residual stocks of vaccine granted a temporary authorisation under Regulation 174 of the Human Medicines Regulations 2012.
2.1 Vaccine product for booster vaccination
Please note that in a statement published on 14 September available here, JCVI advises “a preference for the Pfizer-BioNTech (BNT162b2/ Comirnaty®) vaccine to be offered as the third booster dose irrespective of which product was used in the primary schedule.
Alternatively, individuals may be offered a half dose (50µg) of the Moderna (mRNA-1273/Spikevax®) vaccine, which should be well tolerated and is also likely to provide a strong booster response.
Where mRNA vaccines cannot be offered e.g. due to contraindication, vaccination with the AstraZeneca (ChAdOx1-S/Vaxzevria®) vaccine may be considered for those who received AstraZeneca (ChAdOx1-S/Vaxzevria®) vaccine in the primary course (please refer to the green book for further details)”.
2.2 Safe and secure handling and management of COVID-19 vaccines
COVID-19 vaccines have specific handling requirements which are part of their conditional marketing approval or condition of temporary authorisation. Please refer to the relevant Summary of Product characteristics for each vaccine on the MHRA website.
The characteristics of the different vaccines vary considerably. The product characteristics are in the relevant ‘Healthcare Professional Factsheet’ and patient information in the ‘Consumer Factsheet’.
Maintaining the correct cold chain or preparation technique is critical to the integrity and effectiveness of all vaccines.
Vaccines that have not been transported or stored correctly may be ineffective or harmful; they would therefore no longer be within the conditions of their temporary authorisation and must not be used. The Lead Responsible CCG Chief Pharmacist will support staff by ensuring safe and secure handling and use of vaccines at PCN-led designated vaccination sites, with the Responsible Pharmacist at a Community Pharmacy site assuming that role. Vaccines must be transported only in approved and validated cool boxes. The temperature of the cool box and contents must be monitored and reviewed before use to maintain cold chain requirements. Means of detecting when a temperature excursion has occurred are required and any ‘out of specification’ recordings should be addressed promptly and appropriately, and a full audit trail is maintained. Please see section 2.8 for information on movement of the vaccine.
Avoidance of waste is also of high priority. Providers should ensure processes are in place for the safe and secure handling and storage of vaccines in accordance with principles in the Chief Pharmaceutical Officers letters of 8 December 2020, 31 December 2020, 7 January 2021, 20 May 2021 and 21 May 2021 and any subsequent relevant letters. Specific guidance regarding safe and secure handling of the specific vaccine can be found on the Specialist Pharmacy Services website.
The provider must ensure that appropriate and formal authorisation for vaccine supply, preparation and administration is in place. The following legal mechanisms can be used for these purposes, a summary of which can be found here:
- Patient Specific Direction (PSD) for AstraZeneca (Vaxzevria®), Pfizer/BioNTech (Comirnaty®) (see appendix B) and Moderna (Spikevax®)
- Patient Group Direction (PGD) for Pfizer/BioNTech (Comirnaty®), AstraZeneca (Vaxzevria®) and Moderna (Spikevax®)
- National Protocol for Pfizer/BioNTech (Comirnaty®), AstraZeneca (Vaxzevria®) and Moderna (Spikevax®).
Staff who supply, prepare and administer the COVID-19 vaccine must have signed the relevant PGD or National Protocol that they are working under.
Processes must be in place to maintain product integrity, medicines governance, and risk management of COVID-19 vaccines. It is critical that products are handled in accordance with the detailed SOPs on the Specialist Pharmacy Service’s website. For additional guidance and support, providers should contact the Lead Responsible CCG Chief Pharmacist, who will contact the relevant Specialist Pharmacy Services Regional Quality Assurance Specialist or Regional Chief Pharmacist.
Guidance on safe practice for handling multiple COVID-19 vaccines is published by the Specialist Pharmacy Service.
2.3 Pfizer/BioNTech vaccine (Comirnaty®)
Vaccine should be prepared in accordance with manufacturer’s recommendations (see Regulation 174 Information for UK Healthcare Professionals and Summary of Product Characteristics) and NHS standard operating procedures for the service.
The manufacturer’s product information leaflet is here. SPS information and guidance specific to the Pfizer/BioNTech (Comirnaty®) vaccine can be found here. The letter on the change to the shelf life of the Pfizer/BioNTech (Comirnaty®) vaccine when stored in refrigerators at 2-8C is here.
MHRA issued advice in July around extremely rare and mild reports of myocarditis and pericarditis with mRNA vaccines; further information is available here: COVID-19 vaccines: updates for July 2021. The MHRA has advised that “these events are extremely rare and tend to be mild when they do occur. Our advice remains that the benefits of getting vaccinated outweigh the risks in the majority of people“.
Pfizer has published educational materials for handling, preparation and administration of the vaccine: www.cvdvaccine.co.uk.
AstraZeneca (Vaxzevria®) vaccine should be prepared in accordance with the manufacturer’s recommendations (see Summary of Product Characteristics) and NHS standard operating procedures for the service.
The manufacturer’s product information leaflet is here. SPS information and guidance specific to the AstraZeneca (Vaxzevria®) vaccine can be found here.
2.4 AstraZeneca (Vaxzevria®) vaccine and extremely rare blood clots
The JCVI and MHRA issued advice on 7 May 2021 on the AstraZeneca (Vaxzevria®) vaccine and extremely rare blood clots. While the MHRA and JCVI have made clear that the balance of risk is still very much in favour of vaccination, the JCVI advice stated that it is preferable for adults aged under 40 years to be offered an alternative COVID-19 vaccine, if available. This advice follows this statement of 7 April 2021 relating to the under 30s.
In terms of the use of the AstraZeneca vaccine in booster programme /Phase 3, the JCVI statement of 14 September 2021 stated (bold added):
“After reviewing data on booster responses from different combinations of COVID-19 vaccines, JCVI advises a preference for the Pfizer-BioNTech (BNT162b2/ Comirnaty®) vaccine to be offered as the third booster dose irrespective of which product was used in the primary schedule. There is good evidence that the Pfizer-BioNTech (BNT162b2/ Comirnaty®) vaccine is well tolerated as a third dose and will provide a strong booster response. Alternatively, individuals may be offered a half dose (50µg) of the Moderna (mRNA-1273/Spikevax®) vaccine, which should be well tolerated and is also likely to provide a strong booster response. A half dose (50µg) of Moderna (mRNA-1273/Spikevax®) vaccine is advised over a full dose due to the levels of reactogenicity seen following boosting with a full dose within the COV-BOOST trial. Where mRNA vaccines cannot be offered e.g. due to contraindication, vaccination with the AstraZeneca (ChAdOx1-S/Vaxzevria®) vaccine may be considered for those who received AstraZeneca (ChAdOx1-S/Vaxzevria®) vaccine in the primary course (please refer to the green book for further details).”
The MHRA has also issued information for healthcare professionals on blood clotting following COVID-19 vaccination and Public Health England has updated patient information leaflets giving further advice on symptoms for vaccine recipients to look out for 4-28 days after vaccination and actions to take.
NHS England has written to all PCNs, GPs and Community Pharmacies to advise on the actions required of LVS sites following each JCVI statement. The two letters can be found here and here.
2.5 Moderna vaccine (Spikevax ®)
Vaccine should be prepared in accordance with manufacturer’s recommendations (see Summary of Product Characteristics) and NHS Standard Operating Procedures for the service.
The manufacturer’s product information leaflet is here. SPS information and guidance specific to the Moderna (Spikevax®) vaccine can be found here.
MHRA issued in July some advice around extremely rare and mild reports of myocarditis and pericarditis with mRNA vaccines; further information is available here: COVID-19 vaccines: updates for July 2021. The MHRA has advised that “these events are extremely rare and tend to be mild when they do occur. Our advice remains that the benefits of getting vaccinated outweigh the risks in the majority of people”.
2.6 Anaphylaxis and COVID-19 vaccines
An MHRA protocol for the management of anaphylaxis and an anaphylaxis pack must always be available whenever the Pfizer/BioNTech (Comirnaty®) vaccine is given. Immediate treatment should include administering 0.5mg intramuscular adrenaline (0.5ml of 1:1000 or 1mg/ml adrenaline), with an early call for help and further IM adrenaline every 5 minutes. The health professionals overseeing the immunisation service must be trained to recognise an anaphylactic reaction and be familiar with techniques for resuscitation of a patient with anaphylaxis. Vaccine recipients should be monitored for 15 minutes after vaccination, with a longer observation period when indicated after clinical assessment.
Recipients of both mRNA vaccines should also be monitored for 15 minutes after vaccination, as detailed in the regulatory approval information.
For the AstraZeneca (Vaxzevria®) vaccine there is no requirement for 15 minutes observation unless this is indicated after clinical assessment or where the patient has experienced an adverse reaction to a previous vaccination dose.
2.7 Dosage schedule
LVS sites should follow the dosage schedule set out in the latest version of the Green Book.
2.8 Movement of vaccine
All vaccine movements must be in line with the MHRA conditions of authorisation or summary of product characteristics for the product and the Specialist Pharmacy Service SOPs such as the transportation SOP, conditions set out in the position statement (29 September 2021) and with the oversight of the chief pharmacist and under the guidance of the pharmacy team. Further information is provided in the Standard operating procedure: roving and mobile models. Please also refer to the relevant SPS SOPs for Pfizer here, AstraZeneca here and Moderna here.
The policy on the transfer of COVID-19 vaccines between Hospital Hubs, Vaccination Centres and Local Vaccination Services (‘mutual aid’) can be found here. In general, there should be no mutual aid between any organisations as they are expected to use the supplies made available and delivered directly to them to vaccinate their patients.
PCN groupings must deliver the majority of their COVID-19 vaccinations at the designated site in accordance with MHRA guidelines. Annex B of the policy contains further information on the movement of any vaccine between a PCN grouping and/or a third party, which is a general exception to the policy. See also schedule 3 of the Collaboration Agreement (PCN only).
Community Pharmacy providers wishing to move vaccine between designated sites must have advance permission from their commissioner (and the commissioner of the receiving site if different).
2.9 Staff training
All staff and volunteers involved in the delivery of COVID-19 vaccinations need to undergo appropriate training, the extent and delivery of which will vary depending on the staff member’s role and experience. All vaccinators need to undertake training on the specific vaccine being administered (as outlined in the UK Health Security Agency COVID-19 Vaccinator training requirements here and COVID-19 vaccination training pathway produced by the national programme here).
All vaccinators need to undertake training on the specific vaccine being administered which may be by completing the vaccine-specific e-learning modules within the COVID-19 Vaccination e-learning programme.
They also need to be assessed and signed-off for competency against the PHE COVID-19 Vaccinator competency assessment tool for each specific vaccine they are delivering.
The workforce and training considerations for Phase 3 children’s vaccination are summarised here.
For staff involved in vaccinating children there are specific requirements associated with this (see section 5.3.2).
See section 4.2 for information about co-administration of the COVID-19 vaccine with the seasonal influenza vaccine.
3. Preparation for local vaccination services
Prior to commencing vaccination, commissioners and providers will work together to mobilise LVS sites. Further information on the mobilisation process for designated sites is sent by NHS England and NHS Improvement in advance of site mobilisation. This section should be read in conjunction with those communications. There is a separate Standard Operating Procedure for roving and mobile vaccinations.
3.1 Leadership and governance
All providers must appoint a clinical lead and operational lead who will be responsible for the delivery of all aspects of local vaccination services.
PCN-led sites should determine the most appropriate clinical supervision required, based on local circumstances. It is expected that the majority of vaccinations will be delivered by healthcare professionals under a Patient Group Direction (PGD) or by lay vaccinators under the COVID-19 National Protocol. If other healthcare professionals have the relevant skills and are working under a PGD, the presence of GPs is not an essential legal requirement. However, many have found that the presence of a GP is helpful in a clinical director capacity to assist with consent and complex patients. GP practices remain responsible for the conditions set out in the Enhanced Service for Phase 1and 2 and Phase 3.
Community Pharmacy led sites must have a named Clinical Lead within their organisation. The Clinical Lead must be a pharmacist, registered with the General Pharmaceutical Council (GPhC) and trained in vaccinations and have a clear understanding of the Local Enhanced Service (LES).
The Responsible Pharmacist at the registered pharmacy premises is professionally responsible for the safe delivery of the service at the Designated Site.
If the Responsible Pharmacist is unable to provide sufficient supervision of the service, for example due to workload or where the Designated Site is located in a location other than the main pharmacy premises, an on-site pharmacist(s) supervising the Designated Site must be linked and work closely with the Responsible Pharmacist and Superintendent Pharmacist through an appropriate governance framework. This on-site supervising pharmacist must be registered with the General Pharmaceutical Council and trained in vaccinations, including a clear understanding of this LES.
Agreement
A record must be maintained of who that person is at each site at all times and made available to the Commissioner (NHSE) on request.
Commissioners should offer all possible assistance to providers to mobilise sites and prepare for vaccination administration.
Providers must ensure all staff involved in local vaccination services are aware of escalation processes for clinical incidents and enquiries. This should include reporting any suspected vaccine side effects or adverse incidents related to the use of the vaccines to the MHRA via the Coronavirus Yellow Card reporting site.
3.1.1 Indemnity
PCN led vaccination sites
- Phases 1, 2 and 3. Where vaccinations are administered in line with the General Practice Enhanced Service specification, indemnity for clinical negligence will be provided under the Clinical Negligence Scheme for General Practice (CNSGP). This applies to all staff who are employed or engaged by a general practice to deliver the vaccination programme. This indemnity is not dependent on the location in which the services are being delivered.
Community pharmacy vaccination sites
- Phases 1 and 2. Where vaccinations are administered by community pharmacies in line with the Community Pharmacy Local Enhanced Service agreement, indemnity for clinical negligence will be provided under the Clinical Negligence Scheme for Coronavirus (CNSC). This applies to all staff who are employed or engaged by a Community Pharmacy to deliver the vaccination programme.
- Phase 3. Where a community pharmacy has signed, and is operating under, the Local Enhanced Service (LES), it will be the responsibility of that community pharmacy to source appropriate indemnity arrangements including for clinical negligence. The Government is providing support to community pharmacy by making arrangements with insurance providers in respect of clinical negligence cover from September 2021 to 31 March 2022 to ensure cover is available at an affordable price to those community pharmacies engaged under this LES. Any claims made as a result of services provided under this LES should be reported to your indemnity provider in the usual way. This support is in addition to the clinical negligence state indemnity support available for the Community pharmacy local enhanced service – coronavirus vaccination, COVID-19 vaccination programme 2020/21 LES, and will operate in parallel and independently.
3.1.2 Collaboration agreement
All PCN Groupings must have a Collaboration Agreement in place as detailed in the enhanced service specifications for Phase 1 and 2 and Phase 3.
We have published a template collaboration agreement for GP practices operating as PCN groupings to use and adapt as appropriate. This includes updates clarifying the MHRA rules around the movement of vaccine, and wording to support the use of COVID-19 vaccination designated sites for flu-only clinics.
3.2 Workforce
Providers should consider capacity implications associated with releasing staff to undertake COVID-19 vaccination specific training and the period of time over which staff will need to be trained.
Providers are responsible for ensuring that staff involved in vaccinations are appropriately trained and the appropriate documentation is in place for indemnity purposes, such as a volunteer agreement for engaging volunteers or a staff sharing memorandum of understanding between providers. UK Health Security Agency has developed these training resources and eLearning for staff. Further information regarding indemnity arrangements that apply for PCN COVID-19 vaccination services is on NHS Resolution’s website. It will be the responsibility of the Community Pharmacy to source appropriate indemnity arrangements including for clinical negligence.
To support workforce resilience, both individual and team-level coaching is available through the free-of-charge Looking after you too and Looking after your team programmes.
Workforce planning and skill-mix
Guidance has been issued to help PCN and Community Pharmacy-led vaccination sites on accessing National Workforce Supply Routes. LVS sites should consider how best to maintain workforce resilience including by drawing down on this additional support. Each Integrated Care System (ICS) has a designated workforce lead employer which will act as an operational workforce hub for all vaccination providers in the local area.
The lead employer can help you access the following:
- Vaccinators, clinical supervisors and registered healthcare professionals. These candidates will require local onboarding, training and checks before being deployed to a site.
- Volunteer vaccinators, patient advocates and post-vaccination observers supplied by St John Ambulance. These are provided free of charge.
- Volunteer stewards supplied by NHS Volunteer Responders, delivered by Royal Voluntary Service. These are provided free of charge.
- Clinical and GP returners who have offered support to the NHS by taking part in the COVID-19 vaccination programme.
- Vaccination Operational Support Teams (VOST) can also be requested to support the operational delivery of the vaccination programme. They have been designed as contingency workforce to replicate the rapid deployment support that the military provided to Local Vaccination Services.
Unregistered staff with appropriate training can administer the vaccine under the National Protocol, reducing the need for the clinical team to be composed solely of registered healthcare professionals. Providers should consider the optimum skills and staffing mix, including the use of unregistered vaccinators, to ensure a sustainable and efficient workforce delivery model.
The National Workforce Support Offer Toolkit has been developed to support primary care to access additional staff and to inform their workforce models. The lead employer will work with all providers on workforce communications, management of rostering systems for volunteers and National Workforce suppliers and will have oversight of mandatory and statutory training of these staff. A list of workforce lead employers for each ICS area is available here.
Table 1 presents suggestions on how designated sites could utilise their workforce to support the delivery of local vaccination services, dependent on the legal mechanism adopted for administering the COVID-19 vaccine(s).
Table 1: How designated sites could use workforce to support delivery of local vaccination services
| Roles | Task |
|---|---|
| Registered Health Care Professional (HCP) | Clinical assessment and consent (and vaccinating as required) |
| Diluting /Drawing up vaccine | |
| Directing and managing any medical emergency | |
| Non-Registered Healthcare providers |
Diluting/Drawing up vaccine
Vaccination when appropriately trained, supported and supervised by a clinician. (This will be under a national protocol or under a PSD if supervised by a prescriber.) |
| Infection control / additional cleaning and support of clinical staff
Responsible for managing the post vaccination observation area and providing Basic Life Support |
|
| Administrative support | Patient registration at the site
Assistance with record keeping |
| Reception support | Meeting and greeting people, arrival symptom check |
| Patient marshalling, car parking and advocacy | Directing those being vaccinated, maintaining flow and social distancing
Responsible for answering patient queries and addressing any concerns Support to those requiring additional assistance |
If vaccination providers choose to engage volunteers locally and not through the Royal Voluntary Service, best practice as per the guidance must be followed to comply with Home Office advice and NHS Employment Check standards.
3.3 Site preparation
All providers administering vaccinations will have been designated in line with the relevant Site Designation Process (community pharmacy site designation guidance and General Practice site designation guidance) which includes site requirements
Access
Providers should ensure their local vaccination services are accessible to all members of their community and take reasonable steps to improve access and reduce potential inequalities for people eligible to access vaccinations.
Consideration of any reasonable adjustments required by disabled people is essential during all elements of the care pathway. Ensuring any needs are identified in advance and recorded is paramount to help ensure an appropriate and successful vaccination appointment. Checking any requirements that may need to be taken into account with individuals and/or their carers or families ahead of the appointment is essential. Include clear details on a person’s patient and summary care record and ensure this information is provided to the clinician who will be carrying out the vaccination appointment. This consideration is vitally important for any appointments with disabled or sick children, those with a learning disability and autism, as well as those with SMI and dementia.
Most people with a SMI, dementia, a learning disability or autism will be able to receive their vaccine in the standard way. However, for the minority of individuals where this is not suitable, the reasonable adjustments required should be determined in advance of vaccine provision and be centred on individual needs.
Some proposed adjustments for people with SMI, dementia, a learning disability and autistic people can be found on the FutureNHS platform here. Some individuals may require more substantial adjustments than the ones listed, which may not always be available in local vaccination services/vaccination centres where facilities/resources are limited. Where this is the case, vaccination at home should be considered. In all cases, the priority is to ensure that a person can access the vaccine as quickly as possible while minimising distress.
Patients booked in for vaccination are asked to attend on their own where possible to minimise the risk of COVID-19 infection. However, any individual is allowed to attend with another person, particularly if they need support, for example if they are in a wheelchair, are frail or have a learning disability.
People with young babies or children should not be turned away, unless following a risk assessment by the senior clinician. Denying treatment/intervention, for any reason, is a clinical decision and it must be made by the most senior clinician on duty at the time. They will be able to assess the risks and make a clinical decision which will then be documented. All staff need to be aware of the need to escalate these situations to the senior clinician.
Reasonable adjustments can be made for people bringing children to vaccination appointments, and every effort should be made to ensure that individuals can receive their vaccine at their stated appointment time. Guidance, support and information is on the COVID-19 Vaccine Equalities Connect and Exchange Hub on FutureNHS and resources from PHE here.
This includes access to translation and British Sign Language services as required to support consent, mental capacity and clinical assessments. It may be helpful to have supporting literature available in a range of languages and easy read formats appropriate to the population being served (see in section 4.3 on ‘Communicating for diversity and inclusion’). Contact your commissioner for information about local translation and interpretation services.
Remote British Sign Language interpretation support is available to service users through NHS 119, through InterpreterNow. Guidance is on FutureNHS here.
People do not require an NHS number or GP registration and should not be denied vaccination on this basis. The NHS England and Public Health England letter reassures migrants of their right to the vaccine. Staff should not ask for the immigration status of people accessing the vaccine. See section 4.1 for further guidance on patients who do not have an NHS number.
Flexible hours and days should be available, to meet the needs of different communities.
PCN groupings should note that for COVID-19 vaccination, the Phase 1 and 2 and Phase 3 enhanced service specifications permit the vaccination of eligible unregistered patients to ensure they are able to access local vaccination services. Patients should be encouraged to, but do not have to, register with a general practice. A range of resources are on the FutureNHS workspace to help encourage people to register with their GP.
Community pharmacy providers should seek ways to encourage uptake of vaccine, particularly in populations where uptake is low. Local booking is permissible under the terms of the Local Enhanced Service (LES) in agreement with the NHS England regional team. Vaccination without approval is permissible where the patient is unable or unlikely to use the NBS or appointments previously offered on NBS are unfilled.
PCN and Community Pharmacy sites should work collaboratively to target underserved populations using all providers. A framework for systems, sites and local authorities to help maximise vaccine uptake in underserved communities is also available. A toolkit to help teams to engage the Black African and Black African Caribbean community is also available. The COVID-19 Migrant Guide supports access for this community. These resources build on the Government’s vaccine uptake strategy.
A mobilisation guide for the vaccination of people experiencing homelessness and rough sleeping is available on the FutureNHS workspace here.
Gypsy, Roma and Traveller communities face some of the most severe health inequalities and poor health outcomes in the UK. Friends, Families and Travellers has a service directory on its website, and relevant information on COVID-19.
Vaccine uptake has been disproportionately lower in Black Caribbean, Black African and Mixed Groups. Areas of good practice for engaging with different ethnic backgrounds is on the FutureNHS workspace here.
Information to support vaccination in pregnancy is available here.
COVID-secure, social distancing and patient flow
Please refer to the Health and Safety Executive guidance on making your workplace COVID-secure, government guidance on working safely during coronavirus (COVID-19), guidance on how to stay safe and help prevent the spread of coronavirus and guidance on wearing of face coverings.
Social distancing tips from sites:
- Place marshals and volunteers at entry points, reminding the public of the need to social distance.
- Hold daily updates with staff reiterating IPC guidance in detail.
- Place volunteers throughout the exit process to make sure there is no crowding in corridors and lifts as people leave.
- Use HSE COVID-secure guidance and mark out maximum occupancy in areas with clear signage for the public.
- Oversee flow and compliance.
- People often arrive early for vaccination appointments – plans around queuing and waiting areas should take this into account.
Providers vaccinating in care home settings and patients’ own homes should put in place procedures appropriate to those settings, including considering how to limit the number of different workforce attending these sites to minimise any risk of transmission of COVID-19.
Site security
Providers should take proactive steps to develop and maintain a strong security culture within their vaccine sites. This will play an essential role in promoting the desired security behaviours. To achieve this, providers should take steps to make sure that staff and volunteers have a consistent approach to site security:
- there should be a clear and regularly revised understanding of the main risks
- staff and volunteers should have a clear understanding of what is required of them.
Security Plan
Local Vaccination Services should review their security plan, including arrangements to manage the following risk areas:
- site inductions – making sure that all personnel involved in the site are made aware of the security arrangements and responsibilities
- awareness – making time available for site staff and volunteers to undertake relevant security training, including Action Counter Terrorism (ACT) e-learning
- briefings – involving all site staff and volunteers in regular briefings about the operation of the site and the associated security arrangements
- reporting – creating a culture where site staff and volunteers can easily report any security concerns which they might have and making sure that learning and feedback is shared from these.
Local vaccination services should consider the nature of the site and work with their local police and, if appropriate, local resilience forum (LRF) partners to inform the security-focused SOPs required to ensure the safe operation of the site. Prior to deployment a site-specific risk assessment must be completed which should include as a minimum:
- site security roles and responsibilities, including engagement with local police partners
- site access and external controls. Depending on the nature of the site, this might include issues such as traffic and queue management
- vaccine storage, movement and access to vaccine stores
- emergency responses to a range of possible scenarios, including procedures for site evacuation and to instigate a lockdown of the site
- waste management arrangements (for COVID-19 vaccine products)
- information security (see section 3.8 of this SOP) and awareness of adversaries.
- out-of-hours security arrangements (especially where vaccine products will be stored on-site overnight)
Local Vaccination Services: Make sure there are clear protocols in place to control access, both for staff and patients attending vaccinations. There is a risk that protestors may try and pose as either patients or staff. The protest security guidance can be found in the Site Security section on FutureNHS here.
Staff security: Consider protocols to minimise the risks to staff when on location and conducting vaccination operations.
Incident protocols: Ensure there are clear protocols in place to respond to any security incident. This should include clear roles and responsibilities, including how sites would be locked down in the event of a security incident.
Providers should report any incidents to the police, as well as raising any issues or incidents with their commissioner and Regional Vaccination Operations Centres (RVOC).
Security staffing: Determine requirements for on-site security presence, and make sure that any contracted security staff are SIA accredited. Review levels of security staffing to determine whether it is commensurate with the risk presented to the roving and mobile programme.
A site security risk assessment can be found in the Site Security section on FutureNHS here.
Funding process for security upgrades: The NHS COVID-19 vaccine deployment programme will fund reasonable requests for security enhancements if gaps in arrangements are identified during a routine review. Requests should be submitted to the regional finance team, who will explain the reimbursement process.
LVS sites should refer to the relevant finance guidance for PCN-led sites or Community Pharmacy-led sites on the Future NHS workspace.
Additional information:
- Sites will need to complete a site security risk assessment as part of their preparation for readiness, and link with the police locally and their Local Resilience Forum (LRF).
- Sites are also required to follow the NHSE&I and Department of Health and Social Care (DHSC) guidance below in relation to the security of premises and the personal safety of staff. The DHSC guidance on vaccination site security is available here. The link to the NHS Ops note is available here. Guidance on managing challenging behaviour is available here. Training for Action Counter Terrorism (ACT) e-learning Action Counter Terrorism (ACT). Advice and guidance on the decommissioning of vaccination sites, plus leavers guidance and a checklist can be found in the Site Security section on FutureNHS here.
- PCN Groupings must follow any usual requirements set by the Care Quality Commission (CQC). Community Pharmacy providers must follow any usual requirements set by the General Pharmaceutical Council (GPhC). Any LVS provider must have regard to any other relevant professional regulators involved in the provision and staffing of their site for securing all aspects of the designated sites, and any conditions of Marketing Authorisation for the vaccine.
IT equipment and systems
Prior to starting vaccination, providers should have tested IT equipment and ensured relevant staff have received training and can access clinical and non-clinical systems relevant to COVID-19 vaccination. These include:
- Q-flow National Booking System (not applicable to PCN providers using local booking systems).
- Nationally approved COVID-19 Point of Care system for recording the vaccination event, e.g. Outcomes4Health/Pinnacle Point of Care System.
- NHS Business Services Authority Manage Your Service tool to support the payment of the Item of Service COVID-19 vaccination fee to providers.
- Foundry reporting system or any other systems as directed by the commissioner.
Training material on the main systems is here.
Any issues can be raised via the IT services helpdesk: vaccineservicedesk@england.nhs.uk / 0300 200 1000 open 6am-10pm every day, including Bank Holidays.
3.4 First aid and resuscitation preparation
Providers should reasonably anticipate three medical emergencies associated with vaccination: fainting, hyperventilation, and anaphylaxis.
All designated sites should at a minimum include a registered healthcare professional trained within the previous 18 months in the management of anaphylaxis, cardiopulmonary resuscitation, and use of an automated external defibrillator. PHE has included resuscitation training within the COVID-19 vaccination programme training resources, which can be found on the GOV.uk website.
All designated sites will be provided with resuscitation equipment and medications via the Supply Inventory List; see section 3.7 Some sites may wish to have additional equipment or medicine (included in the ‘Emergency treatment of anaphylaxis’ guidelines) as recommended by The Resuscitation Council UK, due to local circumstances, and can complete a local resuscitation risk assessment to consider as a minimum the following:
- Location (e.g. remoteness)
- Workforce (including the consistent presence of healthcare professionals with advanced skills in resuscitation)
- Volumes of patients presenting (quantities of equipment and workforce requirements)
- Quantities of equipment / medicines held
Facilities for management of anaphylaxis should be available at all vaccination sites (see Green Book Chapter 8).
Access to the following may be helpful:
- Guidance on management of anaphylaxis in the vaccination setting
- Anaphylaxis algorithm chart
- Infographic on resuscitation of adult COVID-19 patients in the primary care setting
In addition, the Royal College of General Practitioners (RCGP) has published resources for all primary healthcare professionals on resuscitation and anaphylaxis which can be used for CPD purposes.
3.5 Occupational health requirements
Providers should ensure they have a local needlestick injury protocol accessible (ideally displayed) on-site which should include contact details for any relevant occupational health service and that staff understand what to do should they experience a needlestick injury. The provider is responsible for ensuring a nominated individual on-site has knowledge and understanding of local needlestick protocols and ensure that they are followed.
3.6 Infection prevention and control (IPC)
Infection prevention and control precautions must be maintained by all staff, in all settings, at all times, for all patients; please refer to the latest IPC guidance. This includes videos and posters demonstrating correct procedures for donning and doffing personal protective equipment (PPE).
If further advice is needed, contact your local infection prevention and control team.
3.7 Supply Inventory List (SIL)
For LVS sites, for Phase 1 and 2 of the national vaccination programme vaccine-related and non-vaccine-related consumables are supplied nationally free of charge.
The Supply Inventory List (SIL) is a centrally-supplied generic equipment and consumables list, providing what is needed to effectively administer vaccinations. The volume of vaccine-related consumables supplied is proportionate to the number of vaccines and is not replenished automatically with each vaccine delivery; designated sites are not required to order items on the SIL.
For Phase 3, the national COVID-19 vaccination programme will continue to supply linked vaccine consumables to all designated sites in Phase 3 (e.g. syringes, and this will now include steret wipes). However, designated sites will need to source non-vaccine linked consumables locally (e.g. PPE, cotton wool, clinical waste bags, sharps bins etc.) as providers would normally do for other vaccination programmes. PPE will continue to be available for practices to order through the DHSC ePortal, which remains free of charge until March 2022.
Community pharmacy and PCN-led sites that have not signed-up for Phase 3 will get nationally sources consumables until the end of October.
The Supply Inventory List (SIL) is a centrally-supplied generic equipment and consumables list providing what is needed to effectively administer vaccinations. The volume of vaccine-related consumables supplied is proportionate to the number of vaccines and is replenished automatically with each vaccine delivery; designated sites are not required to order items on the SIL. Any issues with supply should be raised via the helpdesk CS@nhsvaccinesupport.com or 0800 678 1650 – 7am to 7pm, seven days a week.
More information about the SIL and what equipment and consumables will be provided for different types of sites can be found on our website. Full information will be provided to sites as part of the site mobilisation and onboarding process.
If sites require additional items not on the centrally supplied lists, they should discuss this with their commissioner who may be able to provide the items or reimburse reasonable costs from central funding, where these have been agreed in advance. Further guidance on funding is available separately for PCN and Community Pharmacy providers on FutureNHS.
3.8 Waste management
It is vital that vaccination sites segregate all waste into the proper waste stream. Doing so reduces pressure on the waste services infrastructure, reduces the impact on the environment, significantly reduces costs, and ensures compliance with relevant waste regulations.
All waste should be disposed of into the allocated consumables and stored securely on site or transferred to another site as required (e.g. roving vaccinators) following each vaccination session.
The principles of the COVID-19 waste management SOP should be followed. Specific advice on the COVID-19 vaccination programme can be found on the FutureNHS platform here.
You can contact the national helpdesk to escalate any issues regarding access to the right equipment and consumables: cs@nhsvaccinesupport.com or 0800 678 1650 – 7am to 7pm, seven days a week.
4. Operating model
The operating model below applies to all settings in scope of this SOP; this will need adapting for the type of setting you are delivering local vaccination services in, i.e. whether patients are attending an NHS site (e.g. GP practice, hospital or community pharmacy) or a non-NHS site (e.g. community centre, fire station, museum etc). The principles also apply for roving vaccinations e.g. care homes and patients own homes, but should be read in conjunction with the separate Roving and Mobile Models Standard Operating Procedure.
4.1 Identifying, booking and communicating with eligible patients
JCVI advice on eligible patients is available as follows:
- prioritisation of eligible patient groups in cohorts 1 to 9 is here
- cohorts 10 to 12 is here
- children and young people (cohort 13 within the Enhanced Service specification for Phase 1 and 2) is here. See section 5 for further information on children and young people.
- JCVI guidance for vaccinating immunosuppressed individuals (our letter to general practice and PCN-led vaccination sites regarding assuring implementation of this guidance is available here, with a letter to trusts here).
And for Phase 3 only:
- COVID-19 booster vaccine programme for winter 2021-2 here
Providers should follow guidance from the commissioner on phasing access to different patient groups.
Patients and NHS staff do not require an NHS number or GP registration to receive a vaccination and should never be denied one on this basis, either in person when presenting for a vaccine, or through the design of local booking systems. If an NHS number cannot be found, providers should still vaccinate and record the vaccination event on a nationally approved COVID-19 Point of Care system. A more detailed user guide is available on FutureNHS.
Patients for whom English is not their first language or where they have other communication needs may require additional support with booking and communication e.g. access to British Sign Language.
Staff should take appropriate consideration of the reasonable adjustments and additional communication support that may be required to facilitate vaccination for disabled people and also those that require language interpretation services. See also section 3.3 on reasonable adjustments for access. Further guidance is available here.
Appointment booking
PCN groupings
PCN groupings are responsible under the Phase 1 and 2 enhanced service and the Phase 3 enhanced service for using existing local systems to undertake local call and recall, identifying and inviting all eligible patient cohorts, as advised by the commissioner, on their registered list to book vaccination appointments, or where the PCN grouping has been onboarded onto the National Booking System (NBS) following the NBS requirements to support NBS call/re-call.
Patients registered with practices which have chosen not to sign up to the Phase 1 and 2 and/or Phase 3 enhanced services can be vaccinated by an alternative provider. The patients’ registered practice should co-operate with the commissioner to ensure that patients are advised as to where they can access vaccination. National call and recall communications may also direct these patients to the NBS so they can secure a vaccination through a PCN-led site which is using the NBS, a community pharmacy-led site or vaccination centre.
Unregistered patients who are eligible for vaccination, and who request a vaccination from a PCN-led site should be assessed for eligibility and vaccinated. They should not be turned away or signposted elsewhere. See section 3.3 on access for more information.
As part of the booking process, providers are advised to ensure that eligible patients:
- do not have any clinical exclusion criteria for why they should not be vaccinated.
- for any recommended subsequent doses, patients are booked into the correct vaccine clinic to enable them to have the recommended subsequent dose type, unless there is a clinical exception.
- are offered the required number of appointments for the recommended doses of the vaccine within the required timescales.
- require any additional support e.g. access, translation and interpretation, chaperone, and any reasonable adjustments e.g. for people with a learning disability or who may be autistic (see section 4.1); PCN groupings should prepare their sites to enable support and reasonable adjustments through all aspects of the operating model.
- bring proof of eligibility where they are part of the priority occupational cohort of health and social care workers by providing proof of employment; such as their work ID and a letter / current payslip from their employer if they have not received a call and recall communication.
- for care homes, additional actions have been set out in the separate Roving and Mobile Models Standard Operating Procedure.
Community pharmacy providers
Community pharmacy providers must only provide services to patients under the Local Enhanced Service (LES) Agreement where the patient has made a booking through the National Booking Service unless:
- Alternative arrangements to improve uptake or engagement with communities have been agreed with the commissioner (NHSE);
- The patient is unable or unlikely to use the National Booking Service; or
- Appointments previously offered on the National Booking Service are unfilled and the pharmacy contractor is satisfied that the patient is eligible to receive the vaccination in accordance with the eligibility criteria set out in this LES Agreement.
The pharmacy contractor must have suitable procedures in place to support access for patients who are unable or unlikely to book through the National Booking Service.
Acceptable reasons why a patient is unable or unlikely to book through the National Booking Service includes but is not limited to people who:
- Cannot be authorised by the National Booking Service but who do fall into authorised and announced JCVI cohorts (for example in certain circumstances frontline health and social care workers);
- Do not have an NHS number;
- Do not have easy access to the National Booking Service website and/or telephone booking line;
- Require support with communication; and/or
- Experience other difficulties in accessing healthcare.
See the NBS (Q-Flow) website for vaccination sites for more information and user guides.
Planning for diversity and inclusion
We have a collective responsibility to ensure that the COVID-19 vaccine is accessible to everyone in our community. The COVID-19 Vaccine Equalities Connect and Exchange Hub provides a central place where providers can access information, resources, tools, good practice and webinars to support service planning and delivery.
Providers might find this framework helpful in maximising vaccine uptake in underserved communities. Access to data and population behavioural insights will enable providers to gain a detailed understanding of your local populations and identify gaps in uptake and facilitate targeting of initiatives. Providers may wish to work with their commissioner to support their approach and to account for the needs of the local population. For example:
- Ensure people without access to digital tools, or without an NHS Number, can access vaccine information and know how to get their vaccine
- Use data and local intelligence to choose vaccination sites
- Engage local community and faith leaders, voluntary groups and trusted voices to boost vaccine confidence and sign-post people to trusted sources of information and vaccine centres
- Support diversity within vaccinating teams including volunteers
- Provide information in a range of languages and accessible formats.
In addition to tools and guidance, providers can access the FutureNHS platform for links to a range of resources for the public. This includes links to videos, leaflets and guidance for the public, available in a wide range of languages and formats including BSL and easy read, and information to reassure people about vaccine ingredients or about having the vaccine when planning a baby, pregnant or breastfeeding.
Publicly available resources include:
- COVID-19 information translated into over 60 languages from Doctors of the World.
- Public Health England (PHE) resources to support public communications. These are frequently updated and can be found here.
- PHE leaflet on the use of human and animal products in vaccines
- PHE information on pregnancy and breastfeeding in a range of accessible formats and languages
- Covid Vaccine film by Skills for People and Learning Disability England
- UK Health Security Agency easy read Covid vaccination leaflet
- UK Health Security Agency easy read What to expect after the vaccine leaflet
- PHE easy read Consent form for adults
- Letter to reassure people without an NHS number, including migrants, about their right to access the COVID-19 vaccine, and if they wish to, register for a GP.
The FutureNHS Communications and Engagement pages include the following resources:
- A communications pack to help engagement with diverse audiences, including 16 – 17 year olds
- An easy read template invitation letter for use by PCN-led sites when contacting people with a learning disability and autistic people, together with COVID-19 vaccination easy read resources and easy read consent form
- Tips for people with dementia
Additional training materials for COVID-19 vaccinators and volunteers provide tips on communicating with people with a learning disability and autistic people and any reasonable adjustments that should be considered.
4.2 Vaccination suitability
The Green Book (chapter 14a) is updated on a regular basis. Some recent clarifications have been made including about individuals on stable anticoagulation therapy; patients who are about to receive planned immunosuppressive therapy; and children with neurological comorbidities. Providers should regularly refer to the Green Book for more information.
Patients who are excluded from COVID-19 vaccination
The Medicines and Healthcare products Regulatory Agency (MHRA) and/or the manufacturer of COVID-19 vaccines may provide guidance for certain patient groups who should be excluded from vaccinations.
- Pfizer/BioNTech (Comirnaty®) vaccine: Information for healthcare professionals and the public
- AstraZeneca (Vaxzevria®) vaccine: Information for healthcare professionals and the public
- Moderna (Spikevax®) vaccine: Information for healthcare professionals and the public
All providers are responsible for checking published information about the COVID-19 vaccines. Clinicians should apply professional curiosity to assess, as part of the pre-vaccination clinical assessment, the likeliness of these exclusions applying.
Medical history: contraindications and precautions
- For all patient groups, those whose medical history contains absolute contraindications found within the manufacturer’s recommendations should not receive that vaccine and consideration must be given as to whether other vaccines may be offered. See:
- Pfizer/BioNTech (Comirnaty®) Regulation 174 Information for UK Healthcare Professionals
- AstraZeneca (Vaxzevria®) Regulation 174 Information for UK Healthcare Professionals
- Moderna (Spikevax®) Regulation 174 Information for UK Healthcare Professionals
- A very small number of individuals have experienced anaphylaxis when vaccinated with the Pfizer/BioNTech (Comirnaty®) and Moderna COVID-19 vaccine. All recipients of the Pfizer/BioNTech (Comirnaty®) or Moderna COVID-19 vaccine should be monitored for a minimum of 15 minutes. Facilities for management of anaphylaxis should be available at all vaccination sites (see Green Book Chapter 8) – see section 3.4 for more information.
- The British Society for Allergy and Clinical Immunology (BSACI) has advised that individuals who have a reaction to the first dose of a COVID-19 vaccine may be able to receive a 2nd dose of vaccine. Providers should follow the flowchart provided in the Green Book chapter 14a, reproduced in the figure below.
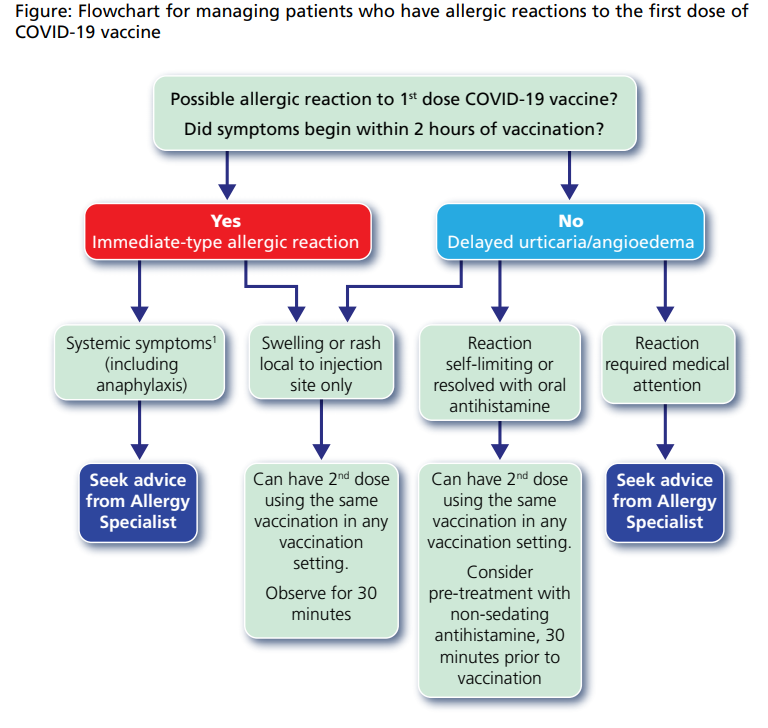
- Syncope (fainting) can occur following, or even before, any vaccination especially in adolescents as a psychogenic response to the needle injection. This can be accompanied by several neurological signs such as transient visual disturbance, paraesthesia and tonic-clonic limb movements during recovery. It is important that procedures are in place to avoid injury from faints.
- On 7 May 2021 the JCVI and MHRA issued updated advice on the AstraZeneca (Vaxzevria®) vaccine. This follows the related JCVI statement of 7 April 2021 which can be found here. The MHRA has also issued information for healthcare professionals on blood clotting following COVID-19 vaccination and Public Health England has updated patient information leaflets.
Pregnancy and breast-feeding
- JCVI advises that pregnant women should be offered the COVID-19 vaccine at the same time as the rest of the population, based on their age and clinical risk group.
- You can read the JCVI statement here.
- NHS England has written a letter outlining next steps in response to the JCVI statement.
- Public Health England’s Green Book has been updated to reflect this latest advice from the JCVI advice.
- The Green Book still advises that pregnant women should discuss the risks and benefits of vaccination with their clinician, including the latest evidence on safety and which vaccines they should receive. Please ensure you have in place a local pathway to ensure that pregnant women can receive the required pre-vaccination advice at their vaccination appointment, as set out in our checklist here.
- The Green Book advises there is no known risk associated with giving non-live vaccines whilst breastfeeding.
Further information is on the GOV.uk website.
Other vaccinations and co-administration
- For advice on co-administration of the COVID-19 vaccine and other vaccinations refer to the Green Book chapter 14a: COVID-19 – SARS-Cov-2.
- “Co-administration of COVID-19 and influenza seasonal vaccines: the JCVI guidance states that “where operationally expedient, COVID-19 and influenza vaccines may be co-administered”. Therefore, systems should consider co-administration wherever eligibility for both programmes, supply and regulation allow. In particular, systems should seek to co-administer in any instances where it improves patient experience and uptake of both vaccines, reduces administrative burdens on services or to reduce health inequalities. The Phase 1 and 2 Enhanced Service specification and Phase 3 Enhanced Service specification for general practice, and the PCN Collaboration Agreement, have been updated to reflect the JCVI guidance, supporting practices working as part of a PCN grouping in using their supply of seasonal influenza vaccine at COVID-19 vaccine designated sites (e.g. at joint clinics). The updated Community Pharmacy Phase 3 Local Enhanced Service Specification (the LES) has also been updated to support co-administration where eligibility for both programmes, supply, and regulation allow.
- The JCVI has advised that “the COVID-19 booster vaccine programme should [not] disrupt or delay deployment of the annual influenza vaccination programme”. Therefore, it is important individuals are offered their COVID-19 and influenza vaccine as soon as they are eligible, rather than delaying for the purpose of co-administration. We recognise there will be some instances where a short delay will ensure that more individuals receive both vaccines, for example in care homes, and sites should use their discretion to maximise these opportunities. Providers should take every opportunity to promote the uptake of both vaccination programmes, including booking individuals in for their influenza vaccine in instances where co-administration cannot realistically be done (taking into account patient choice where eligible patients wish to receive their vaccinations at separate appointments). Vaccination sites should also use appointments as an opportunity for other health promotion activity, ‘Making Every Contact Count’ where practical and appropriate, as well as for co-promotion of flu and COVID-19 vaccine.
COVID-19 symptoms
- For all patient groups, COVID-19 vaccines should not be given to anyone who is suspected or confirmed to have COVID-19 or is awaiting a test result.
- As clinical deterioration can occur up to two weeks after infection, ideally vaccination should be deferred until clinical recovery to around four weeks after onset of symptoms or four weeks from the first confirmed positive specimen in those who are asymptomatic.
4.3 Consent and mental capacity
Consent
As Covid vaccines are new, there may be more questions from the public about their safety and what their own options are. Detailed information about consent for vaccination is provided in Chapter 2 of the Green Book. A briefing on consent issues, including the requirements around consenting children and specific information around consent within the national protocol framework, is on FutureNHS.
Further information on vaccine safety and effectiveness is available from the MHRA. Information for the public is available from PHE.
There is no requirement for consent to immunisation to be in writing, but it is good clinical practice to record that a discussion has taken place and consent has been obtained. The completion of a consent form is not a substitute for the provision of meaningful information sufficient to meet the individual’s needs. Relevant consent forms, other supporting forms and associated information can be found on the GOV.UK website. These forms should be adapted locally to apply to the specific doses (first, second, third, primary dose or booster) being given. Consent remains valid unless the individual who gave it withdraws it. If there is new information between the time consent was given and when the immunisation is offered, it may be necessary to inform the patient and for them to re-confirm their consent.
Patients who lack the relevant mental capacity
Some people may lack mental capacity to make decisions about vaccination. This will include some (but not all) people with dementia, learning disabled and autistic people, people with mental health difficulties and people with acquired brain injury. These people, if they are aged 16 or over, are protected by the empowering, decision-making framework set out under the Mental Capacity Act 2005 (MCA).
Healthcare professionals offering the vaccine to someone who may lack the mental capacity to consent should take all practicable steps to support the person to make the decision for themselves.
Where it has been established that the person lacks capacity to consent, a best interests decision should be taken in line with the best interest checklist in section 4 of the MCA. The decision-maker must consider all the relevant circumstances, including the person’s wishes, beliefs and values, the views of their family where appropriate and what the person would have wanted if they had the capacity to make the decision themselves.
The decision maker should make a record of their best interests decision. Best interests decisions must always be made on an individual basis.
Where appropriate, the person’s deputy or those with a Lasting Power of Attorney (LPA) for Health and Welfare should be consulted. If there is a deputy or an LPA with relevant authority, then the healthcare professional can only give the vaccination if the deputy or LPA has first given their consent. Such consent can only be given if it is in the patient’s best interests.
Consent (given by a deputy or LPA with relevant authority in the person’s best interests), or a best interests decision by a healthcare professional to vaccinate, or not, (informed by advance consideration and information gathering undertaken by carers) should be recorded. This is a required field on the Outcomes4Health/Pinnacle Point of Care system. Where the person giving consent is not the patient, e.g. is their deputy or LPA, the name of that person and their relationship to the patient should also be recorded.
Care homes and care staff
Further important guidance on consent and mental capacity for care home residents can be found in the separate Roving and Mobile Models Standard Operating Procedure.
Health and social care staff
PHE has provided templates for consent forms and letters for frontline social care staff (who may work in care homes, domiciliary care and community care settings) and the wider health care staff.
People with SMI, dementia, a learning disability or autistic people
Further important guidance on consent and mental capacity can be found on the FutureNHS platform here.
Eligible Children and Young People
See section 5.
4.4 Clinical review
The patient must be assessed for their suitability for vaccination following informed consent being obtained.
The principles of the Green Book should be followed as well as COVID-19 vaccine specific guidance.
It is not anticipated that detailed knowledge of the individual’s recorded past medical history or allergy history will be essential to allow for safe decision making about vaccine administration. However, access to the Summary Care Record will be available in all settings. Some conditions may increase local side effects, i.e. bruising and anticoagulants/clotting disorders, but not be inherently unsafe.
4.5 Administration of vaccination
See section 2 for signposting to information about preparation of COVID-19 vaccines.
The patient should be prepared as per usual immunisation protocols and infection prevention and control procedures, and the vaccine delivered as advised by the vaccine manufacturer and as per PHE vaccination guidance for healthcare practitioners.
4.6 Post-vaccination observation
Post-observation periods should follow normal arrangements for observation after vaccination and pharmacovigilance, as set out in chapter 14a of the Green Book.
Patients should be given a post-vaccination record card (delivered to providers alongside the vaccines) with details of their vaccination, and provided with information on the process to follow if they experience an adverse event in the future after leaving the site, including signposting to the Yellow Card service.
The patient should be made aware of possible side effects as set out in the patient leaflets (delivered to providers alongside the vaccines and available online).
The Public Health England (PHE) Immunisation Department is conducting enhanced surveillance of COVID-19 cases in vaccinated individuals in England. Clinicians who are seeing patients face to face are encouraged to report any confirmed cases in partially or fully vaccinated individuals if they tested positive within the preceding 7 days. This provides an opportunity to get early and complete samples from these cases. Further information is available here.
4.7 Records management and data validation
Sites must ensure clinical records are kept up to date. Local vaccination services will be required to document the vaccination event into the Point of Care system. Providers can create an input for any patient using a look up from PDS by NHS number or patient demographic details.
Minimum data capture process:
- Patient confirms consent verbally, from which the applicable consent scenarios can be selected;
- Clinical review and screening questions prompted from within the Point of Care system template, as well as a notification of flu and COVID-19 vaccination status to enable recording of clinical review; and
- Capture of the vaccination event details through manual data entry into system.
There may also be additional fields to complete to support the vaccination programme.
NHS Digital has published FAQs on COVID-19 vaccination record queries here.
Vaccination data from nationally approved COVID-19 Point of Care systems is extracted from the platform and shared with NHS Business Services Authority (which manages the payment process on behalf of NHS England) in order to calculate monthly payments that are due to LVS providers. More information on payments can be found in our financial guidance on FutureNHS.
4.8 Roving vaccinations and clinics not at the designated site
To help improve patient access and to reach patients who are unable to travel to vaccination clinics, PCN groupings and community pharmacy contractors can administer vaccines from locations other than the designated site. This includes the operation of a roving vaccination model to visit housebound patients, care homes e.g., older adults, or people with learning disabilities or mental health conditions, and other residential settings or settings of multiple occupancy e.g. hostels/hotels for people experiencing homelessness.
More detailed guidance on the different roving operating models can be found in the separate Roving and Mobile Models Standard Operating Procedure.
5. Children and Young People
This section must be read in conjunction with:
- Joint Commission on Vaccination and Immunisation (JCVI) statement on COVID-19 vaccination of children and young people aged 12 to 17 years here.
- JCVI statement on COVID-19 vaccination of children and young people aged 12 to 15 years here.
- Vaccination of healthy children aged 12-15. Letter of 15 September here.
- Enhanced service specification: COVID-19 vaccination programme 2020/21 (general practice) here.
- Phase 3 Enhanced Service Specification (GP led vaccinations) here.
- Phase 3 Local Enhanced Service (Community Pharmacy) here.
- The Green Book contains details about the eligibility of individuals aged 12 years to 15 years old on page 17 here.
- COVID-19 vaccination programme: actions for all practices to support vaccinating eligible 12-15 year olds here.
- Letter from Sir Keith Willett on the vaccination of children and young people: here.
- The readiness self-assessment checklist here.
5.1 PCN-led LVS sites
12–15 year olds with underlying health conditions listed in the enhanced service specifications or who are household contacts of individuals who are immunosuppressed
PCN-led LVS sites delivering the COVID-19 vaccination enhanced service (Phase 1 and 2 enhanced service or Phase 3 enhanced service) will be the main providers of vaccinations for eligible 12-15 year olds at increased risk of serious COVID-19 disease that includes those with the conditions listed in the enhanced service specifications; or who are the household contacts of individuals (either adults or children) who are immunosuppressed (cohort xiii).
Household contacts in this context are those used in the Green Book chapter14a: COVID-19 – SARS-CoV-2: “Individuals who expect to share living accommodation on most days…and therefore, for whom continuing close contact is unavoidable. This may include carers.” Members of ‘bubbles’ that do not live with an immunosuppressed person for the majority of the week are not included in the definition of ‘household contacts’ for the purpose of the COVID-19 vaccination programme.
All other 12-15 year olds
The majority of COVID-19 vaccinations for ‘healthy’ 12-15 year olds will be administered by School Age Immunisation Services (SAIS). The Phase 1 and 2 and Phase 3 enhanced service specifications give local commissioners the flexibility to commission a PCN grouping to vaccinate ‘healthy’ 12-15 year olds in collaboration with SAIS where additional capacity is required and where the PCN grouping is able and willing to support. We expect this flexibility to be used in exceptional circumstances only and PCN groupings must not vaccinate this group without commissioner agreement.
16-18 year olds
PCN Groupings which have opted in to vaccinate cohorts 10-12 under the Phase 1 and 2 enhanced service specification, and all PCN groupings which have opted into the Phase 3 enhanced service specification, can vaccinate those aged 16 – 17¾ and 17¾ – 18 years old as these age cohorts have been added to cohort 12.
For sites using NBS, Qflow must be updated if they are NOT vaccinating children. This only applies to sites which have opted out of cohorts 10-12 under the Phase 1 and 2 enhanced service specification; all PCN-led sites opted into the Phase 3 specification are required to vaccinate all cohorts set out in the specification. All PCN-led LVS sites on NBS have been set to show vaccination of children as a default setting.
PCN- led LVS sites are strongly encouraged to review the checklist and ensure they are compliant. The checklist criteria have been developed to help providers identify the minimum requirements for vaccinating children, and cover the following elements: Safeguarding, Consent, Staffing, Environment and processes, Emergency Preparedness, Legal Framework.
5.2 Community Pharmacy LVS sites
Community Pharmacy LVS sites are able to vaccinate 16 – 17 ¾ year olds and 17 ¾ – 18 year olds subject to completion of the clinical criteria in the checklist as a self-assessment process. The checklist criteria have been developed to help providers identify the minimum requirements for vaccinating children, and cover the following elements: Safeguarding, Consent, Staffing, Environment and processes, Emergency Preparedness, Legal Framework.
Once the site is compliant with the requirements outlined in the checklist, their QFlow profile should be updated on NBS. All community pharmacy providers delivering services under the Phase 3 local enhanced service are required to carry out the service in compliance with their Terms of Service.
5.3 For children and young people aged 16 to 17 ¾ years old with severe neuro-disabilities or a Learning Disability
For children and young people aged 16 to 17 ¾ years old with severe neuro-disabilities or a learning disability the preferred model of delivery is via a PCN-led vaccination service, domiciliary teams or school immunisation teams to ensure appropriate reasonable adjustments and access to staff most familiar with the needs of these children and young people. We are developing separate guidance on vaccinating children with learning disabilities and /or autism. Our current guidance is here
5.2 Vaccine advice
At this time, JCVI advice here is that the Pfizer-BioNTech (Comirnaty®) BNT162b2 vaccine is the only vaccine recommended for persons aged 12 to 17 years in the UK.
The National Protocol here and the Patient Group Direction (PGD) here for COVID-19 mRNA vaccine BNT162b2 (Pfizer/BioNTech Comirnaty®) have been updated to reflect JCVI guidance on the vaccination of children and young people. They outline the requirements for the administration of the Pfizer/BioNTech (Comirnaty®) vaccine to individuals in accordance with the national COVID-19 vaccination programme.
The JCVI statement on COVID-19 vaccination of children and young people aged 12 to 17 years here. Young people who receive their first dose above the age of 17 years and 40 weeks may be scheduled to receive their second dose after an interval of at least 8 weeks, as part of the “turning 18 programme”.
In all instances, the offer of vaccination to children and young people must be accompanied by appropriate information to enable children and young people, and those with parental responsibility, to be adequately appraised of the potential harms and benefits of vaccination as part of informed consent (see section 5.3.4) prior to vaccination.
Consideration needs to be given to managing disclosure from the child and young person, and for handling more sensitive topics such as questions around pregnancy and the privacy afforded by the physical space. If interpretation is needed, then providers need to consider using alternatives to a family member. Sites need to consider how they could support children and young people who require translation and interpretation services and wish to answer away from family members (e.g. parents) or carers.
5.3 Specific operating requirements for children and young people
See sections 3 (Preparation for local vaccination services) and 4 (Operating Model) above. The following additionally apply to children and young people.
When inviting children and young people to book for a vaccination appointment. Chapter 2 of the Green Book on consent suggests that “information should be provided in a way the individual can understand, ideally before the immunisation appointment”. This is good practice for all vaccinations but is also a minimum standard within the checklist for vaccinations to the 12–15-year-old group. Information must be given at a different point in time to the gaining of consent.
When contacting patients to book their appointments appropriate information needs to be enclosed. When contacting via a text then a link to the information can be included within the text. The link is here.
If inviting patients to book via a letter then the appropriate literature can be included within the letter. This is available to order and the product codes are found on the same link here.
Identification, booking and communicating with eligible patients
i) Children and Young People aged 12-15 years old in cohort xiii*: PCN led LVS sites only
*Those aged 12-15 year olds at increased risk of serious COVID-19 disease that includes those with the conditions listed in the enhanced service specifications; or who are the household contacts of individuals (either adults or children) who are immunosuppressed.
All GP practices were required to run local searches by 27 September 2021 to ensure patients in cohort xiii are offered the opportunity to receive their COVID-19 vaccination as soon as possible. Once the searches have been undertaken:
- Practices forming PCN Groupings that have opted to vaccinate 12-15 year olds should have ensured that this group is invited to book a COVID-19 vaccination by 30 September.
- Practices forming PCN Groupings that have opted out of vaccinating 12-15 year olds and practices that are not delivering the COVID-19 vaccination enhanced service should follow the next steps detailed in our letter dated 20 September.
Not all eligible 12-15 year olds may be identified through the GP record. Therefore, any approach by parents (or someone with parental responsibility) of children at increased risk should be considered by practices and clinical judgement used around eligibility in line with JCVI advice. If a patient (or someone with parental responsibility) considers the 12-15 year old is eligible under this group but have not been invited for an appointment, we are advising them to contact their GP practice to discuss their eligibility and so that the GP can signpost the patient appropriately. Eligible patients in cohort xiii of PCN-led LVS sites using the NBS booking system will not be able to book an appointment via NBS and appointments will need to be made via a local booking system.
Household contacts of the immunosuppressed aged 12-15 years old: All GP practices should identify individuals on their registered patient lists who fall into the Green Book definition of severely immunosuppressed and write to inform these individuals that any household contacts aged 12-15 years old are eligible to receive the COVID-19 vaccination (using the JCVI definition) in addition to any household contacts aged 16 years and over. All GP practice staff should be informed that 12–15-year-old household contacts of people who are severely immunosuppressed or those with parental responsibility may call the GP practice asking to book a vaccination appointment and they should then be invited to attend a PCN-led vaccination site. Household contacts will need to provide the practice with a copy of the letter here sent by the practice, together with details of address of the immunosuppressed contact which should match the address held by the immunosuppressed person’s GP practice. Younger household contacts may need to confirm their date of birth on the day. This should happen on arrival for their vaccination appointment.
ii) Children and young people aged 16 – 17¾ years old and 17¾ – 18 years old
PCN led LVS sites:
- Sites should invite eligible children and young people to book a COVID-19 vaccination using existing local systems to undertake local call and recall, identifying, and inviting all eligible patient cohorts, as advised by the commissioner, on their registered list to book vaccination appointments.
- Sites can offer COVID-19 vaccinations to all 16-17 ¾ and those aged 17 ¾ year olds as walk ins or via local booking.
- Booking via NBS is available to those aged 16+.
- Patients registered with practices that have chosen not to sign up to the Enhanced Service or to deliver vaccinations to cohorts 10-12 can be vaccinated by an alternative provider. The patients’ registered practice should co-operate with the commissioner to ensure that patients are advised as to where they can access vaccination
Community Pharmacy LVS sites:
- Booking via NBS is available to those aged 16+.
- Community pharmacy LVS sites should offer COVID-19 vaccinations to all 16-17¾ and those aged 17¾ year olds as walk ins or via local booking subject to completion of the requirements in section 5.1.2.
Workforce
Staff working with individuals under the age of 18 must have an appropriate level of disclosure and barring service (DBS) check. Sites should refer to the workforce consideration document available on FutureNHS for further guidance, including how to obtain a fast track DBS check. This requirement for enhanced DBS checks applies to all in the pathway i.e. Registered Healthcare Professionals (RHCPs), unregistered vaccinators, healthcare support workers and SJA volunteers in the role of a vaccinator and post-vaccination observer. Volunteer stewards do not require DBS checks for this cohort if they are supervised by a member of staff with an enhanced DBS check. Further information is available here
Safeguarding
Safeguarding requirements are outlined in the workforce consideration document available on FutureNHS.
Sites should ensure there is a dedicated area available where sensitive questions can be asked in privacy and ensure there are sufficient escalation points (both clinical and non-clinical check points) to ensure patient safety at all stages of the end-to-end journey. These points and processes have to be defined and assured locally.
As per the self-assessment checklist, LVS sites require a dedicated level 3 safeguarding professional and clinical lead. The safeguarding must be trained in L3 Safeguarding Children and Adults. This lead must be immediately available either on- or off-site.
The dedicated safeguarding and clinical lead are responsible for developing local processes to ensure compliance with the requirements set out in the self-assessment checklist, the Patient Group Directives, and the workforce considerations for the vaccination of children.
Consent
See section 4.3. The following specifically applies to children and young people.
Sites should follow the guidance in the Consent Briefing on the requirements around consenting children, which is available here, and ensure they are using the most up to date version. This states:
“As in the case for adults, valid consent will be required before any treatment can lawfully be given to a child. Consent may be given by a competent child, by any person who has parental responsibility for the child or by the court. At 16 years of age a young person is presumed in law to have the capacity to consent, so young people aged 16 or 17 years can [and should] consent to their own medical treatment.
For infants and young children who are not competent to give or withhold consent, consent can be given by a person with parental responsibility, provided that person is capable of consenting to the immunisation in question and is able to communicate their decision.
Clinicians should discuss the risks and benefits of vaccination with a person with parental responsibility, who should be given sufficient information about vaccination of children aged under 16 years to support them with their decision. This should be in more than one form i.e. written information but also verbal, easy read leaflets or videos/online links.
Where someone aged 16 -17 years consents to vaccination, a parent cannot override that consent.
Young people (under 16) who understand fully what is involved in the proposed treatment (referred to as ‘Gillick competent’) can also give consent, although ideally their parents will be involved. It is the responsibility of the registered healthcare professional to determine whether a child under the age of 16 is ‘Gillick competent’ or not. If a Gillick-competent child consents to treatment, a parent cannot override that consent. If the registered health professional taking consent felt a child was not Gillick competent then the consent of someone with parental responsibility should be sought. If a person aged 16 or 17 years or a Gillick-competent child refuses treatment that refusal should be accepted. Further information on this can be found in chapter 2 of the green book.
Emergency preparedness – first aid and resuscitation preparation
The checklist sets-out the requirements that need to be in place, including information on training requirements, the requirement of an emergency protocol, and local risk assessments.
In addition to the requirements in section 3.4 above, see:
- Section A of the checklist for children and young people aged 16-17 here
- Section B of the checklist for children and young people aged 12-15 here
Site requirements
When planning the operational implementation of this guidance, sites must ensure there are appropriate mechanisms for patients aged less than 18 years to be identified on check-in and routed so they are seen by appropriate, competent and trained staff.
While children and adults can use the same facilities such as entrance and post-vaccination waiting areas, it is important that pathways are separated at point-of-care (POC) and that there are dedicated facility and staff.
Post-vaccination observation can take place in the same area that is dedicated for the vaccination of adults. Sites should ensure that information on aftercare is available to the child and/or carers that is age-appropriate and advises them about what to do should the child become unwell.
For disabled young people it is essential that where possible reasonable adjustments are considered with individuals/families and recorded ahead of the vaccination appointment and provided to all members of the Immunising Team involved in the care of the young person involved. Information is available here.
Roving vaccinations and other vaccinations away from the main site
See section 4.8. The following additionally apply to children and young people.
Where children and young people are to be offered vaccination services at roving/pop up sites this is subject to the agreement of the local commissioner and provided the site meets the requirements of the ES and the requirements set out in the checklist.
Where sites can vaccinate children and young people the following applies to roving and mobile models:
- Vaccination of eligible children and young people aged 12-15 (cohort xiii*) – PCN led LVS sites only. The Green Book states that “in secondary school age children, information leaflets should be available for the young person’s own use and to share with their parents prior to the date that the immunisation is scheduled”. This currently means that it is not possible to vaccinate eligible 12–15 year olds in a pop up, roving site or walk in sites unless they have received an information leaflet prior to the date that the immunisation is booked. Further advice will be available on this issue.
- Vaccination of children and young people aged 16-17¾ and 17¾ and over. LVS sites can vaccinate these patients at a roving/pop up site subject to local commissioner agreement.
*Those aged 12-15 year olds at increased risk of serious COVID-19 disease that includes those with the conditions listed in the enhanced service specifications; or who are the household contacts of individuals (either adults or children) who are immunosuppressed.
