Purpose and scope
This guide has been developed jointly by the Department of Health and Social Care (DHSC) and NHS England to detail the national, regional and local management and escalation processes and communication routes for medicines supply issues in England.
It is for NHS teams and professionals who have responsibility for reporting a medicine supply issue, as well as those who need to act when a shortage arises and share information with those patients impacted by the disruptions in supply.
It covers all national processes for managing medicines shortages and discontinuations in the NHS in England. The processes apply to all types of medicines used in the NHS, although alternative processes may be required for some specialist products such as vaccines, clinical trial medicines and specials. These processes are outside the scope of this guide.
The management, escalation and communication processes for non-medicine shortages in the NHS, including medical devices and clinical consumables used to administer medicines, are not within the scope of this document, along with borderline substances.
DHSC regularly shares information about higher impact and critical medicine shortages with the devolved governments to support management of medicines supply issues across the UK. Although not directly within scope, this guide will include some information on DHSC’s information sharing and collaboration with the devolved governments.
Context
We recognise that medicines supply issues are frustrating and distressing for patients and can significantly impact their care as well as the services responsible for managing and mitigating them.
Medicine supply chains are complex, global and highly regulated. There are several reasons why supply can be disrupted – many of which are not specific to the UK. These include manufacturing difficulties, access to raw materials, sudden demand spikes or distribution and regulatory issues. This means that some supply problems with medicines will be inevitable and will require national management and local collaboration across DHSC, NHS England, the NHS and clinicians to help mitigate the risk to patient care.
This guide has been updated to reflect the changes in practice since its first iteration – titled A guide to managing medicines supply and shortages – was published in November 2019. The main changes reflect our working practices since the Covid-19 pandemic and also now that we are accredited to issue National Patient Safety Alerts (NatPSAs).
Background
The escalation and management of medicines shortages and discontinuations, including dissemination of information to primary and secondary care organisations, is vital in ensuring continuity of supply and that patients can access the medicines they need.
DHSC has overall responsibility for the continuity of medicines supply in the UK and crown dependences, including shortages management. However, responsibility for managing the impact of shortages is devolved for Northern Ireland, Scotland and Wales.
Manufacturers have a legal requirement to inform DHSC of any supply problems.
The NHS England Medicines Procurement and Supply Chain Team (MPSC) – formerly the Commercial Medicines Unit (CMU) – is responsible for all secondary care medicines and certain homecare services procured on MPSC frameworks. MPSC may hand over management of a shortage to the DHSC team if the secondary care contract is a small percentage of the market, but this would be agreed on a case-by-case basis.
DHSC and NHS England work closely with the Medicines and Healthcare products Regulatory Agency (MHRA), the wider NHS (including pharmacists), pharmaceutical companies (both manufacturers and suppliers), wholesalers and others in the supply chain to ensure consistency of supply of medicines.
All organisations involved in the national management of shortages and discontinuations must always be mindful of the commercial and competitive nature of the pharmaceutical industry and ensure that exchanged information is handled sensitively and shared appropriately. It is in everyone’s interest that companies are not deterred from providing information that is essential to the management and mitigation of medicines supply issues.
DHSC and NHS England are working with industry and other stakeholders to build robust and resilient future supply chains that can better withstand any shocks or threats to supply. This includes consideration of measures to strengthen UK medicines supply resilience in the short, medium and long term.
1. National management of medicines supply
Overview
1.1 DHSC has overall policy, strategic and operational responsibility for ensuring the continuity of the supply of medicines to the NHS in England. Working closely with DHSC, the MPSC Team in NHS England has delegated responsibility for managing the continuity of supply for medicines procured on MPSC frameworks. These frameworks are used by secondary care trusts in England. Both DHSC and NHS England work with national teams and other supporting teams and bodies across the system to manage and resolve medicines supply issues.
1.2 The Medicines Shortage Response Group (MSRG) was established in 2019. It is a decision-making body that provides the DHSC Medicines Supply Team and the NHS England MPSC Team with governance and oversight as well as support in the management of medicines shortages nationally. Cross-organisational sub-groups may be set up to assist with the management of more complex or disruptive issues.
1.3 This section sets out the national processes for the management and escalation of medicines supply issues. This includes the roles of the national bodies, the different options available to manage supply issues (depending on their severity and scale), and the drafting and sign-off of communications on medicines supply issues to healthcare professionals and procurement teams in the NHS in England.
Role of national teams
DHSC Medicines Supply Team
1.4 This team, comprised largely of pharmacists and pharmacy technicians, is responsible for the management of medicines supply issues across primary and secondary care, including shortages and discontinuations.
1.5 To manage medicines supply issues, the team works closely with NHS England, the MHRA, the pharmaceutical industry and others operating in the supply chain, to help prevent shortages and to ensure risks to patients are minimised when shortages do arise.
1.6 The team regularly shares information about higher impact and critical medicines shortages with the devolved governments to support management of medicines supply issues across the UK.
1.7 The team also works with medicines supply strategy and policy colleagues in both DHSC and NHS England on relevant programmes of work. This includes projects designed to strengthen the medium to long-term resilience of the UK medicines supply chain.
NHS England MPSC Pharmacy and Supply Chain Team
1.8 MPSC is responsible for managing regional and national framework agreements for the procurement of secondary care medicines and certain homecare services by hospitals in England.
1.9 Within MPSC, the Pharmacy and Supply Chain Team works to ensure continuity of medicine supply in secondary care in England. This includes leading the management and communication of MPSC framework medicines supply issues.
1.10 Manufacturers and suppliers or trust pharmacy procurement teams report supply issues for products on an MPSC framework directly to the Pharmacy and Supply Chain Team. The team manages these supply issues in close collaboration with the DHSC Medicines Supply Team.
1.11 Both the NHS England Pharmacy and Supply Chain Team and the DHSC Medicines Supply Team work closely with the NHS England Specialist Pharmacy Service (SPS) and other specialists to seek their advice and support on potential management options.
The NHS Specialist Pharmacy Service
1.12 SPS is a team of pharmacy professionals and support staff who are commissioned by NHS England to provide advice and guidance on all aspects of medicines use. The work of the SPS is outlined in its service specification. The SPS hosts the online Medicines Supply Tool on its website. Please see the Medicines Supply Tool section (from paragraph 1.68) for further detail about this tool.
1.13 Its functions support the management of medicines shortages, as outlined below.
Medicines procurement
1.14 Regional and associate pharmacy procurement specialists act as conduits between local procurement teams and the national teams. They collate and monitor information about medicines shortages relating to secondary care, support local and regional teams to resolve these and escalate issues to the national teams. In doing so, they support the work of the national teams in mitigating issues.
1.15 The SPS Procurement Team is notified of a medicine supply issue by either the DHSC Medicines Supply Team, the NHS England Pharmacy and Supply Chain Team, an NHS trust or NHS foundation trust (collectively referred to as NHS trusts) pharmacy procurement team or a collaborative purchasing organisation. They undertake a supportive and facilitative role with the relevant pharmacy procurement team or relevant collaborative purchasing organisations to ensure mitigation plans are appropriately implemented at a regional level to maintain supply. Support from the regional procurement pharmacist specialist may include analysing use trends; identifying trust, homecare provider, wholesaler or supplier stock positions; redistribution of stock; and facilitating the dissemination of national shortage communications.
Medicines advice
1.16 The DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team approach SPS Medicines Advice (MA) for clinical advice on the impact of a supply issue and possible alternatives to guide the initial risk assessment of a supply issue. SPS MA may provide further clinical input by drafting the clinical advice to be included in official communications of medicines supply issues to the system or to help identify when further specialist input may be required.
Pharmaceutical quality assurance
1.17 The SPS Quality Assurance (QA) Team support the governance arrangements for the safe handling, preparation and purchasing of medicines across the NHS. It may be called on to support the national management of medicines shortages by undertaking a national quality assurance assessment for unlicensed imports. The DHSC Medicines Supply Team or the NHS England Pharmacy and Supply Chain Team will contact the SPS QA Team to request a national assessment of the risk to help organisations use such products.
Medicines use and safety
1.18 The SPS Medicines Use and Safety (MUS) Team supports the safe use of medicines across the NHS by providing resources and outputs that avoid duplication, and by developing and facilitating professional networks. The SPS MUS Team may be asked to support the drafting and review of official communications to the system of medicines supply issues where appropriate. The SPS MUS Director supports the dissemination of national communications to the medicines safety officer network.
Escalating bodies and governance
Medicines Shortage Response Group
1.19 The Medicines Shortage Response Group (MSRG) is a multidisciplinary, clinically-chaired group. Membership of the group includes clinicians and representatives from across DHSC, NHS England and the wider NHS, the devolved governments and the MHRA, as well as from other arm’s length bodies when required.
1.20 The MSRG supports the DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team with the management of supply issues that are categorised as higher impact (Tier 2–4). For more information on the tiers used to categorise medicines supply issues see Appendix A: Clinical escalation categories overview.
1.21 The MSRG is a decision-making body whose role is to:
- have oversight of the number and range of shortages and discontinuations being managed by the DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team
- receive intelligence on medicines supply issues requiring wider discussion
- determine and oversee escalation and de-escalation of Tier 2 and 3 medicines supply issues, in addition to the escalation of Tier 4 issues to a national team, such as Emergency Preparedness Resilience and Response (EPRR), as appropriate
- support and oversee the development of appropriate mitigation plans for managing shortages and discontinuations, including:
- ensuring clinical advice has been obtained, commissioning additional advice where needed, and ratified
- escalating issues and sharing recommendations as necessary with the NHS England National Medical Director and/or DHSC Chief Medical Officer (and nominated Devolved Government representatives where appropriate) for final sign-off
- where a National Patient Safety Alert (NatPSA) is required
- authorising the development of a serious shortage protocol (SSP) – subject to sign-off by Ministers
- agreeing the content and dissemination routes for communications of national medicines shortages
- signing off management and communications plans that are brought before the group for consideration or to agree the sign-off process should senior DHSC and NHS England officials be required to undertake this role
Supporting teams and bodies
Department of Health and Social Care
1.22 Medicine Supply Branch – Strategy and Policy Team: the team works alongside the Medicines Supply Team to assess, reduce, mitigate and communicate risks to medicines supply as well as to strengthen short, medium and long-term medicines supply chain resilience.
1.23 Medicines Directorate – Medicines and Pharmacy Analysis: the team helps to make sure that decisions are informed by high quality evidence. The team supports the design, implementation and evaluation of policies, strategies and programmes.
1.24 Medicines Frameworks and Reimbursement Team: the team has responsibility for a range of policy areas, including community pharmacy reimbursement in England, SSPs, generic medicine pricing and information powers, and has a sponsorship role for the wholesaler sector.
1.25 Supply Resilience Directorate: this directorate works to ensure continuity of supply of medicinal products and promote long-term supply chain resilience.
NHS England
1.26 Medicines Policy and Strategy Unit Medicines Policy (Access) Team: the team supports DHSC and NHS England planning. Policy advice and support is provided where needed in relation to shortages management, winter planning and future medicines supply chain resilience. The team helps co-ordinate shortages mitigation plans, seeking clinical and analytical expertise as necessary.
1.27 Patient Safety Team: the team provides insight and advice in relation to potential patient safety issues that may arise from a medicine supply issue or shortage. The team also advises on draft versions of Medicines Supply Notifications (MSNs) and NatPSAs that have been agreed by the MSRG – please see Medicines supply communications section (from paragraph 1.72). In addition, the National Director of Patient Safety (or Deputy) reviews requests for expert clinical advice on specific high-risk medicines supply issues from the MSRG and acts as the conduit for obtaining this advice.
Medicines and Healthcare products Regulatory Agency
1.28 The MHRA assists the DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team in the management of supply issues. It expedites regulatory procedures to ensure continuity of supply and provides companies with regulatory support if an underlying regulatory issue is contributing to the supply issue. These regulatory procedures may include:
- speeding up the assessment process for new medicine licences (marketing authorisations), variations to existing licences and variations for a specific batch
- granting temporary exemptions from medicine labelling requirements so that medicines packaged for use in an EU country can be used in the UK
- speeding up the assessment process for manufacturers’ and wholesalers’ licences and licence variations
- considering requests to import unlicensed medicines for the treatment of individual patients (known as ‘specials’).
1.29 The MHRA liaises with both the DHSC Medicines Supply Team and NHS England Pharmacy and Supply Chain Team on any likely regulatory action it is proposing to take in circumstances where immediate availability of licensed medicines may be affected. These could be, for example, situations where the MHRA Defective Medicines Report Centre Team issues recalls of defective medicines or where manufacturers and suppliers do not meet good practice standards – these includes good manufacturing practice, good distribution practice and good pharmacovigilance standards.
1.30 The MHRA notifies the DHSC Medicines Supply Team of potential regulatory action. The DHSC team then undertakes a risk assessment of the potential impact on supply of products from that manufacturer and, if required, develops strategies to manage the supply issue.
Management of medicines supply
Overview
1.31 The management and escalation of medicines supply issues follows 3 steps, which are summarised in Table 1. This table gives examples of some of the routes through which the DHSC Medicines Supply Team and NHS England Pharmacy and Supply Chain Team are notified, the types of information considered for risk assessments and the various management options available. Further information about each step is provided below.
Table 1: Shortage management process
| Step 1: Notification of supply issue | Step 2: Initial risk assessment | Step 3: Management options |
|---|---|---|
|
|
|
Step 1: Notification of supply issue
1.32 The DHSC Medicines Supply Team and the NHS England MPSC Pharmacy and Supply Chain Team may be notified of a supply issue through several different routes, including manufacturers, suppliers and NHS intelligence gathering sources.
Manufacturers’ notifications
1.33 Marketing authorisation holders (MAHs) are companies, firms or non-profit organisations that have been granted a marketing authorisation. The marketing authorisation (the formal term for a licence) allows the holder to market a specific medicinal product in the UK. Part 6 of the Health Service Products (Provision and Disclosure of Information) Regulations 2018 was introduced in January 2019. Under these Regulations MAHs are legally required to provide information to the DHSC Medicines Supply Team about the availability of UK licensed medicines and about discontinuation or anticipated supply shortages. Information must be provided at least 6 months before the shortage or discontinuation happens. Where this information is not available 6 months in advance, the MAH must provide it as soon as reasonably practicable after the producer decides to discontinue or becomes aware that there may be a supply shortage.
1.34 These requirements ensure that the DHSC Medicines Supply Team has relevant information from MAHs at the earliest point to help manage supply shortages and mitigate any potential impacts on patients.
1.35 MAHs are expected to be fully accountable for their supply chain to the UK market and are required to understand the potential impact on UK patients should supplies of their products become unavailable.
1.36 MAHs should notify the DHSC Medicines Supply Team about supply issues via the Discontinuation and Shortages (DaSH) reporting portal. If they have not already done so, MAHs can register for DaSH reporting portal access by contacting: DASH@dhsc.gov.uk. The DHSC Medicines Supply Team will acknowledge receipt of the information submitted and companies may be approached for further information.
1.37 More information on the DHSC reporting requirements for medicines shortages and discontinuations can be found in DHSC reporting requirements for medicines shortages and discontinuations.
1.38 Any information submitted by companies, as outlined in Regulation 29(2) of the Health Service Products (Provision and Disclosure of Information) Regulations 2018, is regarded as commercially confidential information and treated sensitively by the DHSC.
1.39 If an MAH is part of an NHS England MPSC framework agreement, then that supplier should also notify the NHS England Pharmacy and Supply Chain Team about anticipated supply issues and discontinuation of products via the usual reporting route.
NHS notifications
1.40 The DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team receive intelligence on medicines supply issues through other, more informal NHS routes. These secondary and primary care routes are detailed in sections 2 and 3 respectively.
Other notifications and intelligence
1.41 The DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team also often receive intelligence from individual practitioners and organisations about issues affecting supply. These include the MHRA, wholesalers, primary care representative bodies such as Community Pharmacy England (CPE), patient groups and clinical networks such as the Royal Colleges.
1.42 There are also organisations that will initially manage a shortage locally or regionally and only escalate it to the DHSC and NHS England teams if appropriate and required. These organisations include:
- Collaborative purchasing organisations (CPOs): initial management of the shortage should be undertaken by the CPO where it is the contracting authority and impacted trusts should escalate any shortages to the responsible organisation in the first instance. The CPO will escalate to the regional pharmacy procurement specialist for advice and support if a broader market-wide supply disruption is identified, escalating further to the national teams if necessary.
- Homecare providers: they should direct queries about the supply of a specific homecare medicine to the relevant contracting authority in the first instance. The contracting authority should escalate to the relevant regional homecare specialist and/or to the regional pharmacy procurement specialist if the issue cannot be resolved, escalating further to the national teams if necessary.
- Health and justice providers: the lead/chief health and justice pharmacist for a health and justice provider should direct queries or concerns about the supply of a medicine to the NHS England health and justice commissioners. If these cannot be resolved, concerns will be escalated to the DHSC Medicines Supply Team.
- Independent aseptic compounders: queries about the supply of a specific medicine used for compounding that cannot be resolved locally or regionally should be escalated to the national teams, either directly or via the appropriate regional pharmacy procurement specialist.
Responsibility for supply issues
1.43 Once notified of an issue through any of the above routes, the team who received the notification will triage responsibility for managing the supply issue; that is, whether it is the DHSC Medicines Supply Team or the NHS England Pharmacy and Supply Chain Team. However, the 2 teams work closely together and so may on occasion work together on issues and assist each other in the management of supply issues where required.
Step 2: Initial risk assessment
1.44 The DHSC Medicines Supply Team and/or the NHS England Pharmacy and Supply Chain Team carry out a thorough risk assessment to determine the potential impact on patients and what type of management options should be considered. The risk assessment considers several factors including, but not limited to:
- the nature of the problem, including the reason for the supply issue and the anticipated duration of the stockout
- the product (formulation, licensed and unlicensed indications, clinical need and patient groups affected)
- assessment of risk, including potential clinical and patient safety impact
- the market, through use figures (using primary and secondary care data), market share and availability of alternative products
1.45 To further assess the medicine supply issue, the DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team may also:
- access prescribing and drug use systems to view NHS trust and homecare provider stock levels and medicines use data
- request NHS Business Services Authority data on the number of patients likely to be affected in primary care by the medicine supply issue
1.46 To support the risk assessment of a medicine supply issue and development of a management plan, the DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team will seek early advice from the SPS MA function. This advice will assist in the risk assessment of the issue, inform selection of appropriate mitigations and form part of the brief to MSRG if the issue is escalated.
1.47 As part of the assessment of a medicine supply issue, the DHSC Medicines Supply Team and/or the NHS England Pharmacy and Supply Chain Team make an initial decision as to the severity of the medicine supply issue and to which tier the supply issue should be allocated. The tier allocated to a supply issue may be discussed and agreed at a MSRG meeting where required.
1.48 Please see Appendix A: Clinical escalation categories overview for the definition of the 4 clinical escalation tiers, the decision-making authorities and likely communication routes.
Step 3: Management options for the supply issue
1.49 Each supply issue has specific characteristics and must be managed on an individual basis while adhering to the clinical escalation categories guidelines.
1.50 If an issue has the potential to impact on patient care, the DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team will consider options to help mitigate and manage any issues. These options may include:
- working with MAHs, suppliers and wholesalers to manage supply of existing stocks
- working with the MHRA to support affected companies with relevant regulatory advice and to expedite regulatory procedures for products deemed critical
- identifying and liaising with other manufacturers to increase production of the product concerned and/or alternative medicines
- commissioning clinical advice from the SPS MA function and national clinical experts via the MSRG and subsequently developing communications to the NHS
- contacting importers to identify sources of product from abroad and expediting import processes with MHRA
- setting allocations for NHS trusts (and homecare providers where appropriate) to manage current stock of a product. If this management option is being considered, regional pharmacy procurement specialists are responsible for identifying and verifying use figures for NHS trusts in their region and assessing how long these stocks are likely to last
- NHS organisations taking a responsible approach to medicine sourcing, distribution and use by implementing mutual aid.
1.51 The DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team may also liaise with the National Homecare Medicines Committee to support national mitigation strategy and standard process for engagement with homecare providers where appropriate.
1.52 All Tier 1 and Tier 2 medicines supply issues are managed by the DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team through the above processes. The MSRG has sight of Tier 1 and Tier 2 supply issues but does not routinely support the management of these unless its support for Tier 2 issues is specifically requested by the DHSC Medicines Supply Team and/or the NHS England MPSC Pharmacy and Supply Chain Team.
1.53 Tier 3 and Tier 4 supply issues are escalated by the DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team to the MSRG, which oversees and decides on management and escalation plans for these issues. These decisions may include:
- commissioning the NHS England Medical Directorate, via the National Director of Patient Safety, to follow the NHS England agreed processes for obtaining further clinical advice in addition to information already gathered from clinical experts, including:
- national clinical directors
- national specialist advisors
- Getting It Right First Time (GIRFT) clinical leads
- Clinical Reference Group (CRG) chairs
- Royal Colleges and other professional bodies
- providing advice on the content and dissemination routes for communications to the NHS and other stakeholders including patient groups
- deciding whether the National Patient Safety Alerting Committee agreed threshold of ‘more likely than one or more potentially avoidable deaths or disability in healthcare in England in a year’ has been met for issuing a National Patient Safety Alert for Tier 3 and 4 issues
- agreeing to the development of an SSP (see Serious shortage protocol section from paragraph 3.6) to help mitigate a shortage
- setting up a short-life working group to support the management of an issue
- deciding when issues need to be escalated to and de-escalated from EPRR teams using the clinical escalation categories framework.
Medicines supply communications
General approach
1.54 The communication option for any medicines supply issue is primarily driven by the tier the issue is assigned. Please see Medicines supply communications section (from paragraph 1.72) and Appendix A: Clinical escalation categories overview for more information.
1.55 The DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team follow a clear operating framework for tier issues and propose appropriate communication routes. These proposals are ratified by the MSRG to ensure that the most appropriate communication route is used.
1.56 The DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team are responsible for drafting communications for medicines supply issues of all tiers of severity. Where a medicine is on an MPSC framework and predominantly used in a secondary care setting, the NHS England Pharmacy and Supply Chain Team are responsible for leading and drafting the communication. The DHSC Medicines Supply Team leads on this process for all other medicines.
1.57 The DHSC Medicines Supply Team and the NHS England Pharmacy and Supply Chain Team may also work closely with MAHs, suppliers, wholesalers, other arm’s length bodies and organisations operating in the supply chain, and relevant patient groups. This helps ensure communications are aligned and supports development of tailored patient communication where needed.
Medicines Supply Notifications
1.58 Medicines Supply Notifications (MSNs) are routinely used to communicate Tier 2 medicines supply issues and those Tier 3 supply issues that do not meet the criteria for issuing a NatPSA.
1.59 The DHSC Medicines Supply Team and/or the NHS England Pharmacy and Supply Chain Team work with primary and/or secondary specialist groups, including patient safety leads, regional pharmacy procurement specialists and the SPS MA function. This ensures that MSNs contain the management options listed above as appropriate for each supply issue.
1.60 The DHSC Medicines Supply Team and/or the NHS England Pharmacy and Supply Chain Team may also request input from the MSRG on specific Tier 2 issues for which more intense management is likely – for example, allocations and therapeutic alternatives. As with Tier 3 and 4 issues, the MSRG Chair is responsible for signing off the final communications
National Patient Safety Alerts via the Central Alerting System
1.61 Some shortages potentially have a more serious patient safety or system-wide impact and therefore require prompt actions by clinicians locally. The MSRG will review all Tier 3 and 4 shortages against the National Patient Safety Alerting Committee criteria for issuing an alert, including the agreed threshold of ‘more likely than not one or more potentially avoidable deaths or disability in healthcare in England in a year’. This will inform a decision regarding whether the issue of a NatPSA to the NHS and independent and social care providers is appropriate.
1.62 All national bodies and teams issuing alerts are required to go through a process of accreditation to issue NatPSAs. This ensures their systems and processes for producing and developing alerts meet the National Patient Safety Alerting Committee’s common standards. The DHSC Medicine Supply Team and the NHS England Pharmacy and Supply Chain Team are both accredited to issue NatPSAs.
1.63 Issuing a NatPSA involves publishing the alert via the Central Alerting System (CAS) website, which generates an email to subscribers. Email alerts are sent to those who need to take action and confirm their actions by logging into the CAS website. Emails are also sent to those subscribers who have requested to receive alerts for information.
1.64 The decision to issue a communication in the form of a NatPSA must be agreed and recommended by MSRG. The MSRG Chair is responsible for signing off the final communications for Tier 3 and 4 supply issues, including NatPSAs where the threshold is met.
1.65 For all Tier 3 and Tier 4 supply issues, national guidance to primary and secondary care will be issued as a NatPSA where the criteria set by the National Patient Safety Committee have been met.
1.66 Where the criteria for a National Patient Safety Alert have not been met, the MSRG will recommend alternative methods of communications, such as an MSN or more bespoke targeted communications.
1.67 The necessary of steps, in order, following the notification of a supply issue for which an NatPSA is to be issued are:
- DHSC MST and/or NHS England MPSC notified of a supply issue. Issue triaged to determine lead organisation
- risk assessment identifies high or critical impact supply issue – Tier 3 or 4
- management options / plan drafted including initial clinical assessment
- escalated to MSRG using MSRG template to provide a summary of the issue and management options
- MSRG reviews issue and decides whether criteria for issuing a NatPSA have been met
- management plan and NatPSA communication route agreed at MSRG
- NatPSA drafted by responsible team (audit trail maintained) with review and input from MSRG sub-groups and appropriate clinical experts
- final draft sent to MSRG Chair or a nominated deputy for sign-off
- NatPSA issued via the Central Alerting System.
The Medicines Supply Tool
1.68 The DHSC and NHS England Medicines Supply Tool is hosted on the SPS website. It is a searchable web-based tool that supports providers of care to manage and mitigate medicines supply issues.
1.69 The DHSC Medicine Supply Team and the NHS England Pharmacy and Supply Chain Team are responsible for adding new entries and maintaining the content of the Medicines Supply Tool. The tool contains entries for all Tier 2, 3 and 4 issues that have also been communicated via MSNs or NatPSAs. Tier 1 supply issues are not routinely communicated but, where appropriate, entries will be added to the Medicines Supply Tool. Entries are maintained regularly as and when updates are available.
1.70 Users of the tool, such as hospital and community pharmacists, can search for medicines supply issues by drug class, tier of issue and new, ongoing or resolved issues. Users can also check when a medicine is expected to be back in stock.
1.71 Only those registered with SPS (using an NHS email address) can access the information in the tool. Registration is open to UK healthcare professionals and organisations that use medicines in providing healthcare.
Communications to the NHS and key non-NHS stakeholders
1.72 The format and dissemination routes for these communication routes in primary care and/or secondary care depend on the severity of the supply issue or shortage and the risk to patients, as shown in Table 2.
Table 2: Dissemination route for communications about supply issues with medicines prescribed in primary care or secondary care
| Tier | Dissemination routes |
|---|---|
| Tier 1 (low impact) |
Primary care:
Secondary care:
|
| Tier 2 (medium impact) |
Primary care: As for Tier 1, plus:
Also circulated to national representative and professional bodies and primary care networks (including Community Pharmacy England, which cascades to its members). Devolved governments are included. Secondary care: As above, plus:
Independent organisations, devolved governments and others are included as required. |
| Tier 3 and 4 (high – critical) |
Primary care and secondary care:
Where the criteria for issuing a NatPSA have not been met, alternative methods of communications, such as an MSN or more bespoke targeted communications, will be used as agreed by MSRG. Also circulated to independent organisations, Devolved Governments and others as required. |
1.73 The following are the primary types of communication, but additional supportive and targeted communications may be necessary depending on the supply issue.
1.74 All Tier 2–4 medicines supply communications will include:
- an overview of the issue including the anticipated out-of-stock date and latest update on the resupply date
- details on the management plan and any local actions required
- links to any relevant clinical advice that has been commissioned, with that advice clearly attributed to the organisation and, if appropriate, the individual who provided it
- information on the availability of potential alternative medicines (including unlicensed imports) that have been recommended as part of the management plan
- signposts to sources of further information such as clinical advice and expertise where available
- a contact for further advice: for example, for secondary care, the relevant regional pharmacy procurement specialists for the region.
2. Managing medicines supply issues in secondary care
Overview
2.1 Local management of medicines supply disruptions in secondary care is led by the pharmacy teams in NHS trusts and collaborative purchasing organisations (CPOs). Support, and escalation where required, is provided by the relevant regional procurement pharmacist specialist who works as part of the SPS.
2.2 NHS trust chief pharmacists are responsible for ensuring that a documented process for responding to medicines supply disruptions is in place. This is led by the NHS trust pharmacy procurement teams.
2.3 This section provides information on the regional and local processes for medicines shortages management, and escalation and dissemination routes for medicines supply communications in secondary care. Organisations may wish to adapt some of these good practice processes for implementation locally, such as detailing the role of the SPS, the CPOs and NHS trust pharmacy teams.
NHS trust pharmacy teams overview
NHS trust chief pharmacist medicines supply and shortage management functions
2.4 The chief pharmacists of NHS trusts are responsible for overseeing their pharmacy team’s local response to and management of medicines supply issues. This includes ensuring that a collaborative approach is taken to consider regional and national supply need, as well as continuity of local supply. The chief pharmacist must take a leadership role in ensuring individual organisations work with national processes and escalate supply issues where needed.
NHS trust pharmacy procurement team
2.5 NHS trust pharmacy procurement teams are responsible for managing any supply disruptions in their trust in accordance with local policies and need to work closely with their SPS regional pharmacy procurement specialist. As some NHS trusts supply medicines locally and to other NHS organisations, such as mental health trusts and community trusts, the teams are also responsible for managing any supply disruptions experienced by these organisations.
2.6 The teams are responsible for NHS trust-level communications to relevant clinicians. They ensure that local risk register reporting and management is undertaken so that clinicians will be aware of any issue and the management plan. The teams should also ensure that medicines governance systems are used for high impact or critical medicines shortages. Most supply disruptions are mitigated locally within an NHS trust.
Medicines supply and shortage management functions
2.7 When an NHS trust pharmacy procurement team identifies a disruption to the supply of a product that is procured and managed by the CPO, it should contact the CPO in the first instance.
2.8 For all other identified medicines supply issues, the NHS trust pharmacy procurement team should first check with all known sources, such as wholesalers and suppliers, for information about current supply issues and take steps to manage the shortage locally. As part of this process, pharmacy procurement teams should consult:
- the online Medicines Supply Tool on the SPS website
- the most recent NHS England Pharmacy and Supply Chain Team supply issues spreadsheet
- all other recent communications on medicines shortages from national teams, for example MSNs and NatPSAs.
Medicines supply and shortage escalation functions
2.9 It is recognised that local capacity to investigate and manage supply issues will vary, but it is important that local action is taken to avoid premature escalation and to enable resolution of most shortages locally. For example, trusts may use the checklist in Appendix D: Checklist for use by NHS trust pharmacy procurement teams, either as it stands or adapted for local use, to support the review of local management and escalation of medicines shortages.
2.10 Only if the medicine supply issue has not been satisfactorily resolved by using the checklist and reviewing all immediately available information, the pharmacy procurement team should escalate the issue to the relevant regional procurement pharmacist specialist. In addition, issues that are likely to persist for longer than 2 weeks should be escalated to the relevant regional procurement pharmacist specialist even if the local pharmacy procurement team have found a resolution, as these issues are more likely to have a larger impact regionally and nationally.
2.11 In the case of medicines shortages that could have an impact on homecare services, teams can use processes such as those described in Appendix B: Regional and local process for reviewing and escalating supply issues that impact homecare and Appendix C: Regional process for implementing national shortage mitigations that impact homecare to support local medicines shortage management processes. These set out the key considerations when managing these specific issues, which includes engagement with the local homecare lead and homecare provider to consider implementation of mitigation plans or escalate as appropriate.
Case study 1: Desferrioxamine 2g injection
Company: Kent Pharma
Date of shortage: October 2021–November 2021
Tier level: Tier 2 (medium impact)
Overview of supply issue: Desferrioxamine injection has a variety of licensed indications including treatment of iron poisoning, aluminium overload in dialysis patients and chronic iron overload. It is predominantly used when compounded as a continuous infusion for patients with established conditions affecting haemoglobin (haemoglobinopathies). Commercial compounders and NHS aseptic units use the 2g vials to aseptically prepare specialised single use infusion pumps, which patients receive via a medicine’s homecare provider. Only 2 suppliers actively market this product in the UK – Kent Pharma and Novartis – and Kent Pharma holds the majority market share. Kent Pharma reported a 3 weeks out-of-stock in late October 2021.
Risk assessment: The NHS England CMU Pharmacy Supply Team (as it was called at the time) worked with NHS England analysts and the DHSC Medicines Supply Team to risk assess the potential impact and management options. The risk assessment identified that an out-of-stock notification was received at short notice due to an unexpected batch failure at the contract manufacturing plant and that this could impact several thousand NHS patients who required ongoing treatment with desferrioxamine. It also identified potential for significant impact on commercial compounders, NHS aseptic units and homecare providers, as the independencies of the supply chain mean a lack of supply of desferrioxamine vials has potential knock-on operational impact across the system.
The NHS England Pharmacy Supply Team led multi-stakeholder engagement with commercial compounders, suppliers and NHS stakeholders, including specialist clinicians, to determine the volume of stock available to prepare infusions for homecare patients, as well as how much was available for patients in the UK supply chain. The NHS England Pharmacy Supply Team ensured all parties involved in the delivery of care were sighted on the exact stock availability in the UK as well as the expected stock delivery schedules over the coming weeks, allowing accurate modelling of stock exhaustion based on actual patient numbers.
Additionally, the NHS England Pharmacy Supply Team worked with the NHS England Clinical Reference Group (CRG) Chair and the 4 lead clinicians for the co-ordinating centres for thalassaemia in England to determine the clinical risks of not having desferrioxamine available for patients. They prepared detailed clinical plans to prioritise stock in the event of a total out-of-stock situation to ensure the most vulnerable patients were safeguarded against unintended clinical risk.
Management plan: A comprehensive management plan was presented to MSRG for ratification. The NHS England Pharmacy Supply Team worked with senior colleagues at Novartis to mobilise every available vial of desferrioxamine and urgently make these available to those organisations with an acute need to access stock. The remaining available stock was allocated based on actual patient requirements and commercial compounders received emergency overnight deliveries. NHS trusts were asked to provide an updated inventory for desferrioxamine presentations at trust level, and mutual aid between hospitals was arranged when required.
The CRG identified the clinical prioritisation of patient need, to be communicated to trusts to ensure all available stockholding would be ringfenced for those patients with the greatest need.
Communications: To ensure prompt and system-wide action, this Tier 2 issue was communicated in an MSN and cascaded via the CMU NHS distribution list to all NHS trusts. Following issue of the alert, the NHS England Pharmacy Supply Team closely managed use and supply of desferrioxamine until available volumes could meet total need. Oversight was provided by the MSRG.Case study 2: Propofol
Company: Various
Date of shortage: February 2021–April 2021
Tier level: Tier 3 (high impact)
Overview of supply issue: The increased demand for mechanical ventilation from the start of the Covid-19 pandemic in March 2020, particularly at any peak in patient numbers, put great pressure on the supply chains of medicines required to treat patients with Covid-19 on mechanical ventilation in critical care settings. Propofol was one of these medicines. By the second peak in January 2021, despite the best efforts of manufacturers, supply of high-volume vials of propofol and syringes was failing to keep up with demand.
Risk assessment: The Allocation and Distribution Group (ADG) – which was established to review stock levels of Covid-19 supportive medicines and allocate medicines as required to ensure continuity of supply – continually reviewed the data relating to supply and demand of propofol and risk assessed the potential impact of this shortage and the management options. This included planning for a worst-case scenario by modelling a continued increase in patient numbers against available stock, considering the need for release of stock from the Covid-19 supportive medicines stockpile and the subsequent equitable allocation and distribution of stock across the system.
Additionally, ADG worked with a clinical group responding to Covid-19, which included the NHS England National Clinical Director for Critical and Perioperative Care and the Royal College of Anaesthetists, to explore actions to conserve propofol, the operational and risk impact of switching presentations and the possibilities of clinical alternatives.
Regular supplier engagement throughout was led by the NHS England CMU Pharmacy and Supply Team (as it was called at the time) and the DHSC Medicines Supply Team. This provided twice weekly validation of supplier stock position as well as influence on suppliers to expedite resupply dates, increase manufacturing outputs where possible and ensure appropriate allocation of stock to the UK. This engagement also gave the NHS England analysis team access to real-time data so the team could frequently remodel the national supply position based on actual patient numbers, identifying what additional stock needed to be secured for the UK to improve the planned delivery schedules.
Management plan: ADG reviewed the data and explored the mitigations to minimise the impact of the shortage. As a result, the NHS England Pharmacy and Supply Team explored the possibility of securing additional deliveries of propofol, made a formal request to release propofol from the stockpile, and continued discussions with clinical colleagues, including the National Clinical Director for Critical and Perioperative Care, and the Royal College of Anaesthetists regarding the use of alternative products.
In addition, organisations were asked to review their current prescribing practice, conserve high-volume presentations of propofol for use in critical care settings and prepare to switch between presentations should their preferred product become unavailable.
Communications: To ensure prompt and system-wide action, this issue was communicated as a Tier 3 issue in a Supply Disruption Alert (SDA) via the MHRA’s cascade system. Following issue of the alert, the use and supply of propofol continued to be monitored weekly, alongside engagement with suppliers by the NHS England Pharmacy and Supply Team. ADG provided oversight. As the situation improved, this group recommended in April 2021 that the original SDA be rescinded, a decision that was ratified by MSRG.Case study 3: Alteplase 10mg, 20mg and 50mg powder and solvent for solution for injection and infusion
Company: Boehringer Ingelheim
Date of shortage: August 2022–autumn 2024
Tier level: Tier 3 (high impact)
Overview of supply issue: Alteplase is used as:
- a fibrinolytic in the treatment of acute ischaemic stroke
- thrombolytic treatment in acute myocardial infarction
- thrombolytic treatment in acute massive pulmonary embolism (with haemodynamic instability)
Boehringer Ingelheim is the sole supplier of alteplase and the manufacturing process for this medicine is complex. Due to an increase in global demand for alteplase, the company informed DHSC that there would be a supply disruption globally until it could increase manufacturing capacity. There is no therapeutic alternative available for the treatment of acute ischaemic stroke.
Risk assessment: The DHSC Medicines Supply Team risk assessed the potential impact of this supply issue and the management options. An important consideration was conserving alteplase stock for thrombolysis in life-threatening indications.
Management options: The clinical criticality of alteplase means disruption to its supply can have a high patient impact. The issue was therefore escalated to the MSRG. The team engaged promptly with clinical specialists, including the NHS England National Clinical Directors for Cardiology, Stroke and Respiratory Medicine, to determine patient prioritisation for treatment and understand the recommended alternative treatment options and implications of switching patients onto alternative treatments.
The DHSC Medicines Supply Team also worked closely with the suppliers of alternative thrombolytics to determine if they could support the market in the absence of alteplase and tenecteplase for certain indications.
MSRG agreed that the preferred option would be to put an allocation process in place to ensure equitable distribution of available stock across the UK. This work was led by the SPS regional pharmacy procurement specialists in partnership with Boehringer Ingelheim. In parallel, DHSC analysts modelled various scenarios to calculate what vial size would best support UK demand while minimising wastage and conserving the limited stock. Boehringer Ingelheim supported this in communicating the correct dosing to avoid wastage and was then able to adjust the stock provision to the UK accordingly, which helped mitigate the situation.
Communications: A NatPSA was issued via the MHRA Central Alerting System to NHS and other organisations, including independent providers of health and social care to:
- advise clinicians to prioritise alteplase for life-threatening indications
- reduce wastage by selecting appropriate vial sizes and using the most appropriate doses, giving consideration to rounding down to the nearest whole vial
3. Managing medicines supply issues in primary care
Overview
3.1 In primary care, community pharmacies and dispensing doctors dispense over a billion prescription items a year and deal with medicines supply issues daily. They work closely with wholesalers and suppliers to support patients to receive the medicines they need in a timely manner. In situations where supplies cannot be obtained, they work with patients and prescribers to ensure patients receive suitable alternatives on an individual patient basis.
3.2 This section will focus on the management of supply issues for medicines that are prescribed and dispensed in primary care. This includes prescribing and administration by GPs, nurse prescribers, dentists, optometrists and pharmacists, and dispensing by community pharmacies and dispensing doctors. It will also cover the communication dissemination routes to support notification and management of a shortage.
Management
Primary care contractors
3.3 In the event of a supply issue, primary care contractors such as community pharmacies and dispensing doctors manage the issue at an individual patient level.
3.4 Alongside following any specific local protocols, supply issues should be managed locally as follows:
- pharmacists, GPs, healthcare professionals and healthcare organisations working in primary care should check their emails, such as nhs.net, for MSNs and other updates or the Medicines Supply Tool to see if the DHSC Medicines Supply Team has already communicated the issue. If so, they should check:
- the proposed management plan and share information with local prescribers and patients as appropriate
- whether there is a SSP in place (see step 3: management options for the supply issue from paragraph 1.49). If so, further information will be given on the NHS Business Services Authority serious shortage protocol webpage. Where an SSP has been issued, community pharmacies and dispensers must consider whether it is appropriate to supply the product in accordance with the SSP rather than the prescription, and facilitate patient access in line with the SSP and in accordance with NHS terms of service. Please see Serious shortage protocols section (from paragraph 3.6) for further information
- if the shortage is not listed on the Medicines Supply Tool, primary care contractors should report the issue via the Community Pharmacy England (CPE) website and CPE will report the issue to the DHSC Medicines Supply Team
- liaise with medicine wholesalers to check availability and resupply dates
- if specific demand management processes have been put in place at wholesaler level, such as prescription validation management, primary care contractors will need to follow these to obtain stock
- consider contacting medicines suppliers directly for an update and to check how supplies can be obtained. In some cases, suppliers may be able to supply direct
- check whether supply can be arranged from other pharmacies locally, if they have stock of the product
- liaise with prescribers regarding the availability of alternative brands, strengths or formulations of the medicine that may be clinically appropriate for the individual, and arrange a new prescription to be sent to the pharmacy.
3.5 In all cases, community pharmacies should endeavour to clearly communicate to patients any supply issues and relevant information about resupply dates and the proposed management plan. They should also counsel and support affected patients where possible.
Serious shortage protocols
3.6 Where an SSP has been issued, community pharmacists can supply a specified medicine in accordance with this rather than a prescription, without needing to seek authorisation from the prescriber. This saves time for patients, pharmacists and prescribers.
3.7 An SSP is only issued when there is a serious shortage of a medicine and it is likely to be out of stock or in limited supply for some time.
3.8 If an SSP is thought by the DHSC Medicine Supply Team or the NHS England Pharmacy and Supply Chain Team to be an appropriate management option for the supply issue, this will be escalated to the MSRG to authorise the development of a SSP. The SSP is then clinically approved by the NHS England National Medical Director and Chief Pharmaceutical Officer for England, before being recommended to Ministers for their authorisation.
Intelligence gathering from primary care
Sources of intelligence
3.9 Intelligence gathering from primary care supplements the formal reporting by manufacturers and close working relationships with wholesalers, collaborative purchasing organisations and homecare medicines providers, as detailed in section 1 (from paragraph 1.41).
3.10 Additional soft intelligence could come from:
- Representative organisations such as the National Pharmacy Association or the BMA Dispensing Doctors Association. They also receive intelligence on medicines supply issues and may provide this information to the DHSC Medicines Supply Team to support the identification of issues and inform management plans. This is not a formal reporting process as representative organisations have no legal requirement to provide this information.
- Community Pharmacy England (CPE) formally collects and provides monthly data on medicines that cannot be obtained at the Drug Tariff price and collates information on shortages. Primary care contractors should notify CPE via its website of any medicines supply issues that they become aware of which are not on the Medicines Supply Tool.
- NHS operational intelligence sources, including NHS England local professional networks and integrated care systems (ICSs) – such as integrated care board (ICB) chief pharmacists, NHS England commissioning teams and others who provide intelligence on medicines supply issues to the NHS England Pharmacy and Supply Chain Team. That team will then report this intelligence to the DHSC Medicines Supply Team.
- Other trusted sources that have national coverage and link into the DHSC Medicines Supply Team providing soft intelligence. For example, in the past DHSC has established and met with a network of pharmacists and buyers who work for community pharmacies to obtain timely market intelligence on potential and actual supply issues.
Case study 4: Enalapril 5mg tablets
Company: Multiple
Date of shortage: November 2021–January 2022
Tier level: Tier 2 (medium impact)
Overview of supply issue: Enalapril is used to treat hypertension and symptomatic heart failure. It is also used to prevent symptomatic heart failure in certain patients.
There are several suppliers of enalapril 5mg tablets in the UK, both in generic and branded presentations. One of the main suppliers experienced issues due to a change in manufacturing site and the other suppliers could not meet the supply gap, resulting in a market wide shortage.
Risk assessment: The DHSC Medicines Supply Team risk assessed the potential impact and the management options. As the use of this product was particularly high, management plans would need to carefully consider how any patient and system-wide impact could be minimised.
Management options: Given the high use, the shortage would put significant pressure on frontline staff including GPs to switch the high number of patients potentially impacted onto alternative treatments. The team therefore engaged promptly with clinical specialists including the National Clinical Director for Cardiology to understand the recommended alternative treatment options and implications of switching affected patients onto alternative treatments.
In parallel, the DHSC Medicines Supply Team worked closely with the suppliers of alternative strengths of enalapril to determine if they could fully support the market for enalapril 5mg tablets. The suppliers of the 2.5mg and 10mg strength tablets were collectively able to support the market. To ensure the appropriateness of halving enalapril 10mg tablets, the DHSC worked closely with the MHRA, which through analysis of scientific data confirmed this practice would be appropriate. The team then liaised with SPS MA to publish advice on the SPS website about halving 10mg enalapril tablets.
As a Tier 2 shortage, the management plan was discussed and agreed at MSRG.
Communications: A Medicine Supply Notification was shared via the NHS England communication pathways to reach all GPs, community pharmacists and the wider NHS, including secondary care.
The DHSC Medicines Supply Team also included information on this supply issue in its monthly supply report (now superseded by the Medicines Supply Tool) cascaded to primary and secondary care networks. NHS organisations were advised that, although enalapril 5mg tablets were out of stock, alternative strengths of this drug were available.Case study 5: Diazepam RecTubes 2.5mg rectal solution
Overview of supply issue
Diazepam RecTubes 2.5mg Rectal Solution is mainly used to treat status epilepticus and convulsions in children.
The sole supplier, Wockhardt, was unable to continue to supply this product due to ongoing manufacturing issues.
Risk assessment
The DHSC Medicines Supply Team risk assessed the potential impact and management options.
An important consideration was the off-label use of the product in emergency situations, including in ambulances, to treat seizures in children. Switching to alternative products needed careful management and appropriate training to be in place before the stock exhaustion date.
Management options
The DHSC Medicines Supply Team worked closely with Wockhardt to explore possible options to reduce any impact from this issue on UK patients. Through careful monitoring and the implementation of allocations, the availability of the remaining stock was extended for several months to allow sufficient time for patients and trusts, including ambulance trusts, to switch to therapeutic alternatives.
The MHRA, DHSC and Wockhardt also all worked together to explore solutions to the ongoing manufacturing issues, but unfortunately no viable option could be identified.
The DHSC Medicines Supply Team quickly engaged with clinical paediatric specialists to obtain advice on clinical alternatives, and with ambulance networks to understand if it would be appropriate for ambulance staff to use any of the suggested alternatives and what information they would need to support the transition from diazepam 2.5mg RecTubes. The team then ensured that the availability of the suggested alternatives was sufficient to support the additional demand for them as a result of this supply issue.
Given the nature and severity of the issue, national patient safety leads were alerted, and they provided input into management options and suggested communications.
As a Tier 3 supply issue, the management plan for this discontinuation was discussed and agreed at the MSRG.
Communications
Healthcare professionals in primary and secondary care, including GPs, and ambulance trusts were alerted to the discontinuation via the MHRA’s Central Alerting System in the form of a Supply Disruption Alert (these have now been superseded by NatPSAs where the criteria for issue are met). GPs contacted their patients and the DHSC Medicines Supply Team ensured that ambulance trusts were fully informed.
Case study 6: Sulfasalazine 500mg gastro-resistant tablets
Overview of supply issue
Sulfasalazine is used to treat ulcerative colitis, Crohn’s disease and rheumatoid arthritis.
There are 2 suppliers of sulfasalazine gastro-resistant tablets in the UK – Pfizer which supplies the branded product and a generic supplier. The generic supplier could fully support the market during this shortage.
During the shortage, the DHSC Medicines Supply Team and Pfizer collaborated to communicate and manage the situation effectively.
Risk assessment
The DHSC Medicines Supply Team risk assessed the potential impact and the management options.
Management options
The DHSC Medicines Supply Team worked with the generic supplier to determine if it could fully support the market for sulfasalazine (Salazopyrin EN-Tabs) 500mg gastro-resistant tablets. SPS MA confirmed that there were no known differences in the bioavailability of the branded and generic products and that they could be considered interchangeable.
As a Tier 2 shortage, the management plan was discussed and agreed at the MSRG, and an SSP that enabled community pharmacists to supply sulfasalazine 500mg tablets against the SSP for eligible patients was enacted and issued in November 2021. The SSP was used over 3,500 times during the shortage period.
Communications
A Medicine Supply Notification was shared via the NHS England communication pathways to reach all GPs, community pharmacists and the wider NHS, including secondary care.
The DHSC Medicines Supply Team also included information on this supply issue in its monthly supply report (now superseded by the Medicines Supply Tool) cascaded to primary and secondary care networks. NHS organisations were advised that, although the Pfizer brand of product was out of stock, they would be able to prescribe and dispense sulfasalazine 500mg gastro-resistant tablets from the generic supplier.
This document was produced by the Department of Health and Social Care (DHSC) and NHS England.
For more information about medicines supply issues, visit the Specialist Pharmacy Service website.
This document was produced by the Department of Health and Social Care (DHSC) and NHS England.
For more information about medicines supply issues, visit the Specialist Pharmacy Service website.
Appendix A: Clinical escalation categories overview
Tier 1
Likely to carry low patient safety risk. Management options should result in patients being maintained on the same licensed medicine.
Characteristics (not all characteristics need to apply when assigning a tier) | Decision-making authority | Potential communication pathways (to be used as appropriate and may only be issued to relevant parties) |
|---|---|---|
|
· Alternative manufacturers of the same medicine (formulation, strength) can cover demand and meet the full supply gap · No differences in licensed indications between the out-of-stock product and alternative suggested · No monitoring requirements related to switching · Clinical review not required · No requirement to amend prescriptions · Alternative strength / formulation of the same medicine is sufficient to meet the supply gap and no further clinical / management advice is required. A simple change of formulation or strength to meet the required dose may require more management advice in primary care (prescription amendments) but no additional support in secondary settings |
· DHSC Medicines Supply Team · NHS England Pharmacy and Supply Chain Team |
Not routinely communicated, however options could be: · online Medicines Supply Tool hosted on Specialist Pharmacy Service website · NHS England MPSC fortnightly branded and generics supply issues report that is distributed to all regional procurement pharmacist specialists and local procurement leads in secondary care in England · email to regional procurement pharmacist specialists · Devolved Governments informed as required |
Tier 2
Likely to carry moderate to high patient safety risk. Requires more intense management options than Tier 1 issues.
Characteristics (not all characteristics need to apply when assigning a tier) | Decision-making authority | Potential communication pathways (to be used as appropriate and may only be issued to relevant parties) |
|---|---|---|
|
· An alternative strength / formulation of the same medicine is sufficient to meet the supply gaps, but further clinical / management advice is required to help manage the switch · Therapeutic alternatives are available and there are moderate clinical and patient safety risks associated with switching. The following should be considered when assessing risk: o if the level of monitoring is more or less than standard of care o level of potential operational input from healthcare professionals o level of clinical risk as advised by expert clinicians · Unlicensed imports of the same medicine can be sourced in sufficient amount to meet expected demand · Issue can be rapidly addressed at source, for example likely to impact very few patients / organisations · Managing available supply via allocations / mutual aid · Use of an SSP might be appropriate |
· DHSC Medicines Supply Team · NHS England Pharmacy and Supply Chain Team · MSRG for issues that require more intense management, for example allocations, therapeutic alternatives |
· Communications issued via an MSN disseminated to primary and/or secondary care via various routes including the NHS England National Operations Centre and NHS mail · Tier 2 issues will also be reported on the online Medicines Supply Tool and MPSC fortnightly branded and generics supply issue report as for Tier 1 issues (England) · Independent organisations, Devolved Governments and others are included as required
|
Tier 3
Likely to carry high patient safety risk and or high operational burden that requires system-wide action.
Characteristics (not all characteristics need to apply when assigning a tier) | Decision-making authority | Potential communication pathways (to be used as appropriate and may only be issued to relevant parties) |
|---|---|---|
|
· Therapeutic alternatives are available but there are clinical risks associated with switching and monitoring is required · High operational burden · No or limited clinical alternatives available · Clinical expert team may be set up to provide specialist clinical advice to manage supply issue · The product is one for which the MHRA designates the patient should be maintained on the same brand, or switching between preparations is particularly difficult · It may be appropriate to request the consideration of exceptional regulatory measures by the MHRA (for example, the extension of product expiry dates) · The patient safety risk is increased because the group affected is likely to be considered a vulnerable population, such as neonates, children or people with a learning disability, or because of their disease, age, social circumstance or access to services |
· MSRG |
Escalated to MSRG for decision on whether to issue a: · NatPSA, where the criteria are met – that is, ‘more likely than not of one or more potentially avoidable death or disability in healthcare in England in a year as defined by National Patient Safety Alerting Committee’ – to the NHS via the MHRA Central Alerting System · Tier 3 MSN disseminated to primary and/or secondary care via various routes including the NHS England National Operations Centre and NHS mail to include, where MSRG agrees, use of the CAS mailing list with an email and a link to online Medicines Supply Tool Independent organisations, Devolved Governments and others are included as required |
Tier 4
Carries very high patient safety risk and requires system-wide action at a national level. This may include additional support from outside the health system.
Characteristics (not all characteristics need to apply when assigning a tier) | Decision-making authority | Potential communication pathways (to be used as appropriate and may only be issued to relevant parties) |
|---|---|---|
|
· Likely to have a life-threatening impact on patients · A supply gap remains (and no viable therapeutic alternatives exist) following the exhaustion of supply and clinical management plans at previous tiers of escalation · Its management may require the support of agencies outside the health system (for example, Department for Transport, police services) · Clinical expert team may be set up to provide specialist clinical advice to manage supply issue |
Escalated to EPRR team by MSRG Chair: · EPRR triggered – including priority links with other agencies outside the health system, and additional project management or communications support · Clear links and command and control mechanism established between the MSRG, NHS England EPRR (when involved), DHSC and devolved governments · Senior responsible officer (SRO) assigned |
· Communications issued via a NatPSA, where the criteria are met, to the NHS via the MHRA CAS · Additional supportive and targeted communications may be required as signed off by the SRO · Independent organisations, Devolved Governments and others are included as required |
Appendix B: Regional and local process for reviewing and escalating supply issues that impact homecare
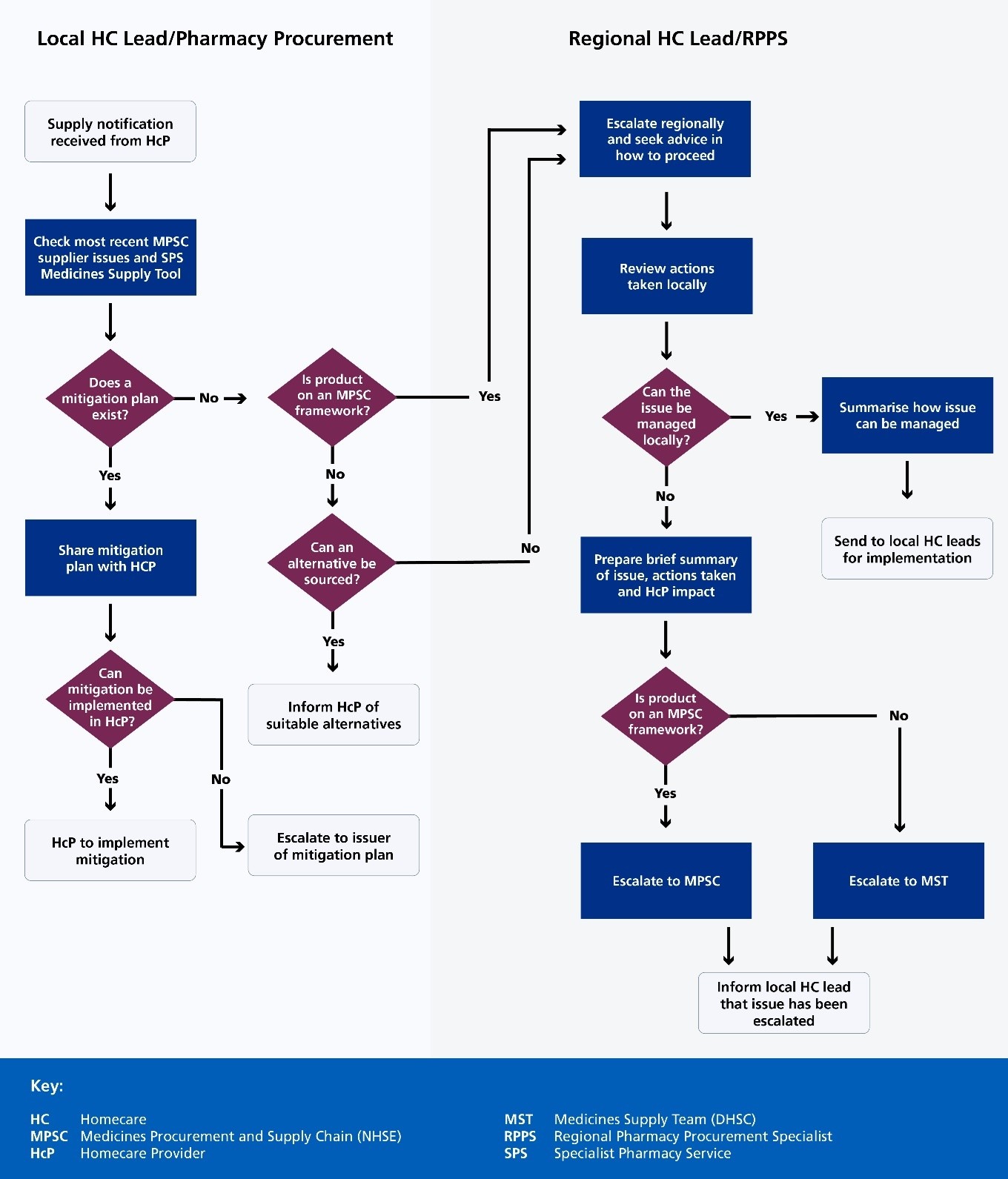
Accessible Flowchart Description: Homecare Medicines Procurement Process
This flowchart illustrates the step-by-step process for managing supply notifications and procurement challenges in homecare medicine supply.
The process is divided into two parallel workflows:
- Local Homecare (HC) Lead/Pharmacy Procurement
- Regional HC Lead/Regional Pharmacy Procurement Specialist (RPPS)
Key decision points:
- initial supply notification triggers a review of medicine supply issues
- multiple decision points assess:
- existing mitigation plans
- product availability on procurement frameworks
- alternative sourcing options
- local management capabilities
Escalation paths:
- local issues can be escalated regionally
- unresolvable local issues are referred to Medicines Supply Team (MST) or Medicines Procurement and Supply Chain (MPSC)
Each step involves careful evaluation, communication, and strategic problem-solving to ensure continuous medicine supply for homecare providers.
Appendix C: Regional process for implementing national shortage mitigations that impact homecare
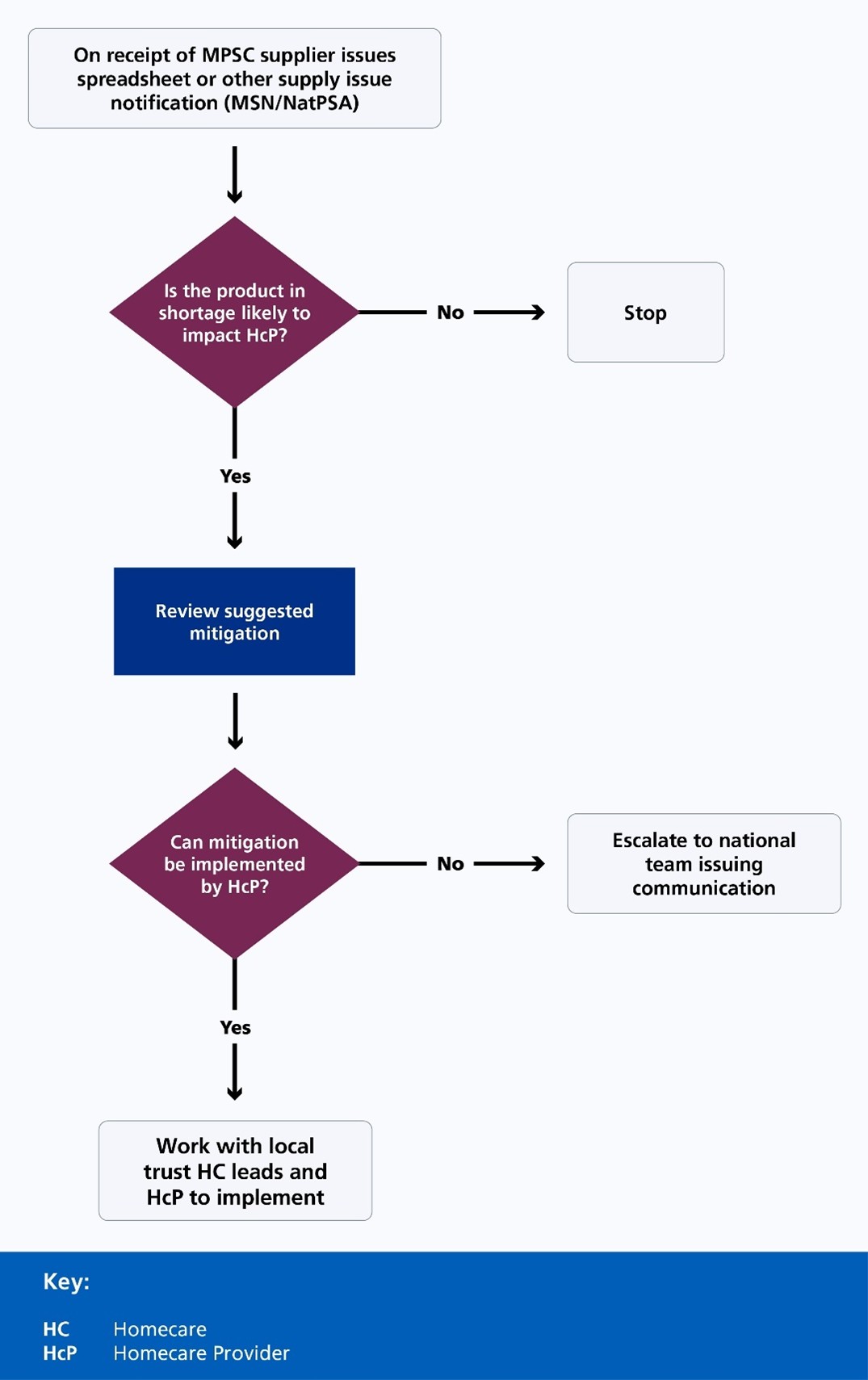
Accessible Flowchart Description: Medicine Supply Issue Management Process
This flowchart details a systematic approach to managing potential medicine supply disruptions for Homecare Providers (HCp). The process begins with receiving a supplier issues notification and follows a structured decision-making pathway.
Key steps:
- initial assessment determines potential impact on Homecare Providers
- if no significant impact is identified, the process halts
- potential shortages trigger a review of mitigation strategies
- critical decision point evaluates local implementation capabilities
- unresolvable issues are escalated to the national team
Decision framework:
- assess supply issue likelihood
- review mitigation options
- determine local implementation feasibility
- escalate if necessary
Appendix D: Checklist for use by NHS trust pharmacy procurement teams
Download a copy of the checklist
Verification of secondary care medicines shortage
|
Section 1: Product details | ||||
|
Product: |
Date: | |||
|
Strength: |
Pack size | |||
|
Form: | ||||
|
Section 2: NHS England Medicines Procurement and Supply Chain (MPSC) oversees and supports management of medicines shortages on NHS England MPSC frameworks where the product is predominantly used in secondary care. The NHS England MPSC supplier issues spreadsheet – distributed fortnightly and managed by the NHS England MPSC. It lists all the supply issues on MPSC branded and generics frameworks that the MPSC is aware of, and should be the first reference point for all supply issues. | ||||
|
Does the product have an NHS England MPSC code – National Product Code (NPC) number? |
y/n | |||
|
If yes, check the fortnightly supply issues spreadsheet for mitigation plan and details of alternative supplier. If no, go to section 3. |
y/n | |||
|
Is the product actively managed by the NHS England MPSC – that is, it appears on the fortnightly supply issues spreadsheet? |
y/n | |||
|
If yes, can the advice be followed? If yes, implement the mitigation plan ensuring all who may be impacted are included (see section 6). If no, go to section 3. |
y/n | |||
|
Section 3: DHSC and NHS England Medicines Supply Tool on the SPS website DHSC Medicines Supply Team and the NHS England MPSC work closely together to do everything possible to support management and mitigation of shortages of medicines whether procured through NHS England MPSC-managed frameworks by acute and mental health providers or sourced by community pharmacists for dispensing to patients in the community. | ||||
|
Is the product on the Medicines Supply Tool for mitigation plan and details of alternative supplier? If no, go to section 4. |
y/n | |||
|
If yes, can the advice be followed? If yes, implement the mitigation plan ensuring all who may be impacted are included (see section 6). If no, go to section 4. |
y/n | |||
|
Section 4: Collaborative purchasing organisations, for example East of England, North of England, London Procurement Partnership | ||||
|
Is the product on contract with a purchasing organisation? If no, go to section 5. |
y/n | |||
|
Has the purchasing organisation contacted trusts regarding the shortage with a mitigation plan and details of alternative supplier? |
y/n | |||
|
Can the advice be followed? If yes, implement the mitigation plan ensuring all who may be impacted are included (see section 6). If no, go to section 5. |
y/n | |||
|
Section 5: Medicine NOT reported on the NHS England MPSC fortnightly update or Medicines Supply Tool and no information available from purchasing hub. Check wholesalers and distributors for stock | ||||
|
☐ |
AAH |
☐ |
Alloga | |
|
☐ |
Alliance |
☐ |
Movianto | |
|
☐ |
Mawdsleys |
☐ |
TPS | |
|
☐ |
Phoenix |
☐ |
Other distributors | |
|
☐ |
Other |
☐ |
Manufacturer | |
|
Consider alternative | ||||
|
☐ |
Pack |
☐ |
Review where stock is stored – can ward stock be used? | |
|
☐ |
Strength |
☐ |
Consider risk assessment for alternative (ensure appropriate clinical pharmacists and SPS medicines information are included in this assessment) | |
|
☐ |
Has the dm+d website been reviewed for possible alternatives? |
☐ |
If the product is a special, has NHS Profile been reviewed for possible alternatives? | |
|
Have you been able to resolve / mitigate the issue locally? | ||||
|
Y |
Go to section 6 |
N |
Escalate to regional pharmacy procurement specialist | |
|
If you have been able to resolve the issue locally but it is likely to persist for longer than 2 weeks, you should escalate to your regional pharmacy procurement specialist. This will ensure the national teams are made aware and risk assess the shortage. | ||||
|
Section 6: Third-party and outsourced suppliers, for example homecare, commercial compounders, outsourced outpatient dispensing Organisations should always be mindful of the impact medicines shortages may have on partner / outsourced providers and how this may impact on patients. When a trust becomes aware of a shortage that may impact on these providers and patients, they must take steps to ensure the third-party suppliers are included in any mitigation plans as appropriate or the issue appropriately escalated to the regional team. | ||||
|
Is the product in short supply likely to be used/dispensed by any of your third-party suppliers? |
y/n | |||
|
If yes, have you notified the supplier(s) of the shortage and informed them of the local mitigation plan and directed them to the Medicines Supply Tool and/or the MSN or NatPSA for further detail on any national mitigation plans? |
y/n | |||
|
If yes, can the organisation impacted follow the mitigation plan and ensure continuity of supply to patients? |
y/n | |||
|
If no, and patient impact is likely (failed deliveries / interruption to service), has the issue been escalated to the regional pharmacy procurement specialist and regional homecare lead, for issues impacting homecare? If no, please escalate urgently. |
y/n | |||

