Classification: Official
Publication reference: PR1627
A strategy for embedding genomics in the NHS over the next 5 years.
Foreword
The last two years have been the most challenging in the history of the NHS. The impact of the COVID-19 pandemic on services, staff and patients are still being felt across the country.
The immediate focus of the NHS is on tackling the inevitable COVID backlogs and meeting new care demands, within the budget set by government and Parliament.
As the NHS does this, it will need to think differently about the way healthcare is delivered.
The solutions are unlikely to be found in the usual places and ambition is required to think about the future as well as the immediate short-term, to continue to deliver a sustainable model of healthcare for patients in England. Genomics will be at the heart of this future and the next generation of healthcare in the NHS.
The investment made by the UK government and the NHS in genomics over the last decade, including through the ground breaking 100,000 Genomes Project delivered by the NHS and Genomics England alongside the existing genomics expertise within the NHS, laid the foundations for the use of genomics in routine clinical care.
That work is now being taken forward through the NHS Genomic Medicine Service (GMS) – a world leading genomic healthcare service, delivering cutting-edge benefits for patients in the NHS.
As a national integrated healthcare system, the NHS is in a unique position to continue to lead the world in implementing genomic medicine, operating as a nationally coordinated, locally delivered network.
Over the next five years, the NHS will push the boundaries to improve care and treatment options for our patients, developing shared clinical and access standards, data platforms and governance, and an interoperable informatics infrastructure.
As we bring the benefits of genomics to patients and our population, we need a comprehensive and ambitious national approach covering prevention, diagnosis and targeted treatments that enables patients, families and carers to participate in shared decision making.
This strategy sets out four priority areas to this approach:
- Embedding genomics across the NHS, through a world leading innovative service model from primary and community care through to specialist and tertiary care.
- Delivering equitable genomic testing for improved outcomes in cancer, rare, inherited and common diseases and in enabling precision medicine and reducing adverse drug reactions.
- Enabling genomics to be at the forefront of the data and digital revolution, ensuring genomic data can be interpreted and informed by other diagnostic and clinical data.
- Evolving the service through cutting-edge science, research and innovation to ensure that patients can benefit from rapid implementation of advances.
This first ever NHS genomics strategy signals the next big step in healthcare in the NHS and the journey to realise the potential of genomics for our patients, our communities and the population we serve.
Amanda Pritchard
NHS Chief Executive
Professor Dame Sue Hill DBE FMedSci FRSB FRCP (Hon) FRCPath (Hon)
Chief Scientific Officer for England and Senior Responsible Officer for Genomics in the NHS
Executive summary
Genomics is the study of a person’s DNA, their genes and how they are expressed and interact to influence the growth, development and the working of the body. Genomic medicine has the potential to offer a greater understanding of how our genetic makeup impacts on our health and to change the way disease is managed and treated.
The NHS has a long history of genomics dating back to the first genetic laboratory services in the 1960s. It is ten years since the ground-breaking 100,000 Genomes Project was announced to sequence 100,000 whole genomes of patients in the NHS to support clinical care and to drive research. Building on this, in 2018 the launch of the NHS GMS created a step change in the use of genomics in the NHS.
Genomics cuts across many clinical specialties and services and so requires a whole system approach to bring the benefits of genomics to patients. Our ambition is that over the next five years, we will accelerate the use of genomic medicine across the NHS, providing a world leading, equitable service to populations and individuals. This will be through a focus on four priority areas.
Embedding genomics in the NHS, through a world leading, innovative service model.
The NHS has developed a world-leading innovative genomics medicine service model. To ensure systematic implementation, efficiency and value for money, as well as the best possible outcomes for patients and the population, this service model cannot stand still. It must evolve from its solid foundations to a service that is embedded across the NHS care continuum, including primary and community care, with education and training at all levels to arm the workforce with up-to-date knowledge of genomics in their field. The NHS will focus on:
- Co-creating services, infrastructure and an operating model with patients and the public.
- Developing a sustainable infrastructure across testing, clinical services and research and innovation.
- Building greater clinical and professional leadership and developing the capacity and capability of the workforce.
- Developing national and international collaborations and partnerships.
Delivering equitable genomic testing for improved prediction, prevention, diagnosis and precision medicine.
Genomic medicine can be used in population health to predict when individuals are at a high risk of developing certain conditions, such as cancer. It can identify individuals carrying certain genes and/or variants that are inherited and run in families. With the appropriate support in place, genomic medicine can empower individuals and family members to access interventions to either prevent the development of conditions, treat the condition, or manage their individual risk to prevent more serious health impacts. It can also provide an earlier and more precise diagnosis which for individuals with rare disease can end years of uncertainty, often termed the ‘diagnostic odyssey’, allowing access to care pathways and support services. In both cancer and rare disease, a genomically-informed diagnosis can provide access to precision medicines early in the patient journey or enable entry into clinical trials. To deliver these benefits for patients, the NHS will focus on:
- Systematically introducing new clinical indications for genomic testing and embedding comprehensive genomic testing within end-to-end clinical pathways.
- Driving the use of precision treatments and optimising the use of medicines through genomics.
- Enabling the rapid evaluation and adoption of affordable, efficient, and innovative genomic technologies.
Enabling genomics to be at the forefront of the data and digital revolution.
The Department of Health and Social Care data strategy, Data saves lives: reshaping health and social care with data outlines how the future of the NHS depends on improving how we use data. This was also outlined in the Office for Life Sciences strategy Genome UK: the future of healthcare. Genomics has an important part to play and the NHS will support this in three key areas:
- Developing an interoperable informatic and data infrastructure that enables the NHS to use and share genomic data appropriately to improve patient care.
- Putting the NHS at the forefront of using genomic data alongside other health data to drive health improvements for individuals and populations.
- Enabling the NHS to use cutting-edge analytical tools and up to date variant databases to maximise diagnosis, access to precision medicine and efficiency.
Evolving the service through cutting-edge science, research and innovation.
To improve the health of future generations and those continuing to access NHS GMS services, it is critical to ensure that genomic research enables scientific progress in diagnostic discovery, translational research and the development of new precision treatments, in partnership with the UK and global Life Sciences industry. To do this, the NHS will support:
- Enabling patients to make informed choices regarding the use of their data for research and innovation.
- Enriching existing and developing new NHS GMS relationships to support innovation and the generation of evidence for adoption and improvements in health and care.
- Ensuring ongoing alignment with clinical trials and national life sciences projects and supporting the growth of life sciences in the UK.
This strategy sets out how more people will be empowered to take preventative action following risk-based predictions, receive life-changing diagnoses and get the support needed to live with genomically-informed diagnoses alongside improved access to cutting-edge precision treatments. It also outlines how the NHS will accelerate future high-quality genomic innovation that can be adopted and spread across the country, leading to positive impacts for current and future generations. Delivering this will require support from a range of partners, and this strategy sets out in more detail how we can work together to deliver the vision and ambition for genomics in the NHS and the patients we serve.
Introduction
Our vision is that the power of genomics in predicting, preventing and diagnosing disease, and targeting treatment is accessible to all as part of routine care in the NHS.
Our ambition over the next five years is to accelerate embedding the use of genomic medicine across the NHS, providing a world leading, equitable service to populations and individuals.
This document explains what genomics is and why we are at a pivotal moment in the development of the genomics service in the NHS in England. It sets out priorities for delivering on the NHS vision and ambition over the next 5 years and how the system will need to work together to improve outcomes for patients and support the continuing scientific and technological advances and discovery in genomics.
Over the course of 2022, NHS England has worked closely with a wide range of stakeholders to gather views, shape priorities and ensure a collective buy-in to the vision for genomics in the NHS over the next 5 years. This has been developed through a range of engagement workshops, a series of tailored engagement with key opinion leaders and a prioritisation survey, which was completed by over 220 organisations and individuals.
The result of this engagement, in particular the areas that were prioritised through the survey, describe how the NHS, with support from NHS England and other partners, will deliver this vision across four priority areas, which are explored in subsequent chapters of this document:
- Embedding genomics in the NHS, through a world leading, innovative service model.
- Delivering equitable genomic testing for improved prediction, prevention, diagnosis and precision medicine.
- Enabling genomics to be at the forefront of the data and digital revolution.
- Evolving the service through cutting-edge science, research and innovation.
What is genomics?
Genomics is the study of a person’s DNA, their genes and how they are expressed and interact to influence the growth, development and the working of the body. Genomic medicine has the potential to offer a greater understanding of how our genetic makeup impacts on our health and the response to treatments.
An individual’s complete set of DNA is called the genome. Virtually every cell in the body contains a complete copy of the approximately 3 billion base pairs or letters that make up the human genome. If the DNA in a cell is mutated it can disrupt the usual processes of the body and lead to a disease, such as cancer.
Genetics relates to the study of inheritance, the genes underlying it and the interplay of genes, variation in DNA and interactions with environmental factors. From the Human Genome Project, it is estimated that the human genome contains somewhere between 20,000 and 25,000 genes. There are other areas of the genome – the DNA letters or ‘bases’ – that are not associated with genes that we now know could be important in human health and disease. Genomics therefore seeks to identify any changes in the human genome that may lead to ‘natural variation’ between individuals, for example differences in eye colour, and those that may be associated with a function that is important for human health, for example, the way in which proteins are made.
There are now a variety of technologies available that allow us to ‘sequence’ the whole human genome or look at parts of it. They are being applied in the diagnosis and treatment of rare, inherited and some common diseases and in cancer tissues, blood and other sample types, such as amniotic fluid.
These technologies look for mutations or variants within the genome which explain why some people have rare and inherited diseases and some cancers. In some cases, there may be a single gene mutation, which causes diseases such as cystic fibrosis and sickle cell anaemia. More commonly, disease results from a complex combination of genetic and environmental factors as is the case for cancers, dementia and cardiovascular diseases.
Genomic medicine is an emerging clinical discipline that involves using genomic information about an individual as part of their clinical care. For example, for diagnosis, making decisions about treatments or interventions or stratifying medicines to tackle different genomic mutations. It has the potential to change clinical care through focusing on the underlying cause of disease rather focusing on the organ in which symptoms might occur.
Benefits of genomic medicine
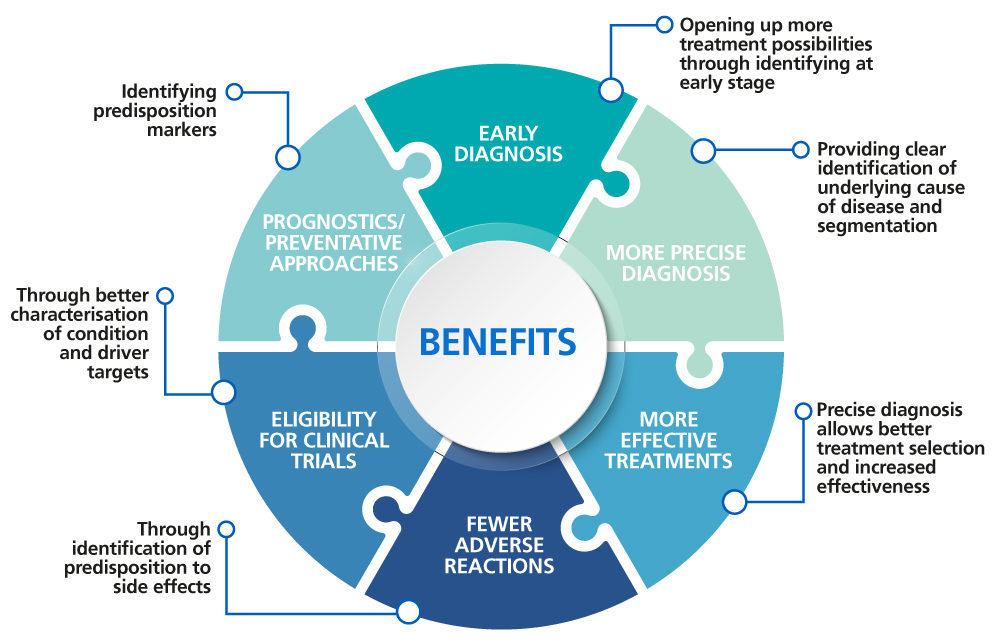
Genomics in the NHS
To realise the potential of genomics in healthcare, in 2018 NHS England established the NHS GMS, which created a step change in the use of genomics in clinical practice in the NHS. The NHS GMS built upon the existing NHS infrastructure and used learnings from the 100,000 Genomes Project. Importantly, it signalled a clear direction for the way in which cancer genomic testing would be funded and provided.
In the NHS Long Term Plan in 2019, NHS England set out that through the NHS GMS, the NHS would use whole genome sequencing as part of routine care, including for seriously ill children who are likely to have a rare genetic disorder, children with cancer, and adults suffering from certain rare conditions or specific cancers. It also committed to extending the use of molecular diagnostics and routinely offering genomic testing to all people with cancer for whom it would be of clinical benefit; expanding access to genetic testing for familial hypercholesterolaemia; and delivering commitments around research and innovation.
The NHS GMS provides consistent and equitable genomically-informed care and treatment for England’s 55 million population with a:
- Consolidated national genomic laboratory network made up of seven NHS genomic laboratory hubs (GLHs) working together with standardisation and quality at the core and driving rapid adoption of technology and covering all types of testing inclusive of cancer genomics.
- Seven NHS GMS alliances working together to support the clinical leadership and embedding of genomic medicine in end-to-end pathways more broadly and the working with other key clinical specialties and structures for example cancer alliances.
- Single mandated National Genomic Test Directory covering use of all technologies from single gene to whole genome sequencing inclusive of cancer and of genomic targets that enable enrolment into clinical trials.
- Clinical genomic services that diagnose and manage complex rare and inherited disease and the impact on both individuals and family members and provide genomic counselling and specialist advice for other specialities.
- National genomic knowledge base to inform academic and industry research and discovery including design and development of clinical trials.
- A partnership with Genomics England in support of delivery of the whole genome sequencing service and ongoing key research initiatives.
- National genomics unit in NHS England that provides strategic oversight, direction, commissioning and funding as well as performance monitoring.
The NHS Genomic Medicine Service infrastructure
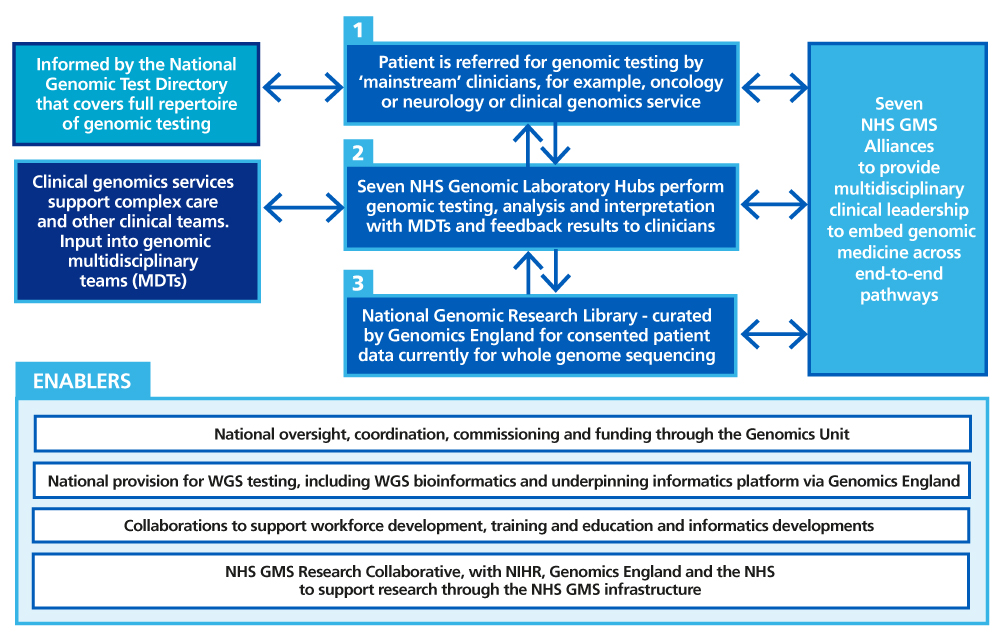
Each NHS genomic laboratory hub, NHS GMS alliance and clinical genomics service is responsible for providing equitable and high-quality services across a defined geography. More information on each element of the infrastructure can be found in Appendix 1. In each geography, there are governance and partnership arrangements, an example of which is shown in Appendix 1.
The NHS Genomic Medicine Service: Geographies
| Geography | Patient population | NHS trusts | NHS ICSs |
| North East and Yorkshire | 8 million | 34 NHS trust | 4 ICSs |
| North West | 7 million | 34 NHS trusts | 4 ICSs |
| Central and South | 10 million | 45 NHS trusts | 14 ICSs |
| East | 8 million | 32 NHS trusts | 13 ICSs |
| North Thames | 7 million | 36 NHS trusts | 11 ICSs |
| South East | 8 million | 29 NHS trusts | 8 ICSs |
| South West | 4 million | 18 NHS trusts | 7 ICSs |
Why now?
A pivotal moment for genomics in the NHS
After decades of investment, ground-breaking discoveries and world firsts, this is a pivotal moment for genomics in the NHS. Technological, clinical and scientific developments alongside increased affordability and value for money have led to an acceleration in the opportunities to improve patient and population health using genomic medicine.
Advances in technology
Technological advances combined with increased analytical capabilities mean that for the first time, the NHS can sequence all 3 billion letters of the human genome, known as whole genome sequencing and is the first clinical service in the world to systematically offer whole genome sequencing as part of routine care for patients who would receive the greatest clinical benefits. This includes seriously ill children who are likely to have a rare genetic disorder, all children with cancer, and adults suffering from certain conditions or specific cancers. Over 35,000 whole genomes have already been sequenced since this service launched with referrals increasing month on month.
The NHS has adopted technologies that can sequence all of the genes that are important for human health, including the national rapid whole exome service for acutely unwell children in neonatal or paediatric intensive care units (NICU/PICU). Since the service launched in 2019, it has provided or confirmed a diagnoses for around 40% of children tested leading to changes in their management and care, who otherwise might not have received a confirmatory diagnosis and spent weeks or months in intensive care. In 2022, the NHS is converting this exome service into a rapid whole genome service to provide even greater diagnostic power.
A world-leading national fetal exome sequencing service has provided or confirmed a diagnosis for around 40% of people tested. The service’s rapid turnaround time means that it can urgently inform the clinical management of a pregnancy, and through non-invasive pre-natal testing (NIPT), it offers families a more informed choice about decisions they may want to take or their clinical care.
Cutting-edge technologies that sequence a large number of genes associated with disease, known as next generation sequencing panel testing, is now available in the NHS for all solid cancers and blood cancers. When combined with additional diagnostic testing for cancer such as imaging and pathology, this comprehensive genomic testing information can provide greater insights into the clinical management of cancer. The ability to use these technologies in the NHS at scale is now possible through an increase in analytical capabilities, providing the ability to handle, store, interpret and make decisions for clinical actionability on the large amounts of genomic data generated. However, further improvements in informatics and interoperability are required to link large and complex genomic datasets with other diagnostic and clinical datasets to support clinical teams in using this wealth of information.
Expanding clinical opportunities
Across prevention, diagnosis and treatment, the clinical applications of genomic medicine are expanding. This includes for an increasing number of conditions across cancer, rare and inherited disease and common diseases. In population health, genomics can be used to predict when individuals are at a high risk of developing certain conditions and enable earlier access to preventative intervention and treatments.
Impact of genomics on clinical services
What clinical services does genomics support?
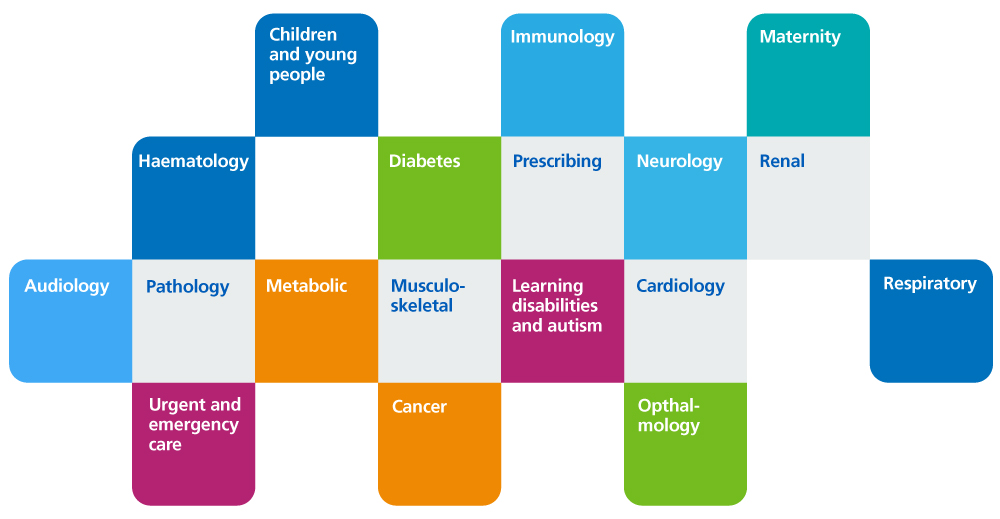
The provision of extensive genomic sequencing technologies is enabling the discovery of the underlying causes of disease, allowing more patients to receive more precise diagnoses and leading to the development of new precision treatments. The use of genomic medicine in the NHS has enabled patients to rapidly access over 12 newly licensed precision medicines, including histology independent medicines in cancer and life-changing medicines for patients with rare diseases.
Overview of precision medicine
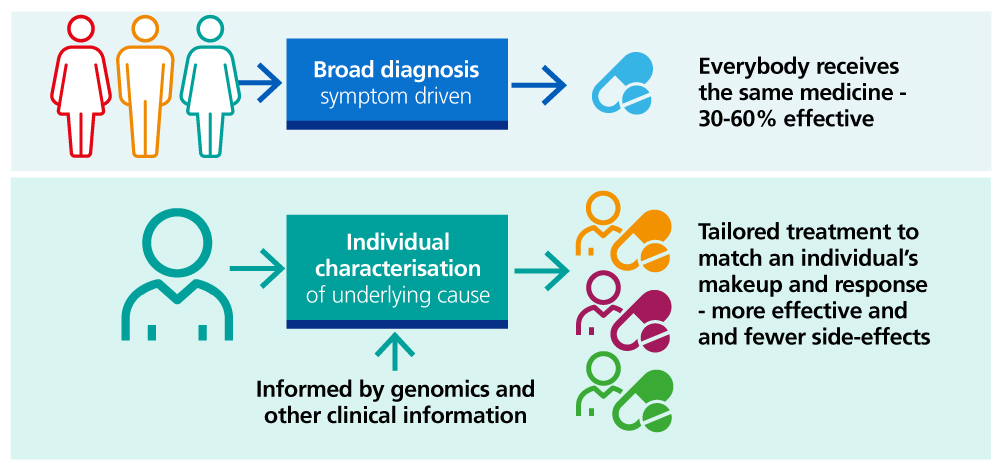
The application of genomics to support precision medicine will create the opportunity to find new purposes for, and better use of, existing medicines and help to use other non-pharmacological treatments, which for some patients may be simple dietary or lifestyle intervention. This will not only improve outcomes for patients but also help to maximise the value from the £17 billion that the NHS currently spends on medicines each year.
Alignment with research and development
As demonstrated throughout the history of the NHS and most recently during the COVID pandemic, research and innovation is critical for improving patient care. The creation and storage of a whole genome sequence for individuals accessing the NHS GMS drives diagnostic discovery by enabling an ongoing evaluation of potentially important genomic mutations for diagnosis, monitoring of disease or for precision treatments based on new and emerging evidence.
The alignment of routine care with research and innovation and with links between clinical and academic researchers is a key strength of the NHS GMS. Working together with Genomics England, over 1,000 new genomic findings have been identified from ongoing research and evaluation of the data that was collected through the 100,000 Genomes Project and of most importance these have been fed back into the NHS to inform clinical care. Furthermore, the NHS GMS infrastructure currently supports over 900 genomic research projects and this number is growing.
Genomic medicine is the most rapidly developing field in medicine. Genomics is continuously discovering new connections between disease, precision treatments and the genome. While studies are also realising the limitations of genomic research given the rare nature of some conditions or genomic variants and so the smaller numbers of eligible patients available in a single NHS trust or even in the whole population of England for studies. It is therefore vital that the NHS GMS works as an integrated system across its different providers and services, and with academia and industry to maximise the opportunities for research and discovery for patient benefit.
Improved affordability
As technology has improved to be able to deliver a higher volume of testing, the cost of genomic testing, storage and interpretation of results has become increasingly more affordable. As affordability continues to improve, this will mean more people are able to access testing and the NHS can continue to deliver better value for money.
The type of testing that can be done is also changing with some genomic testing for cancer being able to be done using a blood sample, known as a liquid biopsy, rather than on obtaining cancer tissue which is more invasive for the patient and costly for the NHS. The availability of liquid biopsies for the detection of fragments of circulating tumour DNA in the blood, mean that in some cases additional biopsies might not be needed to analyse a tumour and/or enabling a tumour to be detected much earlier.
There has also been a continued increase in direct-to-consumer (DTC) genomic testing, where some individuals pay for their own genomic testing via commercial companies. This is creating new understanding and therefore demand for genomic services in the NHS.
Seizing the moment
Through the NHS GMS and the advances it has made to date, the NHS is a world leader in the provision of genomic medicine. However, there are still greater opportunities and challenges that need to be addressed to maximise the full potential benefits that genomics can bring to individuals and populations:
- As described in the previous section, technological and clinical advances in genomics continue at a rapid and exponential pace. This pace presents a challenge for the NHS in terms of implementation and ensuring cost effectiveness.
- Informatics, bioinformatic and decision support developments will continue to evolve, expanding the national genomic knowledge base, to provide ongoing clinical and research insights and to provide more comprehensive and detailed analysis and interpretation of the genome more quickly, with opportunities to apply artificial intelligence (AI) to interrogate genomic and other data. The NHS data infrastructure and expertise will need to be able to adapt to and accommodate these advances.
- In population health, genomics could be used to predict when individuals are at a high risk of developing certain conditions, for example through the use of polygenic risk scores. It could enable earlier access to preventative intervention, monitoring and treatments. However, this comes at a cost and will need to be carefully considered to ensure value for money.
- Patient and public involvement is a key element of the design and delivery of the NHS GMS, but trust must continually be earnt and maintained. Reducing regional variation and improving turnaround times so that clinicians and patients receive results in a clinically relevant timeframe is essential.
This strategy sets out a plan for addressing these challenges and realising our vision for the power of genomics in predicting, preventing and diagnosing disease, and targeting treatment to be accessible to all as part of routine care in the NHS.
The following chapters describe each of our four priorities and the actions that we will take forward under each to realise these benefits for patients, the health service and the wider population it serves.
1. Embedding genomics in the NHS through a world leading, innovative service model
Our priority actions
1.1 Co-creating services, infrastructure and an operating model with patients and the public.
1.2 Developing a sustainable infrastructure across testing, clinical services and research and innovation.
1.3 Building greater clinical and professional leadership and developing the capacity and capability of the workforce.
1.4 Developing national and international collaborations and partnerships.
Why this matters
The evidence to support the use of genomics in new areas of medicine or as part of existing pathways is continually emerging. At the same time, new technologies and approaches to genomic testing, sequencing and bioinformatic analysis are becoming available which are expanding how we can use genomics and how it can be embedded within pathways of care.
The NHS GMS must evolve from the solid foundations developed in recent years, which has positioned the NHS as world leading in terms of what has already been achieved in genomics, to a service that is embedded across the NHS and throughout pathways of care, including in primary and community care. This will require bold clinical and organisational leadership and a multi-professional workforce empowered to use genomics where clinically appropriate to improve patient experience and outcomes.
Priority 1.1 Co-creating services, infrastructure and an operating model with patients and the public
Progress so far
The major development in genomics in the NHS to date has been the development of an integrated service model. This brings together cutting-edge genomic testing, clinical genetics and other services, as well as research and innovation, to deliver the benefits of genomics to NHS patients.
This service model has been developed with patient and public input throughout, including at a regional and national level. Nationally, the NHS GMS People and Communities Forum provides advice to NHS England on the policy and strategy that underpins the NHS GMS, responds to consultations on changes to services and feeds into all the governance structures across the NHS Genomics Programme. At a regional level, each NHS GMS Alliance ensures that patient and public involvement is included within their regional governance structure.
Taking this further
To ensure that the NHS GMS continues to deliver for patients, the proactive involvement of patients and the public from our diverse communities will continue to be a priority. Building on a series of successful events and sharing of powerful patient stories that highlight the benefits of genomics, the NHS will continue to work at a national and regional level to raise awareness of genomics, including the benefits and challenges, and support ongoing and transparent public dialogue on issues relating to genomics.
To understand the impact of genomics on patients, their families and carers an NHS GMS ethics advisory board will be established to consider the introduction of new technologies, return of results, data protection and genomic research, among other areas.
Priority 1.2 Developing a sustainable infrastructure across testing, clinical services and research and innovation
Progress so far
Since the establishment of the NHS GMS in 2018, there has been a considerable focus on developing a sustainable infrastructure. The foundations of the NHS GMS infrastructure are now well established as outlined in the introduction and in Appendix 1. This includes national support and funding for seven NHS genomic laboratory hubs, working as a network including with their local genomic laboratories; a clinical genomics service across the country to provide specialist patient care and to translate genomic findings into clinical actionability; NHS GMS alliances to support partnerships across providers and embedding genomics into clinical pathways; and the partnership with Genomics England to provide national support and infrastructure for whole genome sequencing. Coupled with this are ongoing informatics developments in the NHS GMS that are aligned to the rapid digital transformation underway in the NHS.
Work has been ongoing to develop an updated Genomics clinical service specification which will see closer working of these services with the NHS GMS infrastructure and a greater role for these services in providing their clinical expertise to support the embedding of genomics across NHS services. It is anticipated that following public consultation this service specification will be implemented from April 2023. This will include defined metrics and quality measures.
Taking this further
Over the next 1 – 3 years, the NHS will expand and maximise the use of the NHS diagnostic infrastructure for genomic testing. For example, exploring the role that community diagnostics centres could play alongside the NHS GMS across a number of areas, including the collection of samples from family members for inherited disease genomic testing. As part of this the NHS will also continue to expand the genomic offer into other clinical specialities across the testing spectrum from prevention to treatment.
From financial year 2023/24 the NHS genomic laboratory hubs, NHS GMS alliances and clinical genomics services will establish integrated NHS GMS governance boards with appropriate partnerships and leadership to drive forward the embedding of genomics into the wider NHS. This will support the NHS genomic laboratory hubs, NHS GMS alliances and Clinical Genomics Services to work together with other partners including cancer alliances and other clinical networks to deliver genomics in their geography.
Key to strengthening these local relationships will be aligning the NHS GMS with integrated care systems (ICS). The genomic testing and clinical genomics services are still going through large scale transformation programmes. Work is ongoing to introduce patient level contract monitoring in the NHS genomic laboratory hubs to capture activity, technology and access information which will inform the development and embedding of a sustainable commissioning model. This will also enable the publication of performance data in line with other diagnostic and NHS services.
Once work on the commissioning model is complete, NHS England will consider further delegation to ICSs in the context of its impact on the delivery of genomic services for patients.
Priority 1.3 Building greater clinical and professional leadership and developing the capacity and capability of the workforce
Progress so far
Over 2,000 NHS staff are part of the NHS GMS infrastructure. Clinical and professional leadership is a key feature of the NHS GMS building upon the transformation required to deliver the NHS contribution to the 100,000 Genomes project. To date NHS England has funded over 200 posts in the NHS GMS alliances to support multi-professional clinical leadership, with a further 125 scientific and clinical leadership posts, including in pathology, within the NHS genomic laboratory hubs.
This workforce has been responsible for delivering some of the early successes of the NHS GMS and the move to genomics being embedded across the NHS workforce. For example, engaging different professional groups in the potential of or delivery of key components of genomic services, including nurses, midwives, pharmacists, pathology, and general practitioners (GPs) which have led to specific education and training developments in genomics for this broader multi-professional workforce.
The Health Education England Genomics Education Programme (GEP) was established over a decade ago to develop genomic education and training resources to support the upskilling and development of the multi-professional workforce in genomic advances.
Our approach to developing the workforce
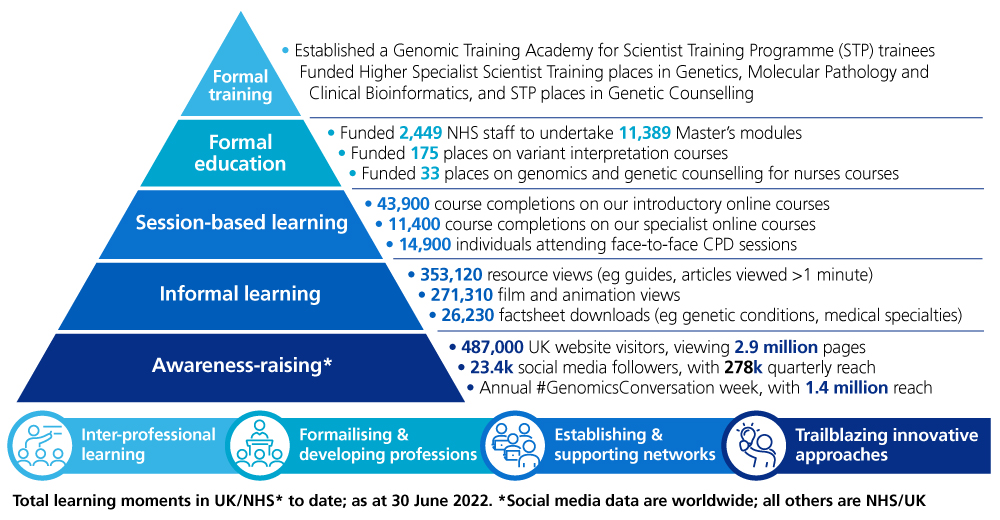
There are a significant number of formal and informal education and training resources available to support healthcare professionals, as the diagram above shows, which will need to continue to be developed to keep pace as new genomic advances are introduced into the NHS.
Case study
GeNotes
GeNotes – genomic notes for clinicians – is a ‘just in time’ educational resource for healthcare professionals working in the NHS.
Launched in a beta phase in 2022, GeNotes provides educational information at the point of need, with opportunities for extended learning.
Initially focusing on oncology, new specialties will be added throughout 2022 and beyond and this will become a vital resource for healthcare professionals as genomics is embedded in the NHS.
Source: Health Education England Genomics Education Programme (2022)
Taking this further
The pace of change and expansion in the scope of genomic medicine means that there is a constant need for capacity building as well as recruitment and retention initiatives in the specialist workforce that supports the NHS GMS, specifically genomic clinical scientists, bioinformaticians and in the medical speciality of clinical genetics and genomic counselling. As well as in those specialities that the genomics services rely on such as pathology for the rapid supply of cancer tissue for genomic analysis. The spending review allocation for the diagnostic workforce provided additional resources for both genomics and pathology services to build capacity and capability and to support training more staff and upskilling others.
Empowering the wider workforce in the NHS to harness the power of genomics is one of the biggest challenges but also biggest areas of potential for the NHS GMS. Not all healthcare professionals need to become genomic experts, but the workforce will need sufficient, up-to-date knowledge of genomics in their field; and the skills and confidence to discern when testing may be relevant for their patients. This includes, how to request it; and how to have conversations with patients, their families and carers about their results and the choices they have and support they need.
Over the next 1 – 3 years the NHS will continue to work with multi-professional groups to deliver the upskilling of the workforce and drive the change in practice needed for the embedding of genomics in the NHS, including through the Academy of Medical Royal Colleges Multi-professional Genomics Partnership Group and through working with specific professional groups, including pharmacists, nurses and midwives.
Over the next year, NHS England and the Health Education England Genomics Education Programme are working together to develop a genomic training academy using the resources allocated as part of the Spending Review diagnostic workforce settlement and to reduce the burden of training on the system.
Over the next 1 – 3 years, the NHS will explore opportunities to recruit and retain specialist and other key staff and develop a plan for increasing capacity and capability based on a robust assessment of what is required across multi-professional groups and recognising the spending review investment plans in this workforce. This will be done together with the NHS GMS funded education and training leads and relevant Health Education England leads.
Over the next 3 – 5 years, the NHS will explore the future training and development model with academia and industry, particularly for very specialist staff such as bioinformaticians, to secure a future supply for the NHS and share experience and knowledge.
Priority 1.4 Developing national and international collaborations and partnerships
Progress so far
The NHS GMS is part of a rich genomics ecosystem in England and its success is based on strong partnerships, for example with Genomics England, the NIHR, clinical academics and the wider academic system, industry partners and others. The NHS will continue to strengthen and build on these partnerships where they are relevant and appropriate for the NHS and its leadership of the NHS GMS.
As a world leader in the development of genomic services, there is interest from international partners to share the NHS GMS learning and best practice.
There have also been opportunities for international collaboration with the Global Alliance for Genomics and Health (GA4GH), a coalition of over 500 organisations working together to establish frameworks and standards for responsible, voluntary and secure sharing of international genomic and health related data and the Global Genomic Medicine Consortium (G2MC) is a community of global leaders dedicated to advancing genomic medicine implementation in clinical care.
The NHS works closely with these groups, as well as with others, implementing genomic medicine initiatives within healthcare systems across the world to drive innovation and learning to ensure patients in the NHS can access cutting-edge services.
NHS England also works closely with the Department of Health and Social Care, Office for Life Sciences, other government departments and the health departments of the other nations of the UK to ensure harmonisation of approaches in genomic medicine services where appropriate and to explore opportunities for collaboration.
Taking this further
The NHS will continue to share learning and best practice with national and international partners and align to and inform international standards. The NHS will continue to gather insights from other health systems across the world to inform the NHS GMS strategic direction.
NHS England will continue to work strategically across government and with other countries of the UK to explore and support harmonisation of services and opportunities for UK wide approaches, for example shared tumour genomics advisory boards and for broader genomics initiatives, such as precision medicines initiatives.
2. Delivering equitable genomic testing for improved prediction, prevention, diagnosis and precision medicine
Our priority actions
2.1 Systematically introducing new clinical indications for genomic testing and embedding comprehensive genomic testing within end-to-end clinical pathways.
2.2 Driving the use of precision treatments and optimising the use of medicines through genomics.
2.3 Enabling the rapid evaluation and adoption of affordable, efficient, and innovative genomic technologies.
Why this matters
The demand for NHS diagnostic services is increasing. The right genomic medicine services at the right time in a patient pathway can speed up diagnosis and improve patient and family experience and outcomes. It can provide access to precision treatments, enable an informed decision to be made on intervention or reproductive choice and ensure an equitable offer to participate in clinical trials. Unnecessary clinical appointments, diagnostic testing and other interventions or treatments can be avoided. Equity in access to genomic medicine services is critical, which is why the genomic testing that patients and their families can access through the NHS is outlined in a single National Genomic Test Directory, see Appendix 1.
For the NHS to realise the potential of genomics, the genomic testing offer needs to be embedded into end-to-end pathways. While these are cross cutting priorities, it is important to assess the specific considerations for cancer, rare and inherited disease, and precision medicine.
Cancer
Comprehensive genomic sequencing offers patients with cancer the ability to receive a more precise diagnosis at the start of their treatment pathway. This can provide prognostic information, guide faster access to precision treatments or surgical intervention based on the complete genomic profile of the cancer together with other diagnostic information, avoid drug toxicities and enable access to molecularly stratified clinical trials. Genomic advances can arm clinicians with the information needed to provide optimal treatments for their patients at point of diagnosis, as well as if a patient is no longer responding to a treatment or following signs of relapse. This can improve the quality of life and survival outcomes for those individuals and monitoring of reoccurrence.
Case study
James, a one year old with mixed phenotype acute leukaemia was one of the first children to receive whole genome sequencing through the NHS at Great Ormond Street Hospital. The test detected a gene fusion that conventional testing would not have shown up and means their condition can be accurately monitored and the child has the opportunity to quickly access a targeted therapy with a memin inhibitor if conventional therapy fails.
Rare and inherited disease
Many individuals and families with a rare or inherited disease or a suspected genetic condition can experience years of uncertainty with no confirmed diagnosis, often referred to as the ‘diagnostic odyssey’. A rapid and accurate diagnosis is essential for improving health outcomes and reducing the need for multiple diagnostic investigations. Comprehensive genomic sequencing can provide a diagnosis to individuals with rare and inherited diseases, who might not have received one otherwise and can inform reproductive choices or determine the potential impact for other family members. This can enable access to precision treatments, specialist care pathways, and broader support services and communities.
Some inherited conditions occur more frequently in the population such as familial hypercholesteremia where the estimated prevalence is 1 in 250 of the population. The condition can be well managed with treatment already available in the NHS, however, if left undiagnosed it can lead to sudden death. Genomic testing can identify the genomic variants associated with this condition and inform different approaches to treatment as well as finding other family members who may be affected.
Driving the use of precision medicine and optimising the use of medicines
Genomics can provide insights into how to optimise or direct prescribing as part of precision medicine and stratifying treatments, and also on how an individual will react to a medicine or intervention. The potential to detect adverse drug reactions is an emerging field known as pharmacogenomics; this application of genomics can inform prescribing for common drugs such as pain medications, where individuals might require tailored dosing or to avoid specific medicines.
Case study
Sarah, a 75-year-old started coughing up blood in November 2021 and on seeing the NHS lung cancer campaign went to see their GP. She was diagnosed with lung cancer and was referred for genomic sequencing and was diagnosed with a ROS1 fusion. She was given Entrectinib, a histology independent treatment, and is currently on a course of this and doing well. She has had almost no side effects aside from losing her taste, but it really does not bother her. She has been feeling well and living a much better life than she expected with lung cancer and is being monitored every three months instead of every month, showing how well she is doing.
Priority 2.1 Systematically introducing new clinical indications for genomic testing and embedding comprehensive genomic testing within end-to-end clinical pathways
Progress so far
The National Genomic Test Directory covers around 3,200 rare and inherited diseases and over 200 cancer clinical indications. An annual review process, supported by a horizon scanning function, ensures the NHS genomic testing offer continues to evolve as new science and technologies emerge.
The NHS genomic laboratory hubs have put in place the testing infrastructure to deliver the Test Directory, covering the full repertoire of genomic testing from predictive testing, diagnosis, and identification of treatment, through to monitoring of disease and identification of patients eligible for clinical trials. The NHS GMS has focused on offering the full continuum of genomic testing technologies ranging from single gene testing to large gene panels, whole exome sequencing and whole genome sequencing.
Multimodal approach
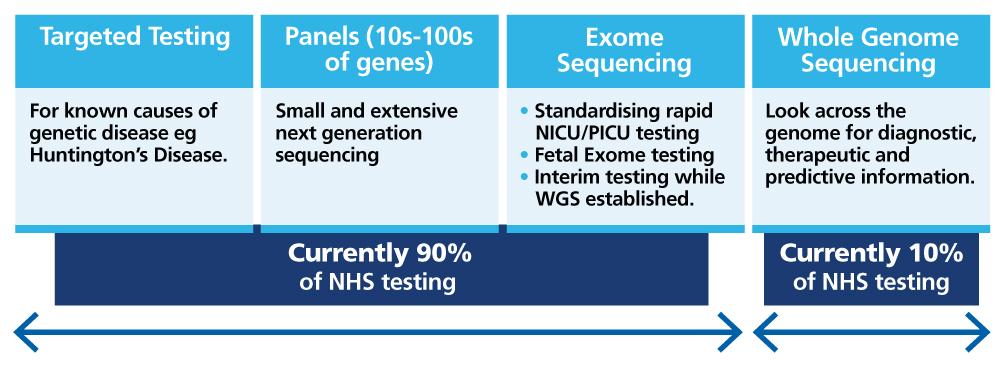
The NHS GMS has also started work to embed genomic medicine, including genomic counselling, within existing clinical pathways and created new clinical pathways so that more individuals are now able to access genomic testing and receive a more accurate diagnosis and/or have support and timely access to precision medicines.
To support more extensive cancer genomic testing there has been a drive to ensure closer collaborative working between colleagues in pathology and genomics. This has highlighted the need to address several issues including capacity, networking and optimisation of cancer tissue pathways. Solutions to these issues are vital to ensuring pathology laboratories can deliver rapid preparation of tissue samples for genomic testing to the NHS genomic laboratory hubs. Action is underway to address these issues, including through bringing together the NHS genomic laboratory hub pathology leads to review workflow and challenges and establishing a national pathology accelerator project in conjunction with Royal College of Pathologists.
Taking this further
The policy and process to update the Test Directory will continue to operate to ensure that the NHS GMS remains up to date with the latest scientific, clinically and cost-effective advances. This is likely to include more extensive use of technologies in cancer and where appropriate rare and inherited disease and a move to increased genomic testing for more common disease as the evidence is generated.
Over the next 1 – 3 years the NHS genomic laboratory hubs, NHS GMS alliances and clinical genomic services will continue to transform clinical pathways and service models to embed genomics where there is the greatest impact on clinical outcomes and pathway efficiencies. This includes working across clinical specialties and services to define clinically meaningful use cases for genomics. Pathways will need to be redesigned to enable for example the collection of biopsies for cancer genomics early in the diagnosis pathway to ensure the timely return of genomic test results to inform clinical care and decision making. The NHS GMS and cancer alliances will need to work together to deliver rapid turnaround times for certain types of cancer where timely results are needed to inform diagnosis and treatment.
Over the next 3 – 5 years, the NHS will explore the utility of genomic testing to support population screening for cancer, including reviewing the evidence from population based studies to detect cancer earlier, such as the research trial of the GRAIL Galleri test, and piloting to expansion of BRCA testing in higher risk populations. The NHS GMS will aim to expand predictive and preventative care to achieve earlier diagnosis and improve public health and patient outcomes.
Over the next 1 – 3 years the NHS will drive equity in access to genomic testing. For example, by exploring opportunities to increase genomic testing referrals from primary and community care where clinically appropriate and supported by appropriate education and training. This will be with a particular focus on unmet need and supporting undiagnosed populations. As part of the patient level contract monitoring data collection, activity data and turnaround times will be monitored and made publicly available. The NHS GMS alliances will have an explicit role in supporting the NHS GMS in their geography to reduce inequities in access and to use genomics to reduce health inequalities.
The NHS Genomic Medicine Service approach to the adoption of new technologies

Priority 2.2 Driving the use of precision treatments and optimising the use of medicines through genomics
Progress so far
Genomic capabilities have accelerated the development of new precision medicines and repurposing or optimising the use of existing medicines where there are genomic variants that can be targeted. To bring precision medicines to patients, the NHS has supported access to clinical trials where eligibility is based on genomic variants and supported the adoption and spread of innovative medicines through an innovative genomic testing service and commercial medicines framework.
In cancer this has led to NHS patients accessing a new generation of cancer fighting medicines and in rare disease, hundreds of NHS patients with spinal muscular atrophy (SMA) are now able to access effective medicines, while in more common chronic conditions, patients are accessing targeted treatments for renal disease after receiving a genetic diagnosis.
Genomic medicine has allowed medicines already available in the NHS to be repurposed. For example, the repurposing of existing NHS medicines for COVID-19 patients, such as Baracitinib frequently used in the treatment of rheumatoid arthritis, which was identified through the whole genome sequencing of severely affected individuals during the UK led RECOVERY Trial. Not only did this speed up patients accessing the benefits of these medicines, but it also saved the considerable time and costs involved in developing new medicines.
Case study
Jen, a 45-year old transport business owner from Wigan was revealed to have monogenic diabetes rather than Type 1 diabetes after having a genomic test. Jen said: “Since I was diagnosed and my treatment was changed from insulin injections to tablets, I feel much better in myself as my blood sugar levels are now stable and I do not have the highs and the lows that I used to.
“I used to have damage to the blood vessels at the back of my eyes due to my diabetes, but since getting the correct genetic diagnosis, my blood glucose control is much better with the tablet treatment and that has dramatically improved. I have also managed to lose two stone in weight, which I was never able to do before!”
Where evidence already exists, genomic tests that drive the optimal use of medicines to reduce adverse reactions or to inform dosing (known as pharmacogenomics) have been included on the Test Directory. For example, the introduction of DPYD testing for approximately 38,000 patients undergoing cancer treatment with the commonly used class of chemotherapies called fluroropyrimidines has enabled the NHS to prevent adverse reactions to the treatment, which can be fatal. More generally in optimising the use of chemotherapy, a national initiative led by the NHS GMS alliances to optimise and standardise the equitable implementation of testing has helped to prevent significant and life-threatening toxicity to patients.
Taking this further
Over the next 1 – 3 years, the NHS will work with partners to ensure that prescribing decisions are informed by genomic testing where appropriate. This will involve working with partners across the medicines landscape, for example key clinicians such as pharmacists and GPs and existing medicines governance structures, such as the regional medicines optimisation committees.
Key to embedding pharmacogenomics will be the adoption of standardised pharmacogenomic information into electronic patient health records so that it can inform real-time clinical decision making as part of routine clinical care and precision medicine pathways as appropriate. Over the next 1 – 3 years NHS England will review the evidence from pilot projects currently being undertaken in the NHS GMS alliances, alongside evidence generated by other programmes and organisations, to inform potential wider implementation.
Over the next 1 – 3 years the NHS will drive equity in access to clinical trials by aligning clinical trial targets with standard of care NHS testing. In appropriate circumstances this will involve partnering with clinical trials units and industry to support identification of eligible patients. This will require putting in place a mechanism to systematically horizon scan upcoming clinical trials to ensure the correct targets are added to the National Genomic Test Directory, while also having the data sharing infrastructure in place to share genomic data safely where appropriate and with the necessary patient consent.
The NHS will work with the horizon scanning function that includes Medicines and Healthcare Products Regulatory Agency (MHRA), the National Institute for Health and Care Excellence (NICE) and the Accelerated Access Collaborative to identify and prepare for the rapid introduction of companion diagnostic genomic testing which is critical to provide access to innovative precision medicines and technologies.
Priority 2.3 Enabling the rapid evaluation and adoption of affordable, efficient, and innovative genomic technologies
Progress so far
In recent years NHS England has made a significant investment in cutting-edge high throughput sequencing technology to deliver genomic testing at scale and reduce the time it takes for individuals to receive a genomic test result.
The NHS GMS has implemented next generation sequencing pan-cancer panels for solid tumours and haematological malignancies, as well as whole genome sequencing for cancer patients, including all paediatric cancer patients. This has enabled testing for a larger number of genetic variations, the identification of biomarkers to target treatment and improved eligibility for clinical trials. The adoption and clinical use of next generation sequencing technologies supports the future evolution of genomic testing to allow a rapid response to scientific advancements that improve understanding of genomic mutations in cancer biology.
For rare and inherited diseases, the NHS has introduced comprehensive genomic sequencing to enable a quicker diagnosis for patients and their families. Implementation of cutting-edge sequencing technologies is allowing more individuals to receive an accurate diagnosis to access appropriate clinical care and support.
Case study
Jake, a football-mad Newcastle fan, was born with Leber’s Congenital Amaurosis (LCA), a form of retinal dystrophy, in which patients carry a gene mutation that means the retinal cells can’t produce a pigment that allows us to see. He received a gene therapy, voretigene, in which the faulty gene is replaced by a functioning gene. Some 18 months on and Jake went from seeing nothing to being able to see emails, write letters and even watch his beloved Newcastle United on the television.
Alongside the current national whole genome sequencing service which continues to support an increase in testing volumes, the NHS is establishing pathways to provide rapid whole genome sequencing via the NHS genomic laboratory hubs. This has the potential to increase the detection of diagnostic variants and offer more individuals a diagnosis to access life-saving treatments more quickly. This could result in fewer days in hospital, fewer invasive procedures and improve efficiencies in the NHS by reducing the need for multiple diagnostic tests inclusive of other genomic tests and through enabling earlier decisions to be made on the care and management of patients.
The NHS is exploring the introduction of innovative genomic sequencing techniques that can be applied to a range of clinical applications, including cancer. For example:
- RNA sequencing – used to examine the expression of genes encoded in an individual’s DNA, which can be used to access precision treatments.
- Long read sequencing and optical mapping – currently being explored to sequence parts of the genome that cannot easily be sequenced by other techniques for example methylation in brain cancers.
- Liquid biopsies – using blood samples to test for circulating tumour DNA (ctDNA), see box below.
Taking this further
Over the next 1 – 3 years the NHS will evolve the genomic testing strategy to develop an integrated diagnostic model and a multi-modal multi-omics testing approach, utilising multiple DNA and RNA based technologies across existing care pathways, clinical conditions and disease areas. This will support preventative healthcare, earlier diagnosis and faster access to precision treatments. It will also explore whether other diagnostics associated with the functional genomics pathway should be introduced, for example proteomics.
Over the next 1 – 3 years the NHS will explore the future model of delivery for whole genome sequencing in the NHS considering requirements for rapid whole genome sequencing to reduce the time to return results and inform clinical care.
Case study
Liquid biopsies
Increasingly it is becoming possible to use blood samples to test the circulating tumour free DNA (cfDNA) for the presence of disease-causing mutations in ctDNA.
There are several use cases where ctDNA testing could benefit patients, such as earlier detection and faster diagnosis of cancer including earlier detection of relapse. It could be used alongside other diagnostic testing to fully profile the tumour and enable precision treatment. ctDNA testing can be used for patients or tumour types where obtaining a sample is challenging, for example brain tumours or non-small cell lung cancer and where a tissue biopsy is not feasible, for example cancer of an unknown primary source. ctDNA also provides new opportunities for ongoing disease monitoring to identify genomic changes that could confer drug resistance.
NHS England has funded a transformation project through the NHS GMS alliances to explore the implementation of ctDNA in the NHS, starting in stage 3/4 non-small cell lung cancer patients, whilst continuing to define additional clinical use cases.
In November 2020, NHS England announced a research trial of the GRAIL Galleri test, which uses a blood test to detect multiple types of cancer through looking for ctDNA markers in individuals with no cancer symptoms, potentially identifying the type and location of the cancer, supporting earlier diagnosis.
3. Enabling genomics to be at the forefront of the data and digital revolution
Our priority actions
3.1 Developing an interoperable informatic and data infrastructure that enables the NHS to use and share genomic data appropriately to improve patient care.
3.2 Putting the NHS at the forefront of using genomic data alongside other health data to drive health improvements for individuals and populations.
3.3 Enabling the NHS to use cutting-edge analytical tools and up to date variant databases to maximise diagnosis, access to precision medicine and efficiency.
Why this matters
Case study
The future of the NHS depends on improving how we use data for four purposes:
- For the direct care of individuals.
- To improve population health through the proactive targeting of services.
- For the planning and improvement of services.
- For the research and innovation that will power new medical treatments.
Source: Data saves lives: reshaping health and social care with data
Significant amounts of data are generated through the NHS GMS, which falls into three broad categories:
- Genomic data – generated from genomic testing, including phenotyping and staging data to enable interpretation, is becoming increasing complex as technology develops and as more next generation sequencing and whole genome sequencing takes place. The value of this genomic data can only be maximised if linked with other diagnostic and clinical data sources across the life course often at a national level and in standardised formats.
- Clinical data – where additional patient data, for example clinical symptoms, and longitudinal health data are used to inform genomic analysis and ongoing research insights.
- Management data – for operational improvement and evaluation – national collection of the genomic testing activity, variation in access, turnaround times and, in time, linked with outcomes and treatment data. The NHS will also use these data to generate evidence of the value of genomic medicine services.
This priority focuses on the clinical value of the first category of data, however the NHS GMS will continue to collate robust performance management data, with a focus on equalities and health inequalities data, to underpin service delivery and service improvement. Where appropriate, this data will be made publicly available. In all cases the NHS will continue to put patient choice at the heart of all decisions on how genomic data in used.
Data and digital solutions can enable the NHS GMS to maximise its capacity and capability. This includes enabling clinicians to easily understand what genomic tests are available through an online National Genomic Test Directory, ordering genomics tests quickly and digitally, performing cutting-edge bioinformatics on genomic data and delivering results to clinicians quickly and accessibly, to inform treatment decisions or access to clinical trials.
With the introduction of a national genomic medicine service the requirement for IT systems to work together and enable the sharing of data and information is critical.
Innovative informatics solutions are needed to maximise the benefits of genomics for individuals and population, including supporting research and innovation. This will enable evidence and insights to continue to grow our use of genomics in medicine and make the NHS a destination of choice for clinical trials and other research and development activities. For example, building on the genomic diagnostic discovery work through the NHS and Genomics England partnership.
Given the pace of scientific discovery in genomics and the advances expected over the next decade in computing and data analytics, the informatics infrastructure that supports the NHS GMS needs to be robust, safe and continuously improving if it is to keep in step with the new service models and clinical pathways that will evolve.
Priority 3.1 Developing an interoperable informatic and data infrastructure that enables the NHS to use and share genomic data appropriately to improve patient care
Progress so far
Building on the 100,000 Genomes Project informatics infrastructure, NHS England partnered with Genomics England to develop the National Genomics Informatics System (NGIS). NGIS is an informatics platform that provides a standardised suite of analytical tools for interpreting whole genome sequences by the NHS genomic laboratory hubs. It includes a mechanism for NHS genomic laboratory hubs to order whole genome sequencing and receive targeted bioinformatics results.
Beyond whole genome sequencing, the majority of NHS genomic testing activity is undertaken in the NHS genomic laboratory hubs, which currently use a variety of different informatics systems that are part of wider provider trust and laboratory systems and connected or otherwise to patient records, with varying digital maturity that means NHS genomic laboratory hubs still receive significant numbers of paper based requests and are unable to follow and report to clinicians where a sample is within the system, for example in cancer. Investment has been made in enhancing the Laboratory Information Management Systems (LIMS); this, together with standardisation of the coding for testing procedure, has enabled the implementation of the National Genomic Test Directory and begun the process of standardising test reporting. These enhancements will provide a foundation to deliver future interoperability.
Taking this further
To improve patient care and expand the use of genomic medicine across clinical specialties, the NHS will support digital interoperability and an appropriate data sharing infrastructure and improve the accessibility to order, follow, and return genomic test results.
Over the next year, the NHS will develop shared data standards, as a first step to developing a fully interoperable data infrastructure. During 2023, the NHS working with partners such as Genomics England will publish a genomics informatics implementation plan, outlining how the NHS genomics data infrastructure will continue to develop to support interoperability with other NHS systems and drive efficiencies in the service. Delivery of the plan will be overseen by a newly established NHS Genomics Data and Digital Board.
The implementation plan will outline for both whole genome sequencing and non-whole genome sequencing testing:
- Improvements and developments required across the NHS for existing NHS GMS services, for example test ordering, and core capabilities to meet volume objectives and improve the clinical experience.
- Initiatives that will introduce step-changes to the existing NHS GMS model and core capabilities, including, identifying technological opportunities to reduce the demand on the NHS GMS workforce and therefore increase capacity.
- Systems that enable clinicians to understand when a sample has been received by the NHS genomic laboratory hubs, where it is in the testing pathway and the expected timeline for the return of the result.
- The developments required by the NHS GMS providers within each NHS GMS geography and by partners including Genomics England and regional NHS systems, to enable interoperability with genomic systems, adoption of standardised bioinformatic pipelines, and to inform population health and service development.
- The managed convergence and simplification of the complex landscape and levelling up of digital maturity.
Priority 3.2 Putting the NHS at the forefront of using genomic data alongside other health data to drive health improvements for individuals and populations
With the introduction of new genomic technologies such as next generation sequencing panels, whole genome sequencing and whole exome sequencing more genomic data is being generated than ever before. To gain a comprehensive view of an individual’s diagnosis to inform their care the ambition is to integrate genomic data with other health and care data, for example other diagnostic information (phenotypic data and additional genomic data), pathology data, physiological data and imaging data with over time environmental and social factors.
Bringing together the multiple maps of the various stages of the functional genomic pathway in an individual, together with related environmental and social information, our ambition is to provide a panoramic view of an individual, offering an even richer opportunity to shape precision care.
Progress so far
To support the move towards mainstreaming genomics in the NHS, human phenotype ontology terms – a standardised and common vocabulary – is being introduced into routine care, initially for whole genome sequencing, to describe characteristics associated with rare and inherited diseases. Utilising this common terminology, rare disease genomic multi-disciplinary teams have been established to enable discussion of complex cases and phenotype genotype relationships to inform and direct reporting and downstream care. This is supporting clinicians across a broad range of clinical specialties who have referred individuals for genomic testing.
As part of the National Genomic Research Library consented deidentified clinical and genomic data is stored in a secure data environment that was developed and is curated by Genomics England in partnership with the NHS. This supports ongoing research and discovery from approved researchers, academia and industry.
Genomics England are undertaking developing a digital archive of cancer radiology and pathology images alongside genomic data with around 40,000 images generated in the NHS scanned to date. The data generated will look to identify new features of cancer that drive prognosis or response to specific treatments. NHS England will review the evidence generated and develop a plan to implement it where it is clinically and cost effective to do so.
Taking this further
To maximise the clinical utility of genomic sequencing and to enable re-analysis of sequencing generated over the next 1 – 3 years, the NHS working with partners will explore the potential benefits of developing a genomic healthcare record to establish a longitudinal genomic record across the life course of an individual.
Over the next 1 – 3 years, the NHS will review the evidence generated by Genomics England’s multi-modal diagnostic initiative and explore the further development of integrated reports including use of digital images and digital diagnostic data.
As appropriate and in consultation with patients and the public, over the next 1 – 3 years, the NHS will expand the use of NHS generated genomic data to support approved research.
Priority 3.3 Enabling the NHS to use cutting-edge analytical tools and up to date variant databases to maximise diagnosis, access to precision medicine and efficiency
Using cutting-edge analytical tools and machine learning creates opportunities to generate new insights from large datasets and create efficiencies by reducing the time required to undertake manual and complex clinical analysis. This computing and analytical field is rapidly advancing – making available a range of tools and resources to the NHS GMS will be critical in ensuring that it can undertake the most comprehensive analysis in the shortest time possible.
Progress so far
To date the NHS GMS has supported the continuous development of bioinformatics pipelines by Genomics England for analysis of whole genome sequencing and by the NHS GMS for non-whole genome sequencing data to improve diagnostic yield.
NHS England has also launched an NHS GMS version of PanelApp, a resource that contains a list of all the genes that relate to genomic tests listed in the NHS National Genomic Test Directory. This links with a version of PanelApp curated by Genomics England that enables researchers to add evidence and suggest additional genes or entities for a panel that can then be considered by NHS England.
Taking this further
Over the next 1 – 3 years, the NHS will work with partners, including Genomics England, to enhance a gene agnostic bioinformatics pipeline into appropriate clinical indications for whole genome sequencing to improve diagnosis rates.
Over the next 1 – 3 years, the NHS will work with partners, including Genomics England to evaluate the potential for the clinical implementation of artificial intelligence (AI) and machine learning in genomics, through a series of pilot schemes in the NHS GMS.
Over the next 3 – 5 years the NHS will work with partners to understand what is required to store and analyse NHS data from multiple genomic analyses and develop a model for analysis and interpretation of genomic data using standardised bioinformatics pipelines that is sustainable and utilises the skills and expertise of clinical scientists in the most efficient way
4. Evolving the service through cutting-edge science, research and innovation
Our priority actions
4. 1 Enabling patients to make informed choices on the use of their data for research and innovation.
4.2 Enriching existing and developing new NHS GMS relationships to support innovation and the generation of evidence for adoption and improvements in health and care.
4.3 Ensuring ongoing alignment with clinical trials and national life sciences projects and supporting the growth of life sciences in the UK.
Why this matters
To improve the health of future generations, it is critical that equitable access to genomic research enables scientific progress in diagnostic discovery, translational research and the development of new precision treatments for all. This is particularly relevant for both cancer and rare and inherited disease and increasingly in common diseases.
NHS Genomic Medicine Service innovation pathway
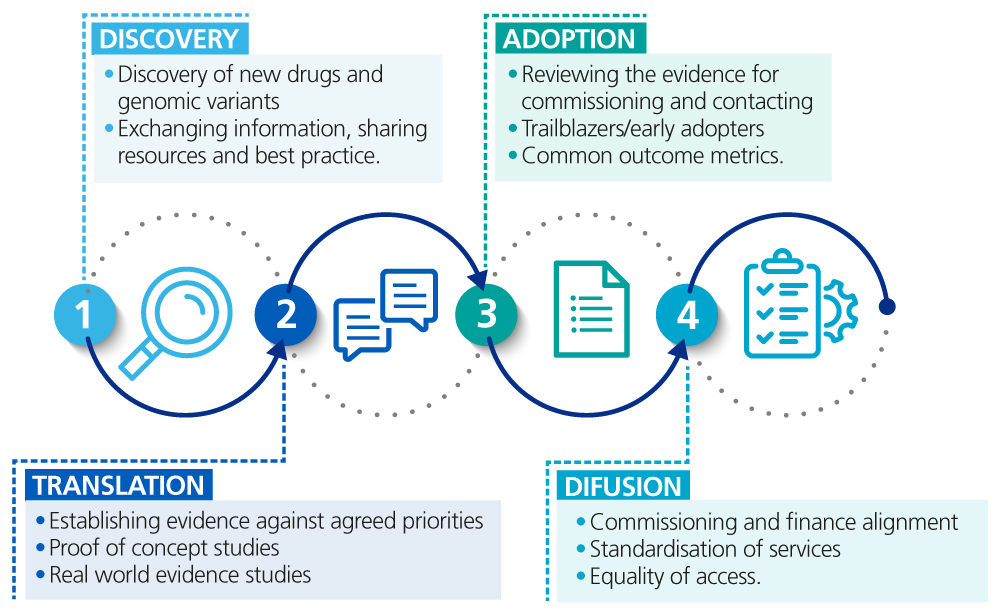
There is an opportunity to establish a systematic approach to embedding research and discovery for patient and societal benefit. This includes putting in place mechanisms to review the latest evidence from diagnostic discovery and where there is information that supports clinical care, for example providing a diagnosis or precision treatment, ensuring that this information is provided to clinicians and individuals using the NHS GMS.
Case study
Noah was a six year old boy with moderate to severe learning difficulties after showing signs of delayed development from an early stage. As an early participant in the 100,000 Genomes Project, Noah has benefited from whole genome sequencing. Following a reanalysis of Noah’s whole genome sequencing data, this has identified a likely pathogenic variant which has been revealed following new research into development disorders caused by FOXP4 variants, that was published in March 2021.
The NHS GMS infrastructure currently supports over 900 genomic research projects across the testing spectrum, from small scale research to national projects and clinical trials. This number is growing and there is an opportunity to increase coordination and the delivery of these research and innovation developments from a national perspective.
Priority 4.1 Enabling patients to make informed choices on the use of their data for research and innovation
Progress so far
In partnership with Genomics England, patients and clinicians, NHS England developed a national patient choice framework, that supports clinicians, regardless of clinical specialty, to discuss having whole genome sequencing with their patients, and whether they would consent to their genomic data being accessible for research via the National Genomic Research Library. The framework built on the experience from the 100,000 Genomes Project and currently this is available for all patients receiving a whole genome sequencing test. To date over 90% of patients who have had the opportunity to discuss participating in research have consented.
Taking this further
The focus of the existing national patient choice framework is whole genome sequencing. There is a significant opportunity to expand the data available for research by using the framework to seek consent from patients accessing next generation sequencing for whole exomes and large panels, particularly in cancer. This data could help inform clinical trial design, development and enrolment in trials, and ultimately drug and therapeutic developments for many conditions.
Over the next 1 – 3 years the NHS will work with patients, the public and key partners to evolve the patient choice framework and put in place mechanisms to enable the consent to and collation of NHS genomic sequencing data for research and innovation purposes at a national and regional level. This will include having appropriate information and support available to patients to make an informed decision as to whether they consent to their data being available for research.
Priority 4.2 Enriching existing and developing new NHS GMS relationships to support innovation and the generation of evidence to improve health and care
Progress so far
In December 2021, the NHS GMS Research Collaborative was established as a partnership between NHS England, the NHS GMS, Genomics England and the NIHR to facilitate and fulfil the research mission. The NHS GMS Research Collaborative aims to make it easier for researchers in academia, the NHS and the life sciences industry to conduct interactive genomic research and validate new genomic technologies, diagnostics and treatments to drive improvements for patients and the NHS.
The 2022/23 planning guidance for the NHS genomic laboratory hubs and NHS GMS alliances includes supporting research and innovation as a priority area. This sets out the requirement that the NHS genomic laboratory hubs and NHS GMS alliances have the infrastructure in place and outline support for research and innovation within their business plans. This will continue to be a priority to ensure ongoing function of the NHS GMS Research Collaborative and support of clinical trials.
Case study
CRUK Cambridge Innovation Centre
The Cancer Research UK Cambridge Innovation Centre, is at the cutting-edge of leveraging science to drive the implementation of genomics into standard of care clinical practice, using the NHS genomic laboratory hub infrastructure. The Centre has developed a personalised Breast Cancer programme using whole genome sequencing (both DNA and RNA sequencing).
The model includes using whole genome sequencing for patients with early or later stage breast cancer, before discussing the results at an Omics Review Board (ORB), before returning results to clinicians and patients. This is driving personalised treatment, including prophylactic surgery, as well as screening for other cancers and enrolment in clinical trials.
This approach is leading to increased rate of referral to genetics services; around 40% of patients seeing a change in management of their cancer; and increased identification of family members needing screening for breast cancer or other cancers.
From a research perspective it allows scientists and clinicians to return to the data as new evidence accrues; to stratify patients based on molecular classification; increasing the knowledge pool regarding variants of unknown significance; and identifying areas of need for a clinical trial focus.
Taking this further
The NHS GMS Research Collaborative will continue to be developed to ensure it responds to the requirements of genomic research projects and initiatives.
During 2023/24 the NHS will, as part of the evolving NHS GMS alliance infrastructure, establish ‘NHS genomic networks of excellence’. The networks of excellence will bring together the NHS GMS, academia, universities, industry and other partners in networks to deliver genomic research from discovery to adoption and spread, in specific priority areas designated by NHS England and aligned to NHS priorities. Areas of focus will include cancer, fetal and pre-natal medicine, cardiovascular disease and neurological disease.
Networks of excellence will be designated based on expertise and capability following an application process that will be initiated in early 2023. They will support the closer alignment between NIHR research networks, clinical research networks and biomedical research centres, as well as other major centres or initiatives created by other third sector organisations.
Priority 4.3 Ensuring ongoing alignment with clinical trials and national life sciences projects and supporting the growth of life sciences in the UK
Progress so far
Closer engagement and alignment is needed with industry – with a systematic mechanism in place – to signal the needs of the NHS in genomics and to provide an opportunity for industry to respond to those needs. This will bring about tangible benefits for NHS patients through the development of innovative sampling, sequencing, analysis and diagnostic and therapeutic technologies, alongside the need to be innovative with regards to genomics data and workforce requirements.
Taking this further
To support increasing focus on genomics and the critical role of the life science sector, over the next 1 – 3 years the NHS will explore new opportunities to work with industry to signal the needs of the NHS GMS.
The NHS will contribute to and review the evidence generated from key research initiatives to inform any future decisions regarding commissioning of services. This includes:
- Whole genome sequencing pilot for newborns. The Newborn Genomes Programme will be co-designed by Genomics England with the NHS and will run as an ethically approved research pilot embedded in the NHS to explore the benefits, challenges and practicalities of sequencing babies’ genomes to accelerate diagnosis and access to treatments for rare genetic conditions.
- Diversity in genomic data. Genomics England is undertaking a diversity in genomic data initiative, which has been created with the purpose of enriching genomic datasets by engaging with relevant communities, sequencing consented cohorts from diverse backgrounds and developing analytics to derive the most value possible from the data.
- Cancer long read sequencing proof of concept. Genomics England, in partnership with the NHS, are currently developing the evidence for the use of DNA next generation long read sequencing for cancer patients in the NHS. The use of this technology could lead to an increase in the number and speed of cancer patients receiving genomic information to inform their diagnosis or treatment.
Our Future Health. The project will generate evidence that will help the NHS understand the impact of giving people personalised health and risk information and contribute to decisions on whether and how polygenic risk scores should be implemented at scale in the health service.
Call to action
Our vision is that the power of genomics in predicting, preventing and diagnosing disease, and targeting treatment is accessible to all as part of routine care in the NHS is ambitious. As set out in this document, genomics in the NHS is at a pivotal point, and the opportunities are there to maximise the benefits of genomic medicine and make this vision a reality.
This strategy has set out four priorities areas for action which will give us the best possible change of making it happen:
- Embedding genomics in the NHS, through a world leading, innovative service model.
- Delivering equitable genomic testing for improved prediction, prevention, diagnosis and precision medicine.
- Enabling genomics to be at the forefront of the data and digital revolution.
- Evolving the service through cutting-edge science, research and innovation.
Delivering these actions will require a collaborative and coordinated approach with a range of partners, and action from people, teams and organisations across the NHS.
Providers should:
- Work together to coordinate and embed genomics across NHS pathways and clinical specialties to inform earlier decision making in conjunction with the NHS GMS infrastructure.
- Identify areas of unmet need or inequalities that require concerted action.
- Utilise the available and funded genomic testing for all defined conditions in all NHS trusts and across the care continuum.
- Support the tissue pathway developments that are required in pathology to enable the comprehensive genomic analysis of cancers by the NHS genomic laboratory hubs for all patients and alignment with stratification of precision medicines working in conjunction with the NHS GMS alliances and the cancer alliances.
- Develop and support the multi-professional workforce to be leaders in genomics and to be upskilled to use and interpret genomic information and to support patients and their families.
- Enable the monitoring of access to precision treatments and interventions based on genomic data;
- Invest in informatics, analytical and data capabilities in NHS trusts to enable genomic data to be shared and integrated with other diagnostic and clinical data.
- Support continued research and innovation projects and initiatives in the UK life sciences sector and enable the ongoing population of a national genomic research library.
Healthcare professionals should:
- Engage with their NHS GMS alliance to understand the genomic services available in their area and where their particular profession can contribute.
- Seek opportunities to learn more about the potential of genomics, for example through the Health Education England Genomics Education Programme.
- Champion the use of genomics and sharing genomic data among colleagues and patients and members of the public.
- Inform the development of clinical guidance and practice on the use of genomics in different conditions and specialities.
- Understand the clinical trials and projects and initiatives that are available and ensure patients under their care have access to participate.
- Share good practice with the NHS GMS alliances or get involved in projects and initiatives and promote case studies demonstrating benefits.
- Support the need to monitor access to genomic testing and monitoring of access to precision medicine.
Patients and patient groups should:
- Become involved in the NHS GMS governance structures at a regional and national level.
- When offered genomic testing through the NHS, speak to their healthcare professional about consenting for genomic research.
- Help raise awareness of the genomic testing available on the NHS and the benefits.
Academia, industry and charities should:
- Work with the NHS GMS Networks of excellence when established to generate evidence from research for further adoption and spread across the NHS.
- Work with the NHS to support research in areas identified as priorities through horizon scanning and demand signalling.
- Engage with the NHS GMS Research Collaborative to utilise the NHS infrastructure for research and innovation projects and initiatives.
- Work with the national genomic research library to continue to inform diagnostic discovery and to provide insights to evolve healthcare.
NHS England will:
- Ensure patients and the public are involved at all levels of the NHS GMS.
- Continue to invest in genomics and provide national and regional leadership, including multi-professional leadership.
- Ensure equity of access for all patients, through coordination, sharing of best practice and driving a standardised model of delivery across the country.
- Continue to develop the capacity and capability of the workforce in conjunction with our partners.
- Be responsive to innovation and new technologies through working with partners to identify opportunities and challenges.
- Be clinically and scientifically led, informed by data-led insights.
- Continue to review and monitor delivery of this strategy to provide the benefits of genomics for our patients and populations.
- Work collaboratively with government departments including the Office for Life Sciences and Department of Health and Social Care, health organisations, UK health services, other key partners and with international partners and initiatives in genomics and precision medicine to ensure alignment and adoption of best practice and sharing of knowledge and experience.
Glossary
Bioinformatics: The application of computer science and information technology to analyse and interpret biological data.
Circulating tumour DNA: Fragments of DNA shed by the cancer that have entered the bloodstream.
Clinical geneticist: A medically trained doctor who specialises in diagnosing and managing patients with genomic conditions through a combination of medical knowledge and a specialist understanding of molecular biology.
Diagnostic odyssey: A term used in genetics and genomics to describe the often long period of time it can take for a patient to receive a diagnosis for their condition.
Deoxyribonucleic acid (DNA): The chemical that contains, or ‘encodes’, genetic information. DNA is made up of four different chemical bases.
Expanded carrier screening: Genetic screening for would be and soon to be parents that can detect whether the parents unknowingly carry genetic conditions that may be passed onto family members.
Functional genomics: Functional genomics is the study of how genes and intergenic regions of the genome contribute to different biological processes.
Gene: A segment of DNA that that contains the biological instructions for the production of a polypeptide chain, usually a specific protein or component of a protein.
Genomics: The study of the genomes of individuals and organisms that examines both the coding and non-coding regions. This term is also used when talking about related laboratory and bioinformatic techniques. The study of genomics in humans focuses on areas of the genome associated with health and disease.
Genomic medicine: Genomic medicine (or healthcare) is the use of genomic information and technologies to determine disease risk and predisposition, diagnosis and prognosis, and the selection and prioritisation of therapeutic options.
Health Education England Genomics Education Programme: Exists to deliver and advise on learning and development opportunities that prepare current and future NHS professionals to make the best use of genomics in their practice.
Histology independent treatments: A treatment that targets all solid tumours with a certain genomic mutation, regardless of where the primary tumour is in the body.
Inherited condition: A condition caused by a genetic variant that has been passed down from parent to child.
Liquid biopsy testing: Using blood samples to test the circulating free DNA for the presence of disease-causing mutations in circulating tumour DNA.
Multi-modal data: Bringing together data from different sources, including genomics, pathology and radiology, to look at them as a whole.
Multi-omics: An approach to bringing together different omics, including genomics, proteomics, transcriptomics, epigenomics and microbiomics for analysis.
Multi-technology: Utilising a range of different technologies, for example whole genome sequencing and whole exome sequencing, to study DNA.
National Genomic Test Directory: A directory that specifies which genomic tests are commissioned by the NHS in England, the technology by which they are available and the patients who will be eligible to access each test.
NHS genomic laboratory hub: Responsible for coordinating genomic testing services, across one of seven regions in England.
NHS Genomic Medicine Service Alliance: Responsible for overseeing and coordinating the embedding of genomics into mainstream clinical care, across one of seven regions in England.
Personalised medicine: Medicine targeted towards an individual or group of individuals, which uses knowledge of genetic, environmental and lifestyle factors to determine suitable methods of prevention, diagnosis and treatment of disease.
Pharmacogenomics: The use of genetic and genomic information to tailor pharmaceutical treatment to an individual.
Phenotype: An organism’s observable physical and biochemical characteristics directly influenced by the genotype (genetic factor) and/or environment. In humans, this is often the observed signs and symptoms of a condition.
Population screening: The process of identifying healthy people who may have an increased chance of a disease or condition
Precision medicines: The application of emergent technologies to better manage patients’ health and to target therapies to achieve the best outcomes in the management of a patient’s disease or predisposition to disease.
Rare and inherited disease: A disease that affects less than 1 in 2,000 of the general population (EU definition). In the UK, approximately 3.5 million people will be affected by a rare disease at some point in their life.
Ribonucleic acid (RNA): Chemically similar to DNA but a single-stranded molecule. RNA is made up of four chemical bases.
Sequencing: A technique used in laboratories to determine the order of bases in DNA.
Solid tumour: A mass of tumours, which represent approximately 90% of adult human cancers.
Targeted gene testing: Using testing technology to focus on specific genes rather than large panels.
Whole exome sequencing: Sequencing only the protein-coding regions of the genome (around 2% of all DNA bases).
Whole genome sequencing: A type of genetic sequencing that has the potential to sequence every DNA base in a genome.
Appendix 1 – overview of the NHS GMS
Patients and the public
Patients and the public are at the heart of the NHS GMS infrastructure and have played a key role in the development and delivery of the NHS GMS. For example, a national NHS GMS People and Communities Forum informs national decision making, while regional patient and public involvement groups and representatives on governance bodies drive this at a local level. The NHS also has ongoing dialogue with various patient and charity groups with an interest in genomics.
NHS genomic laboratory hubs
The NHS genomic laboratory hubs are seven consolidated laboratory networks with defined geographies that operate as part of a national genomic laboratory network, commissioned by NHS England to deliver the genomic testing outlined in the National Genomic Test Directory. This includes the delivery of specialist rare and inherited disease, cancer and pharmacogenomic testing. Their aim is to provide a cutting-edge and comprehensive genomic testing service using the latest technology, standardise genomic testing and reduce variation, ensure equity of access and be able to the meet growing demand through driving efficiently and value for money. The NHS genomic laboratory hubs deliver an estimated 650,000 genomic tests each year and this is expected to continue to increase year on year as genomic testing becomes embedded across clinical pathways and medical specialities and across the care continuum.
Each NHS genomic laboratory hub is funded to include a multi-disciplinary clinical and scientific leadership infrastructure led by a medical, scientific, operations and informatics director, as well as a number of other roles covering cancer (solid and haematological malignancies), rare and inherited disease, bioinformatics, pathology and education and training. These multi-disciplinary teams work across the genomic testing pathway from referral and receiving a sample, through to interpretation and reporting of results and supporting genomic multi-disciplinary team (MDTs) meetings for both rare diseases and cancer and to enable complex genomic information to be discussed across clinical teams.
The National Genomic Test Directory
The National Genomic Test Directory (Test Directory) outlines the range of genomic tests that are commissioned by NHS England for patients in England. The Test Directory covers the full repertoire of testing from targeted testing, to panel next generation sequencing and up to the level of whole genome sequencing. The Test Directory currently includes 357 rare disease clinical indications and 203 cancer clinical indications and set outs the patient eligibility for a test, the clinicians who can order a test and standardises the test method used and genes that are looked at.
NHS England, supported by a genomics clinical reference group and test evaluation working groups, reviews the Test Directory on an annual basis to keep pace with scientific and technological advances, while delivering value for money for the NHS. A robust, evidence-based process and policy is in place to ensure testing continues to be available for all patients for whom it would be of clinical benefit. This is supported by a horizon scanning process and fast track application system to ensure the Test Directory can respond quickly to emerging developments.
NHS GMS alliances
Seven NHS GMS alliances were established in December 2020. An NHS GMS alliance is a collective made up of a small number of key NHS providers with an recognised track record and expertise in genomics working in partnerships to support the strategic systematic embedding of genomic medicine in end-to-end clinical pathways and clinical specialities for a given population. They drive this embedding across all providers within their geography from primary and community care to secondary and tertiary care.
Each NHS GMS alliance is funded by NHS England to deliver national and local transformation projects that for example support piloting new genomic technologies, such as RNA sequencing technology, support implementation of new pathways, such as the introduction of comprehensive genomic testing for sudden cardiac death or support workforce transformation, for example in nursing and midwifery. Governance arrangements are in place reflective of their geographies and with aims and objectives to drive system leadership.
The infrastructure includes a clinical director, research director, programme and project management support, communications and patient involvement and a multi-disciplinary clinical leadership to secure input from a range of professions including clinical leads for different areas such as cancer, nursing, midwifery and allied healthcare professionals and pharmacy.
To support implementation, each NHS GMS alliance has an alliance network to facilitate engagement with all other NHS providers and organisations across their geography, including, primary care networks, cancer alliances, pathology networks, ICSs, academic health science networks and academia.
Clinical genomics service
NHS England commissions seventeen NHS clinical genomic services (NHS CGSs), including genetic medical consultants, specialist trainees and genetic counsellors. They deliver a comprehensive clinical genomic and counselling service that directs the diagnosis, risk assessment and lifelong clinical management of patients of all ages and their families who have, or are at risk of having, a rare genetic or genomic condition, including, inherited cancer.
Each NHS CGS is responsible for a defined geographical area, accountable to its host NHS trust and, through them, to NHS England. Clinicians are usually clinical geneticists or genetic counsellors, but some services also have specialist nurses and family history coordinators.
To support the embedding of genomics across specialties each NHS CGS is expected to work with and upskill other clinical services in genomics, for example cardiovascular disease, neurology, renal, through education, training and support to embed genomics in end-to-end patient pathways.
The Clinical genomics service specification defines the standards of care expected from NHS England. An updated Genomics clinical service specification will be implemented from April 2023 that will be more reflective of the current requirements of the clinical genomics service and aims of the NHS GMS and include defined performance and quality metrics.
NHS GMS Research Collaborative
The NHS GMS Research Collaborative was established to provide a systematic approach to evidence generation, research, innovation and discovery for genomics in the NHS and advance clinical care for patient and societal benefit through the NHS GMS structures. The NHS GMS Research Collaborative is led by NHS England, Genomics England, the NIHR and partners in the NHS GMS including the NHS genomic laboratory hub and NHS GMS alliance representatives.
The NHS GMS Research Collaborative aims to facilitate genomic research with a focus on academic research projects; data and tooling infrastructure; exploratory clinical studies; clinical trials support; industry and biotech research and development; review and validation of emerging technology and diagnostic discovery. The outcomes from the research and evidence generated is then shared directly with NHS to support rapid adoption of research into NHS clinical practice to improve services for patients.
Genomics England
In a number of areas, the NHS works with Genomics England – a company established and wholly owned by the Department of Social Care in 2013 to deliver the 100,000 Genomes Project. Genomics England provide key services to NHS England and to the NHS GMS, managed through a Master Service Agreement that underpins the delivery of whole genome sequencing in the NHS. This includes the provision of whole genome sequencing by Illumina, an IT system from ordering to reporting (referred to as NGIS) and bioinformatic analysis. NHS England also works with Genomics England on a national trusted research environment, the National Genomic Research Library and a number of proof of concept studies outlined in this document.
Health Education England Genomics Education Programme
Health Education England and specifically the Genomics Education Programme support the upskilling of the multi-professional workforce in genomics. This include through the development of resources, educational materials and best practice sharing to increase education and training.
The Academy of Medical Royal Colleges
The Academy of Medical Royal Colleges – through the Genomics Professional Partnerships Group – provide clinical leadership across all medical royal colleges and with other professional groups within the multi-professional workforce to support the embedding of genomics across clinical specialties.
Health system partners
There are many health system partners the NHS works with to support the delivery of services, for example NHS Blood and Blood Transport, who are responsible for the collection and transportation of genomic samples from NHS genomic laboratory hubs to sequencing partners. Similarly, the UK Health Security Agency works closely with the NHS on host pathogen sequencing initiatives.
The NHS also works with others including the Medicine and Healthcare Products Regulatory Agency to understand the regulatory elements of the NHS GMS and the National Institute for Health and Care Excellence to align on horizon scanning activities and to ensure the adoption of new testing into the NHS when the evidence shows benefit.
Academia, researchers and industry
To ensure that the NHS continues to align innovation and a clinical service, the NHS GMS brings together academia, research and industry inclusive of NIHR to support innovation and the development of new technologies and treatments as well, driving research endeavours through a dynamic, diverse and inclusive system through the NHS GMS Research Collaborative.
Appendix 2 – summary of commitments
0-1 year commitments
| Priority | Commitment |
| Co-creating services, infrastructure and an operating model with patients and the public | The NHS will continue to work at a national and regional level to raise awareness of genomics, including the benefits and challenges, and support ongoing and transparent public dialogue on issues relating to genomics. |
|
Developing a sustainable infrastructure across testing, clinical services and research and innovation | The NHS genomic laboratory hubs, NHS GMS alliances and clinical genomics services will establish integrated NHS GMS governance boards with appropriate partnerships and leadership to drive forward the embedding of genomics into the wider NHS. |
| Building greater clinical and professional leadership, and developing the capacity and capability of the workforce | Over the next year, NHS England and the Health Education England Genomics Education Programme are working together to develop a Genomic Training Academy. |
| Developing an interoperable informatic and data infrastructure that enables the NHS to use and share genomic data appropriately to improve patient care | Over the next year, the NHS will develop shared data standards. During 2023, the NHS working with partners such as Genomics England will publish a genomics informatics implementation plan. |
| Enriching existing and developing new NHS GMS relationships to support innovation and the generation of evidence for adoption and improvements in health and care | NHS England will, as part of the evolving NHS GMS alliance infrastructure, establish ‘NHS genomic networks of excellence’. |
1-3 year commitments
| Priority | Commitment |
| Co-creating services, infrastructure and an operating model with patients and the public | An NHS GMS Ethics Advisory Board will be established. |
| Developing a sustainable infrastructure across testing, clinical services and research and innovation | The NHS will expand and maximise the use of the NHS diagnostic infrastructure for genomic testing. NHS England will consider further delegation to ICSs in the context of its impact on the delivery of genomic services for patients. |
| Building greater clinical and professional leadership, and developing the capacity and capability of the workforce | The NHS will continue to work with multi-professional groups to deliver the upskilling of the workforce and drive the change in practice needed. The NHS will explore opportunities to recruit and retain specialist and other key staff. |
| Developing national and international collaborations and partnerships | The NHS will continue to share learning and best practice with national and international partners and align to and inform international standards. NHS England will continue to work strategically across government and with other countries of the UK to explore and support opportunities for UK wide approaches. |
| Systematically introducing new clinical indications for genomic testing and embedding comprehensive genomic testing within end-to-end clinical pathways | The NHS genomic laboratory hubs, NHS GMS alliances and clinical genomic services will continue to transform clinical pathways and service models to embed genomics. The NHS will drive equity in access to genomic testing. |
| Driving the use of precision treatments and optimising the use of medicines through genomics | The NHS will work with partners to ensure that prescribing decisions are informed by genomic testing where appropriate. NHS England will review the evidence from pilot projects currently being undertaken in the NHS GMS alliances, alongside evidence generated by other programmes and organisations, to inform potential wider implementation. The NHS will drive equity in access to clinical trials by aligning clinical trial targets with standard of care NHS testing. The NHS will work with the horizon scanning function that includes Medicines and Healthcare Products Regulatory Agency (MHRA), the National Institute for Health and Care Excellence (NICE) and the Accelerated Access Collaborative. |
| Enabling the rapid evaluation and adoption of affordable, efficient, and innovative genomic technologies | The NHS will evolve the genomic testing strategy to develop an integrated diagnostic model and a multi-modal multi-omics testing approach. The NHS will explore the future model of delivery for whole genome sequencing in the NHS considering requirements for rapid whole genome sequencing to reduce the time to return results and inform clinical care. |
| Putting the NHS at the forefront of using genomic data alongside other health data to drive health improvements for individuals and populations | The NHS working with partners will explore the potential benefits of developing a genomic healthcare record. The NHS will review the evidence generated by Genomics England’s multi-modal diagnostic initiative and explore the further development of integrated reports. The NHS will expand the use of NHS generated genomic data to support approved research. |
| Enabling the NHS to use cutting-edge analytical tools and up to date variant databases to maximise diagnosis, access to precision medicine and efficiency | The NHS will work with partners, including Genomics England, to enhance a gene agnostic bioinformatics pipeline into appropriate clinical indications for whole genome sequencing. The NHS will work with partners, including Genomics England to evaluate the potential for the clinical implementation of artificial intelligence (AI) and machine learning in genomics. |
| Enabling patients to make informed choices regarding the use of their data for research and innovation | The NHS will work with patients, the public and key partners to evolve the patient choice framework. |
| Ensuring ongoing alignment with clinical trials and national life sciences projects and supporting the growth of life sciences in the UK | The NHS will explore new opportunities to work with industry to signal the needs of the NHS GMS. |
3-5 year commitment
| Building greater clinical and professional leadership, and developing the capacity and capability of the workforce | The NHS will explore the future training and development model with academia and industry. |
| Systematically introducing new clinical indications for genomic testing and embedding comprehensive genomic testing within end-to-end clinical pathways | The NHS will explore the utility of genomic testing to support population screening for cancer. |
| Enabling the NHS to use cutting-edge analytical tools and up to date variant databases to maximise diagnosis, access to precision medicine and efficiency | The NHS will work with partners to understand what is required to store and analyse NHS data from multiple genomic analyses. |
Ensuring ongoing alignment with clinical trials and national life sciences projects and supporting the growth of life sciences in the UK | The NHS will contribute to and review the evidence generated from key research initiatives to inform any future decisions regarding commissioning of services. |

