1. Purpose
Biologics offer a new treatment for chronic obstructive pulmonary disease (COPD) patients with frequent exacerbations, elevated eosinophil counts, and ongoing symptoms despite optimised care.
With dupilumab receiving a positive NICE recommendation in March 2026 and further biologic therapies expected, we can reduce exacerbation-related harm and improve COPD outcomes.
The purpose of this guidance is to:
- provide integrated care board (ICB) commissioners, clinical and service leads with a robust evidence base for the use of COPD biologics and the benefits for patients and services
- provide cost and health benefit information to help create business cases for setting up services that support the use of COPD biologics and ensure rapid and equitable access across England
- provide ready-to-use content that local teams can copy into business cases, outlining the case for change and how access to biologics aligns to key strategic priorities
We consulted stakeholders across all 7 regions and reviewed existing evidence and guidelines to develop a recommended clinical pathway for COPD biologics. The model pathway, in Appendix A, supports ICBs design and deliver the most appropriate local pathway and understand the potential cost and health impacts.
See the accompanying COPD commissioning strategies and resources publication for more information about recommendations for frontline care, service transformation and population health approaches.
2. Case for change
COPD costs the NHS around £2 billion a year and is the second leading cause of hospital admissions, placing significant strain on services, particularly during winter, and disproportionately impacting deprived communities. Exacerbations account for 45% of total COPD spend.
ICBs must ensure funding is available for the provision of new biologic medicines recommended by NICE within 90 days of publication of the technology appraisal (unless NICE explicitly states otherwise in the guidance) given their impact on improving patient outcomes and reducing exacerbations as demonstrated in the clinical trials (see under research evidence section below for links to the two trial outcomes).
Patient experience and outcomes
COPD has a major impact on quality of life, morbidity and mortality. In 2021, it was the 4th leading cause of death worldwide and the 4th leading cause of disability-adjusted life years (DALYs) lost in the UK.
Each DALY represents 1 year of full health lost. In the UK, COPD accounts for 1,192 DALYs lost per 100,000 people.
73% of COPD patients report being unable to work at some point due to breathlessness, creating financial pressures that are likely to impact access to care, heating, and overall wellbeing.
As well as having COPD, 74% of patients were found to have comorbidities of which 44% were musculoskeletal and 25% were mental health conditions.
COPD is also associated with significantly increased risks for other conditions and events; lung cancer risk is 5 times higher as is the risk of myocardial infarction following a community managed exacerbation.
Research evidence
The biologic treatments being developed for COPD have the potential to significantly reduce the rate of exacerbations – the main outcome measured in clinical trials.
Dupilumab reduced annual exacerbations by 30% and 34% in 2 trials looking at its use in COPD and is the first biologic to receive a positive NICE Technology Appraisal (TA).
Other biologics are expected to be considered for a TA as they are demonstrating significant impacts on exacerbation rates in late-stage trials.
The TA process for mepolizumab is underway, and in its phase 3 MATINEE trial showed a statistically significant reduction in annualised rate of moderate and severe exacerbations compared to placebo, with a reduction in annual exacerbations of 21%. It also showed a 35% reduction in annual exacerbations that would have resulted in an A&E visit, hospitalisation or both.
Healthcare inequalities
COPD is 1 of the 5 priority areas (for adults) in the Core20PLUS5 approach. There are strong links between deprivation and increased emergency healthcare use and costs.
There is a strong association between smoking and deprivation, with 90% of COPD diagnoses associated with smoking. People living in the most deprived areas of England are more than twice as likely to smoke as those in the least deprived areas.
Age-standardised COPD mortality rates are the highest in deprived areas and in men, particularly Bangladeshi men.
COPD may also drive people into deprivation; 31% of people with COPD report they had to give up work early due to their lung condition, and 52% of COPD patients have a household income below £20,000 a year.
Other protected characteristics also need to be considered, including sexual orientation and gender reassignment as those within the LGTBTQ+ community have a higher reported prevalence of COPD.
Efforts to proactively identify and target specific populations, should start with these at-risk groups in their local population.
3. Strategic fit
10 Year Health Plan for England
One of 3 shifts in the 10 Year Health Plan is moving from sickness to prevention. It also highlights the role of new medicines in reducing demand on the health services.
Adopting COPD biologics supports this priority mission by significantly reducing exacerbations associated with A&E attendances and hospitalisations.
The plan also highlights the importance of the NHS being “deliberate in its approach to innovative medicines from horizon scanning to implementation, so that NHS patients can rapidly benefit from game-changing treatments.” The plan also indicates a shift to a more proactive approach to introducing new medicines, to facilitate early and rapid adoption rather than traditional slower adoption curves.
As care shifts in line with the plan from hospital to community, COPD biologics can be delivered at home, supported by a virtual a hospital assessment.
Life Sciences Sector Plan
The Life Sciences Sector Plan includes the target that, by 2030, the UK will be 1 of the top 3 countries in Europe for rapid patient access to medicines and MedTech.
This includes interventions that support access to clinically and cost-effective innovations, such as plans for a Single National Formulary (SNF) to reduce unwarranted variation and delays to patients accessing treatments.
Urgent and emergency care plan
Clinical trials show COPD biologics reduce exacerbations, particularly those that lead to hospitalisations. Their use therefore supports the urgent and emergency care plan by reducing the burden on services such as ambulances, A&E and hospitals (if admitted), including intensive care services for severe COPD exacerbations. The rate of exacerbations is particularly pronounced during winter when services experience the greatest demands.
4. Expected system impact
The primary finding from the clinical trials for the COPD biologics is reduced annual exacerbations by 30% (BOREAS trial) and 34% (NOTUS trial). In the BOREAS trial, in addition to reduced exacerbations, dupilumab was associated with other benefits such as:
| Benefit observed | % reduction or improvement | Impact |
|---|---|---|
|
Patient reported outcomes (measured by St George’s Respiratory Questionnaire) total score improvement of at least 4 points at week 52 |
19% improvement |
There is a significant correlation between improved St George’s Respiratory Questionnaire score and lower COPD exacerbation risk. |
|
Reduction in the severity of symptoms (measured by Evaluating Respiratory Symptoms in COPD). |
69% reduction |
Likely there is a correlation between improved Evaluating Respiratory Symptoms in COPD score and lower COPD exacerbation risk. |
|
Increased lung function (forced expiratory volume in 1 second) |
108% improvement in prebronchodilator forced expiratory volume in 1 second |
There is a significant correlation between improved forced expiratory volume in 1 second and lower COPD exacerbation risk. |
A longer-term study, over 7 years looked at the benefits of dupilumab in a multicentre cohort study and found the following wider systems benefits:
| Benefit observed | % reduction or improvement | System impact |
|---|---|---|
|
Lower all-cause mortality |
47% reduction |
Supports respiratory programme priority to reduce mortality, particularly avoidable deaths. |
|
Fewer emergency department visits |
22% reduction |
Supports the Urgent and Emergency Care Plan objective to reduce demand for urgent care. |
|
Reduced risk of respiratory infections such as pneumonia |
35% reduction |
Supports the Urgent and Emergency Care Plan objective to reduce demand for urgent care. |
|
Reduced risk of COPD-relevant comorbidities: heart-failure |
31% reduction |
Supports the Urgent and Emergency Care Plan objective to reduce demand for urgent care. |
|
Reduced risk of COPD-relevant comorbidities: new onset anxiety |
30% reduction |
Reduces pressures on general practice and mental health services. |
In addition, patient reported benefits have been observed, including improved quality of life over 6 to 12 months of continued biologic therapy. These findings suggest an overall positive system impact with potential reduced need for healthcare services to manage exacerbations (in the community or in hospital) or respiratory infections.
5. Service model
Specific service models will be selected at trust and ICB level to meet the specific needs of the local population. The examples provided illustrate innovative care models that can support the design, costing and implementation of COPD biologic services.
This summary table below shows the model clinical pathway (full version of the pathway is available in Appendix A). It is designed to offer flexibility for local adaption, with suggestions (in square brackets) based on previous stakeholder consensus. The pathway for biologic initiation starts at week 52 because it is assumed patients are diagnosed with COPD and fully optimised before being referred for biologic treatment over a maximum of 52 weeks.
| Week | Pathway step | Who | Dose(s) | Notes |
|---|---|---|---|---|
|
52 |
Blood test |
Appropriate health professional |
N/A |
Eosinophil count, ideally historic trends should be available |
|
52 |
Decision to initiate |
Respiratory specialist in secondary care or integrated community setting |
N/A | Patient identified in line with NICE technology appraisal criteria [Initiation led by a respiratory specialist in secondary care or integrated community setting] |
|
56 to 58 |
Supervised administration of first doses |
Home care service |
1 and 2 | Carried out by a homecare nurse who will teach and supervise 1st and 2nd doses in a monitored setting for anaphylaxis risk. Dupilumab is administered fortnightly but other biologics will vary between 2 and 8 weeks. |
|
60 to 81 |
Patient self -administered doses |
Patient |
3 to 13 |
Where a patient is unable to self-administer home care support will need to be provided |
|
82 |
6-month review |
Appropriate specialist |
N/A |
May also be patient initiated follow up. [Can be led by a nurse remotely] Depending on outcome of review, patient may require additional support or reviews. |
|
82 to ongoing subject to reviews |
Patient self-administered doses |
Patient |
14 to 26 |
Where a patient is unable to self-administer home care support will need to be provided |
|
108 |
12-month review |
Appropriate specialist |
N/A |
Efficacy assessment, measure will be number of exacerbations. [Can be remote]. Biologic will continue if evident they are responding to treatment. |
|
13 |
6-month review |
Appropriate specialist | 27 to 39 |
If patient is switching between biologics. May also be beneficial for other patients who may not initiate follow up. [Can be remote.] |
|
160 |
12-month review |
Appropriate specialist |
40 to 52 |
Annual efficacy assessment. [Can be remote.] |
6. Finance and activity impact
NICE have developed a Resource Impact Assessment (RIA) as part of the resources available following the positive appraisal for dupilumab. This provides systems with a framework to estimate the following:
- local eligible populations for dupilumab
- the change in pathway or service delivery cost for the people receiving biologics
- costs per exacerbation
- potential savings from a reduction in exacerbations
In addition, the RIA will include medicine costs, including any associated homecare costs. There will be functionality to tailor the costs and any potential savings to the local population size and proposed service model. There will also be functionality to change the workforce grade and type according to the local service model.
7. Evaluation and monitoring
Suggested monitoring for local business cases to support biologic services
As part of measuring impact and outcomes for local business cases, some example metrics are suggested.
Financial:
- tracking cost savings (from a reduction in activity from reduced exacerbations) and return on investment
- workforce costs (staff required to deliver any additional services for COPD biologics)
- medication costs (actual cost of medication per dose, administration costs)
Operational:
- reduction in COPD exacerbations clinical capacity created (number of additional clinical outpatient slots)
Biologic uptake:
- number of patients initiated onto COPD biologics
Suggested additional monitoring for broader COPD clinical outcomes
Insights from the National Respiratory Audit Programme (NRAP) can be used to identify regional variability in COPD care delivery, regional benchmarking of service performance, and inform strategic commissioning decisions for biologics. NRAP data covers:
- hospital admissions
- pulmonary rehabilitation
- primary care
It can support population health management, highlight unmet needs, and guide equitable service development.
8. Appendix A – Model pathway
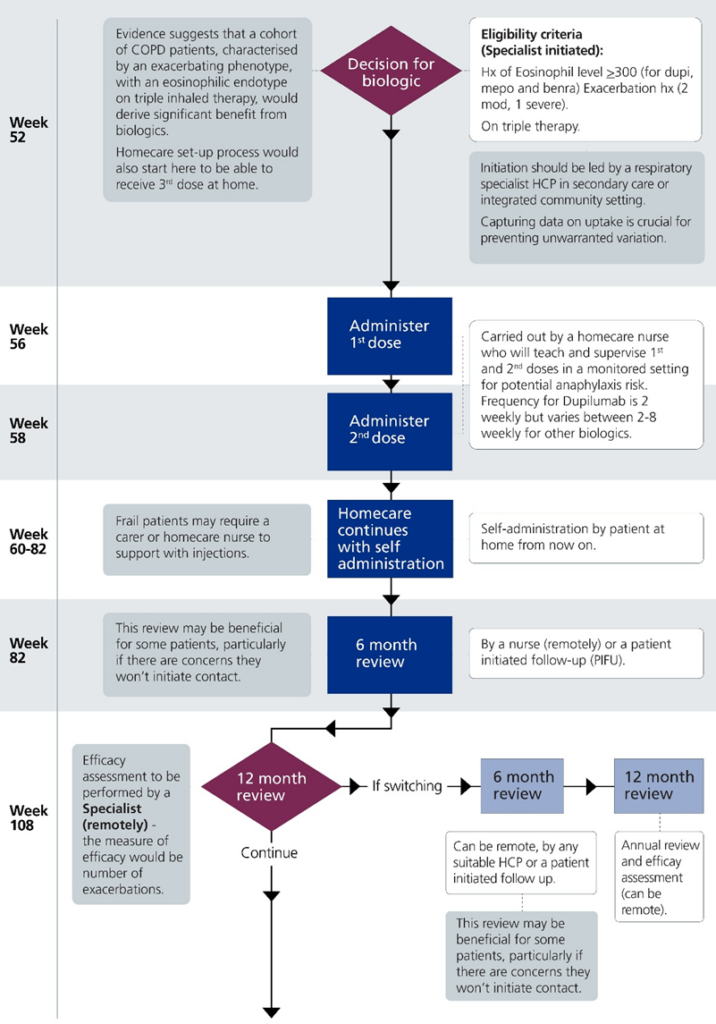
This model outlines a patient pathway for initiating and monitoring biologic treatment for chronic obstructive pulmonary disease (COPD) over approximately 1 year. The pathway for biologic initiation starts at week 52 because it is assumed patients are diagnosed with COPD and fully optimised before being referred for biologic treatment over a maximum of 52 weeks.
Publication reference: PRN02144_ii

