Organisation objective
- NHS Long Term Plan
- Governance
Working with people and communities
- Recruited patient and public voice (PPV)
- Consultation/engagement
- Quantitative data and insight, for example national surveys
- Partnership working with voluntary, community and social enterprise organisations
The national dementia team aims to utilise patient and carer voices to inform policy making. This is achieved via regular engagement with voluntary sector organisations including Dementia UK, Alzheimer’s Research UK, and Alzheimer’s Society. These organisations are also represented in the series of roundtables on dementia organised by NHS England, and there are efforts underway to include smaller voluntary sector organisations. National policy on dementia is also informed via the National Audit of Dementia (NAD) that is commissioned by NHS England. The national dementia team works closely with DHSC to ensure outputs from public consultations pertinent to dementia, such as the recent consultation for Major Conditions Strategy, are used to shape the programme of work.
Proactive stakeholder engagement is also a key part of the national Alzheimer’s Disease Modifying Treatments (DMT) programme team’s role and regular meetings are held with charities with a direct interest in dementia, including Alzheimer’s Research UK and the Alzheimer’s Society. This enables the programme team to learn and build upon the charities’ insights and the extensive knowledge and experience that patients and their carers bring, and to identify opportunities for collaboration as we prepare for the potential roll out of new treatments, for example working together on public campaigns to provide helpful, timely and accurate information on the treatments themselves, and on decisions being made on treatment access in the NHS. PPV partners are being recruited to support specific national programme activities, for example joining the group that will co-produce a template clinical access policy, one of the ‘do once’ activities being undertaken by the national programme, alongside ICBs, to support local planning and implementation. The programme has also been able to access NHS England’s Lived Experience network, through the Community Health Services team, to ensure that public communications to date are accessible to all; for example through inclusion of an easy-read summary of our December 2023 blog.
Executive summary
This paper provides an overview of the national dementia programme and specifically updates on progress on work to improve rates of dementia diagnosis.
The paper also covers the preparations underway to plan for, and support implementation of, potential new disease modifying treatments for mild cognitive impairment associated with Alzheimer’s Disease and / or mild Alzheimer’s Disease. This nationally led programme is enabling a more proactive and targeted approach to building system awareness and readiness, including more formal engagement with a range of stakeholders, whilst key regulatory decisions are awaited.
Background
1. Rates of dementia are growing; dementias (including those caused by Alzheimer’s disease) are already the biggest driver of mortality after coronavirus in England, and place significant burden across NHS services. For example:
- 25% of acute hospital beds are occupied by people with dementia
- People with dementia stay in hospital twice as long as other people over age 65
- 90% of people with dementia found admission to hospital frightening and confusing
- 43% of people with dementia in hospital were due to urinary tract and chest infections (treatable in the community)
- 25% of people with dementia living in their own homes were admitted to hospital with a potentially treatable condition over a one-year period
2. Alzheimer’s disease is the most common sub-type of dementia diagnosed. For December 2023 it was reported that Alzheimer’s disease represented 44.6% of all dementia diagnoses.
3. A timely diagnosis of dementia is vital. It enables a person to access the advice, information, care, and support that can help them (and their carers) to live well with the condition.
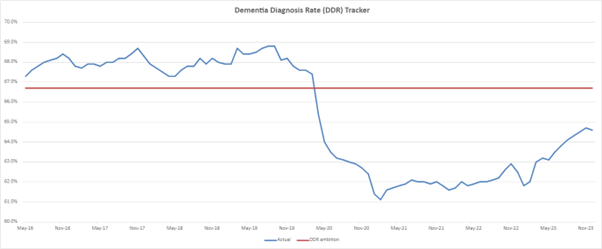
4. The national ambition for dementia, for at least two thirds (66.7%) of people with dementia to have received a formal diagnosis, was put in place in 2015.
5. Prior to the pandemic, the dementia diagnosis rate (DDR) had tracked above the ambition since 2016, however the pandemic had a significant impact on the numbers of people coming forward to primary care with dementia symptoms. In April 2020, the rate fell below the ambition.
6. In response to this, the December 2022 NHS Priorities and Operational Planning Guidance reinstated the focus on recovery of the DDR. We are now seeing a consistent, but gradual increase in the diagnosis rate. As of December 2023, the rate was 64.6%. With recent media focus on potential disease modifying treatments, we are also seeing an increase in referral rates to memory assessment services (MAS). From April to December 2023 there were 28,834 new referrals to memory clinics, a 17% increase compared to the same period in 2022.
7. At a regional level, London, the North West, and North East and Yorkshire regions met or exceeded the national ambition of 66.7% for December 2023. All other regions remain below this, with the South West region more than six percentage points below. However, based on recent trajectory there is confidence that the ambition of diagnosing 66.7% of people over 65 will be met in 2024.
National dementia programme priorities
8. The Dementia Policy Team is supporting a range of activities to aid recovery of the DDR.
9. NHS England is funding DiADeM (Diagnosing Advanced Dementia Mandate) tool pilots within two trusts in each region (14 sites in total). Trusts are using the DiADeM tool to improve diagnosis of dementia in care homes and to create an alternative pathway for diagnosing dementia. Early evidence suggests that this is having a positive impact on the DDR, and as a result we are seeing organic expansion of the DiADeM initiative across services in England.
10. The DiADeM tool was developed by the Yorkshire and Humber Dementia Strategic Clinical Network and is supported by the Alzheimer’s Society. For those with advanced dementia, a referral to memory services may not be feasible or desirable and is likely to be distressing for the individual. However, they can still benefit from a formal diagnosis. A diagnosis may enable access to appropriate care to meet individual needs and prompts staff to consider mental capacity and deprivation of liberty issues where appropriate.
11. Given the level of inequalities known within dementia diagnosis, NHSE has commissioned the Office for Health Improvement and Disparities (OHID) to develop a resource to support investigation of the underlying variation in dementia diagnosis rates, including the assessment of underlying population characteristics such as rurality, ethnicity, and age. The aim of this work is to provide context for variation and enable targeted investigation and provision of support at a local level to enhance diagnosis rates.
12. NHS England also released a number of resources during Dementia Action Week 2023, to help narrow the health inequalities gap for people living with dementia from an ethnic minority background. These were promoted during various NHS England communication channels.
13. NHS England’s RightCare team is soon to publish the updated RightCare Dementia Scenario. This details optimal and sub-optimal approaches to supporting people living with dementia and their carers through the dementia well pathway (diagnosing well through to dying well). This supports planning and commissioning decisions. A model pathway based on data for each component of the dementia well pathway is also being developed.
14. In addition, NHS England’s Analytical team has developed a dashboard for management information purposes, supporting commissioners and providers of memory services with several metrics to track their local DDR, an additional dementia pathway metrics.
15. To have maximum impact across the NHS, and this is especially true when managing co-morbidities, it is critical that dementia is integrated into everything we do. This presents huge opportunities to support wider NHS England pressures by managing their health needs at the right time in the right place.
16. On that basis, NHS England has started a rolling programme of Dementia Roundtables with external stakeholders and senior executives from across NHS England. Key objectives to be met through this process are:
- Effective commitments that make a tangible difference to patient’s lives and NHS England objectives, based on robust evidence
- Integration of the approach to dementia within wider strategies
- Improved external communication of this agenda
Disease modifying treatments in development for early Alzheimer’s disease
17. Alzheimer’s is a progressive neurodegenerative disease associated with an abnormal build-up of toxic clumped proteins in and around the brain. Over time, as more parts of the brain are damaged, more symptoms develop and there is further decline, initially interfering with activities of daily living and ultimately resulting in total dependence on others. Alzheimer’s is therefore devastating for patients, their families and their carers, and in most cases there is currently no cure. It is a life limiting illness with significant economic and societal costs.
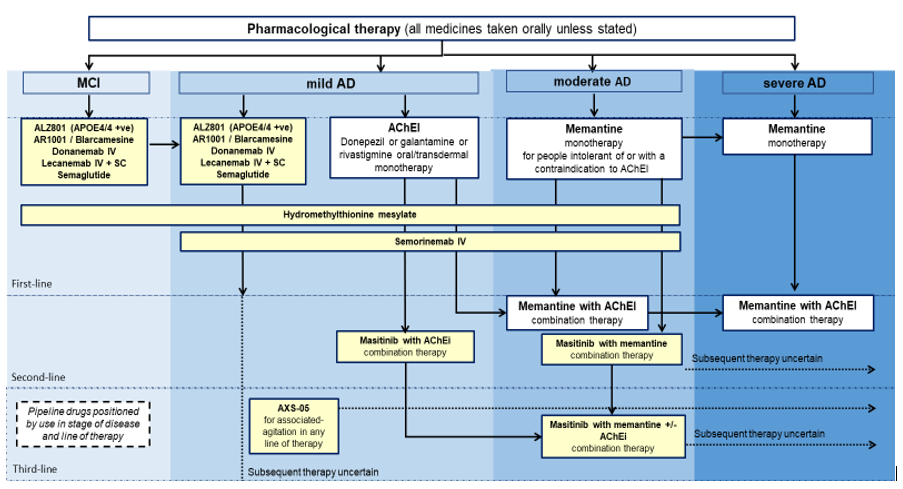
18. Currently licensed medicines offer support in the management of Alzheimer’s disease symptoms, but do not alter the course or duration of this devastating disease. Understandably, there is therefore significant excitement about the current pipeline of 28 potentially disease modifying treatments for Alzheimer’s disease in late-stage trials (please see Annex A for medicines in development for potential launch by 2027).
19. Of these, Eisai / Biogen’s lecanemab (brand name, Leqembi®) and Eli Lilly’s donanemab (brand name Kisunla®) are currently subject to UK licensing applications with the MHRA for use in the earliest stages of Alzheimer’s disease. NICE technology appraisals (TAs) for both products are also underway and may conclude by summer and autumn 2024, respectively. Lecanemab has so far been licensed in the United States, Japan and China.
20. Whilst there is understandably significant public interest in these potential ‘first in class’ treatments, the published trial results so far suggest a relatively modest (and possibly less than a ‘minimally clinically important’) benefit, with Alzheimer’s progression being slowed at the earliest stages, rather than the medicines either stopping or reversing decline, or offering a cure. It is important to recognise that it is possible for a new medicine to, appropriately, be given ‘breakthrough’ status whilst, at the same time, only delivering a limited clinical benefit.
21. There are also important safety considerations, with material rates of (potentially life threatening) brain swelling and brain bleeds reported.
22. Treatment costs are likely to be significant and the treatment regimen will also be intensive for individuals and their families, with frequent clinic visits for infusions, outpatient visits and regular MRI scans as part of safety monitoring, potentially continuing over several years. Further research is being undertaken to better understand the length of time for which treatment might offer benefit.
23. Given the uncertainties over materiality of benefit, safety and cost effectiveness, NHS adoption of these medicines will be determined, subject to licensing, through the usual NICE TA process. NICE has a number of potential recommendation options, including for routine adoption, for a period of access under a managed access agreement whilst additional data are collected, or for use only in research. A positive TA recommendation for routine adoption would place a statutory funding obligation on Integrated Care Boards (ICBs), as the main commissioners, and NHS England, as the responsible commissioner for PET-CT scanning and genetic testing, following publication.
24. Costs of implementation are currently estimated at between £500m and £1bn per year, with 50-60% of the total estimated cost relating to drug cost (with remaining costs relating to patient assessment, diagnosis and administering treatment). It should be noted that, ahead of an agreed ‘England’ price being available, indicative drug costs used in financial modelling have been based on publicly available information from the US. Actual costs will instead be subject to specific prices agreed for England, following a NICE cost-effectiveness evaluation. Total implementation cost will therefore be materially affected by drug prices agreed for England.
25. The very significant costs of making these treatments available to all eligible patients, together with the associated scale of implementation, mean that even with additional Government funding, a phased mobilisation, beyond the statutory 90-day funding requirement will be necessary.
Indicative diagnostic and treatment pathway
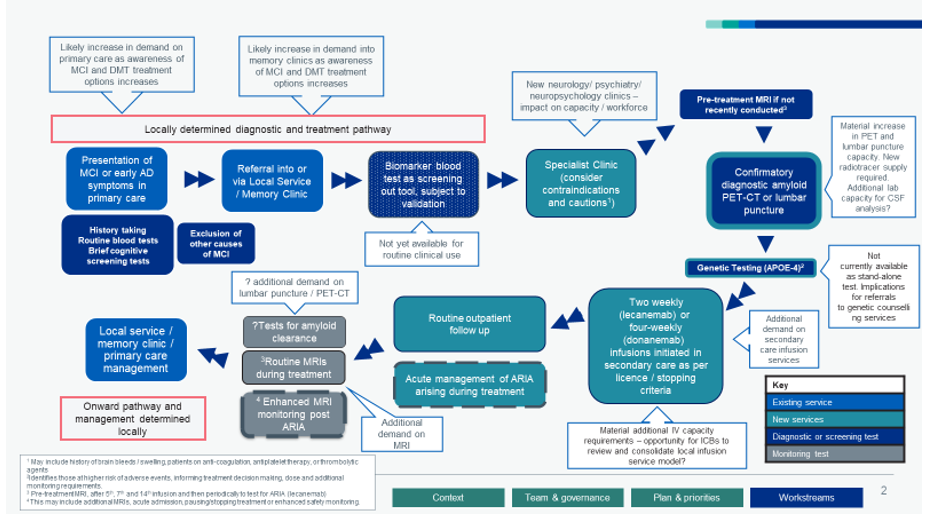
26. The diagnostic and treatment pathway will differ slightly depending on the disease modifying treatment concerned, and local ICB commissioning decisions, but will typically begin when an individual presents in primary care with early cognitive symptoms. Where it is thought that symptoms are persistent and potentially linked to Alzheimer’s disease, individuals will be referred on for more comprehensive cognitive and diagnostic testing under local clinic arrangements. To be eligible for treatment, patients will need to have a baseline MRI scan and then either a PET-CT scan or lumbar puncture that confirms the presence of beta-amyloid proteins in the brain, which are associated with the Alzheimer’s disease process. Individuals wishing to progress to treatment (and not contraindicated under the medicine’s licence) will then progress to fortnightly (lecanemab) or 4-weekly (donanemab) infusions, with accompanying scheduled MRI scans to monitor for potential adverse treatment effects.
27. Whilst not yet validated for front line clinical use, it is possible that biomarker-based blood tests will be available in the future to be used in the pathway as potential screening, diagnostic or monitoring tests. This means we should be cautious about driving a massive expansion, for example in amyloid PET-CT capacity, when this could become redundant in the longer term. Further blood-based biomarker research, and specifically achieving test validation for clinical use, are a key focus of the government’s Dementia Mission and recent NIHR research calls.
28. As early Alzheimer’s disease and / or mild cognitive impairment (MCI) associated with Alzheimer’s disease are not currently well recognised by the public nor consistently coded in NHS clinical practice, there are only very broad population estimates available to assist in planning. Estimated MCI and early Alzheimer’s population estimates range from 200,000 to 1.6 million. The estimated number of patients that might be determined eligible for disease modifying treatment by NICE ranges from 50,000 to 280,000.
Formalised Alzheimer’s DMTs Programme arrangements and activities
29. NHS England has established a programme team hosted within the specialised commissioning function, to co-ordinate the contributions of multiple NHS England teams into the planning and preparations for the potential introduction of these treatments. As a significant national and multi-directorate transformation programme, the programme steering group reports directly to the Strategy, Planning and Investment Committee.
30. Though ICBs will be the primary commissioners of the new treatment pathway, NHS England will be supporting ICBs in their rollout of approved treatments (as required) building on other rapid service mobilisations, such as the roll out of COVID-19 vaccines and treatments, and experience with more challenging service transformations such as the pioneering ‘find and treat’ approach to hepatitis C elimination, rapid rollout of personalised ‘CAR-T’ cancer treatments at highly specialised NHS centres, and commissioning of cutting-edge gene therapies like Libmeldy® for the treatment of metachromatic leukodystrophy.
31. Key activities being undertaken by the national programme whilst regulatory decisions are awaited include:
- A regular rhythm of meetings with national agencies and other key partners, including the medicines manufacturers, to share information and co-ordinate and collaborate on regulatory and other preparatory initiatives.
- Joint work with regional and ICB leadership teams to support local planning and awareness raising, and collaborate on a range of ‘do once’ activities such as specifying new service requirements, developing decision aids, and creating standardised eligibility criteria and treatment guidance. ICBs are the primary commissioners of the future diagnostic and treatment pathway and are therefore responsible for determining how treatments are planned and delivered locally.
- Articulation and refinement of the current and future diagnostic and treatment pathway to support engagement with clinicians, commissioners and patient groups. This includes deep dives in specific areas, for example determining best practice in the management of ARIA (brain swelling or bleeding as a potential side-effect of treatment) and introducing a new stand-alone APOE-4 genetic test and associated treatment advice (please see Annex B).
- Completion of detailed modelling and costing work to properly inform the early phase of the NICE appraisal and better understand the additional national and local diagnostic and treatment volume requirements at each stage in the care pathway (for example, how many additional infusion sessions should ICBs plan for each week).
- Development of a commercial strategy to maximise value over the medium to long term
- Working with charities to prepare messaging on topics including regulatory and TA processes and potential NHS access, benefits of treatment, risk profiles, and wider pathway design.
- Communication of remaining evidence gaps impacting on clinical policy formation or front-line clinical decision-making to inform the very welcome, material new research investments being made by NIHR, charities and the Dementia Mission.
- Work to build upon the well-established medicine horizon scanning process to better understand the equivalent development pipelines for blood-based biomarkers, digital cognitive testing and radiotracers (for PET-CT scanning).
32. The programme has identified a number of areas of work and analysis to support future planning and decision making, including:
- increasing understanding of the size of the potentially eligible population and the resulting number of patients who may progress through each stage of the diagnostic and treatment pathway;
- gaps in the clinical evidence, including published long-term outcome data;
- increasing understanding of cost pressures for NHS England and ICBs;
- increasing understanding of additional capacity and workforce requirements across a range of specialties;
- increasing understanding of medicine supply into the UK; and
- increasing understanding of mobilisation/lead times where key investment decisions need to be made following potential regulatory approval (such as commissioning and mobilising additional PET-CT capacity).
Annex A
Medicines for Alzheimer’s disease for potential launch 2024 to 2027
Annex B
Draft diagnostic and treatment pathway