1. Introduction
Patient initiated follow-up (PIFU) is key to personalising outpatient care and, by giving patients more control over when they receive care, can reduce unnecessary routine follow-up appointments and free up clinician time for more complex patients.
This guide outlines a PIFU model of care for patients with mild to moderate heart valve disease (HVD) or following valve intervention who require ongoing surveillance imaging but not necessarily frequent clinician review. It supplements the generic Implementing patient initiated follow-up guidance for local health and care systems.
Patients with HVD are commonly asymptomatic and often require surveillance imaging as part of their routine management. This ensures that any asymptomatic changes to the patient’s condition are identified and acted on in a timely manner by the heart valve team. A heart valve PIFU pathway can work effectively alongside a surveillance imaging pathway (predominantly echocardiography), by enabling the patient or their carer to self-manage their condition but also have the safety net of being able to contact the heart valve team if they develop concerning symptoms or have questions about their condition. These pathways are not appropriate for patients with severe valve disease or symptomatic disease.
2. Why use PIFU in heart valve disease?
The number of patients with HVD is predicted to double by 2046, and given the chronic nature of HVD, ageing population, improved access to diagnostics/screening and increasing percutaneous valve implantations, valve surveillance services risk being significantly impacted.
Heart valve services need to be structured according to patient need so all patients can be seen by the right experts at the right time. Over 70% of patients followed up in one NHS heart valve clinic had non-severe HVD or prosthetic valves (Leeds Teaching Hospitals audit data, 2021); this indicates that reducing the number of consultations for stable patients with mild to moderate HVD or following valve intervention will allow heart valve clinic resources to be directed to those with severe HVD who are at risk of heart failure and death (NICE guidance 2021). Patients with mild to moderate disease or following valvular intervention are usually asymptomatic for many years and may only need dedicated imaging (usually echocardiography, with results reviewed by a HVD specialist) at pre-specified intervals and not necessarily a consultation with a HVD specialist.
PIFU should never be used as an alternative to clinical discharge. Suitable patients should be discharged following routine trust guidelines.
3. Which patients could benefit?
Patients who are suitable for a heart valve PIFU pathway
- A patient who is aged 18 or over.
- A patient or carer who understands and/or accepts the meaning and principles of a PIFU pathway.
- Depending on local service needs and the expertise of the heart valve team, patients with the following conditions:
- bicuspid aortic valve with non-severe valve disease
- mitral valve prolapse with non-severe valve disease
- moderate mitral valve disease and moderate aortic regurgitation
- low to moderate aortic stenosis (Vmax <3.5mol/s with preserved left ventricular function): normally functioning prosthetic valves, bioprosthetic valves, mechanical heart valves, valve repair, percutaneous valvular intervention including TAVI and TEER.
Patients who are not suitable for a heart valve PIFU pathway
- Patients with severe valvular disease.
- Patients with valvular disease symptoms.
- Patients with left ventricular impairment.
- Patients with outstanding investigations or decisions still to be made regarding options for further care.
- Patients with complex HVD or who would benefit from regular review of their clinical status.
- Patients who do not have the knowledge, skills and confidence to manage their follow-up care.
- Patient who cannot easily contact the service (e.g. no access to telephone/ internet, language barriers).
- Patients deemed by their clinician to be unsuitable for valvular intervention (e.g. because of frailty and multiple co-morbidities). They should be discharged back to their GP.
4. Shared decision-making – being aware of risks and symptoms
The patient needs to understand the risks, benefits and any consequences of this before deciding, jointly with their clinician, whether PIFU is right for them. This understanding is achieved through a shared decision-making conversation between the clinician and patient and/or carer, covering:
- Why the patient is suitable for a heart valve PIFU pathway.
- The PIFU process, including:
- How and when to contact the heart valve PIFU helpline.
- Giving the patient or carer a leaflet that includes a contact phone number, is written in understandable language and signposts them to online information.
- Checking the patient and/or carer understands how the service works, including by encouraging them to ask questions.
- Emphasising that if the patient is placed on a PIFU pathway and they find it does not work for them, they can change their mind and revert to traditional follow-up.
- Comprehensive information and education about symptoms that may indicate progression or decompensation of HVD and the patient needs to be aware of, e.g. red flag symptoms, including of endocarditis (examples can be seen in the Appendix or patients can be signposted to online resources and third sector organisations).
- Appropriate education and support in condition self-management, including for endocarditis symptoms.
- The importance of good dental and skin hygiene.
- Lifestyle advice:
- pre-pregnancy planning for females of childbearing age
- sport and exercise
- dental antibiotic prophylaxis where applicable
- healthy lifestyle, including weight and smoking.
Shared decision-making conversations should be recorded in the medical records and communicated to the GP and other healthcare professionals involved in the patient’s care. Should a patient and/or carer accept joining a PIFU pathway, the clinician should also clearly record that the patient is now on a PIFU pathway on the trust clinical outcome form (COF).
5. Designing a PIFU model for a heart valve service
We recommend that organisations adopt a consistent approach for PIFU, but that this is tailored to work for the heart valve clinic and adapted to suit local needs.
A dedicated clinician or clinical team with competency in managing patients with HVD should have overall responsibility for the development of clinical guidance, risk stratification protocols and a standard operating procedure (SOP) for the implementation and delivery of PIFU within their service.
Heart valve services should take a standardised cardiac network-based approach to ensure equality of services. While patients on a PIFU pathway are generally managed by secondary care, this will depend on local expertise and service provision. Secondary care heart valve services provide overarching responsibility with close links to the local heart valve centre.
Design considerations
What does a patient on a PIFU pathway need to do when their circumstances change?
Patients on a PIFU pathway, alongside imaging surveillance pathway, should contact the HVD service if their health condition changes and be reviewed by their heart valve team. This review should include re-evaluation of whether a PIFU-led approach is still a suitable care option, with the clinician and patient together deciding if it is best for the patient to remain on or come off PIFU.
If a patient’s circumstances unrelated to HVD change, e.g. they become unwell from another clinical condition or there is a change in their personal circumstances such as a patient’s carer is no longer able to provide care, the patient or carer should contact the HVD service for further advice about the suitability of remaining on a PIFU pathway.
Target response times
Clinical job plans must allocate time to manage patient queries and administration tasks for patients on PIFU pathways. Some PIFU services have clinical staff running helplines, and others are run by administrative or secretarial staff who can manage administrative concerns and refer on any clinical problems.
- Patients with urgent problems should be evaluated within 2 weeks.
- The clinician in charge of the patient (referred to as the ‘responsible clinician’) will decide if the patient requires any tests and/or a consultation and the timescales within which these should occur.
- A robust audit process should be in place to clinically assess and monitor the turnaround time between patient contact and clinical review.
- Target response time delays or lack of clinic capacity for patients needing to be seen should be raised urgently with departmental management according to agreed local protocol.
Managing HVD patients on a PIFU pathway alongside a surveillance pathway
- The responsible clinician determines if the patient is suitable for a PIFU pathway, with the patient and/or carer then deciding whether to accept placement on a PIFU pathway after considering the risks and benefits of PIFU as explained by their responsible clinician.
- The patient and/or carer have the option to decline PIFU if it does not suit their needs and should be reassured that their decision will not affect their ongoing care.
- The PIFU pathway should support patient self-management and enable direct patient/carer access back to the heart valve service should the patient develop any of the concerning symptoms their heart valve team has advised them to look out for.
- Patient tracking using the local patient administration system should enable the responsible clinician and/or heart valve team to identify which patients are on a heart valve PIFU pathway.
- We recommend that there should be a PIFU HVD helpline for patients and/or carers to call should they have questions or develop symptoms. Its organisation and day-to-day management will depend on local resources. Ideally, this should be a dedicated phone number (and possibly also email address) with direct access to a clinician, although it could be the number of the valve clinic’s admin team or secretary. The helpline could also be run by other members of the heart valve team such as heart valve nurses or clinical scientists. Whatever its arrangement, the helpline should be the patient or carer’s route to rapid decision-making and review.
- Any PIFU response time delays should be communicated to the patient and/or carer and, if there are safety concerns about the patient’s condition, an alternative plan should be made and communicated to the patient and/or carer.
- The Appendix has flowcharts for how a patient could be managed on an PIFU pathway (see Appendix 1).
- A clinician should be responsible for regular evaluation of the PIFU service, development of patient education material, setting up policies and protocols, and investigating any clinical incidents/complaints.
- We recommend a dedicated PIFU co-ordinator oversees the pathways and ensures the smooth running of the service, with appropriate administrative support provided.
Managing HVD patients on a surveillance imaging pathway alongside a PIFU pathway
- The surveillance imaging pathway ensures that a patient is imaged (predominantly echocardiography) at predetermined time points, under the overarching care of the dedicated heart valve service. This allows the clinician to monitor the patient and treat them in a timely manner should abnormalities be detected.
- The trust needs to decide the local procedures for regular surveillance imaging requests, who books repeat surveillance imaging and who acts on imaging results and communicates the results to the patient. Imaging requests and review of results should remain the responsibility of a heart valve specialist in a dedicated heart valve clinic.
- Local SOPs need to define the imaging follow-up interval for specific valve conditions included in the surveillance imaging pathway. Imaging type, intervals and/or clinical triggers also need to be decided and adopted according to individual service need; see Appendix 1 for an example SOP.
- Procedures and policies should be in place to ensure that surveillance imaging occurs at regular intervals, and if it does not (due to cancellations or Did Not Attends, for example) there are protocols to ensure the patient is not lost in the system.
- A clinician should be responsible for regularly evaluating the surveillance imaging pathways, development of patient education material, setting up policies and protocols, and investigating any clinical incidents/complaints.
- We recommend appropriate administrative support is provided, and a dedicated surveillance imaging co-ordinator oversees the pathways and ensures the smooth running of the service.
- The imaging service is the backbone of HVD surveillance and needs to be appropriately resourced, continually evaluated and workforce planning regularly reviewed. Dedicated heart valve imaging templates may streamline the service depending on local need and expertise.
6. Health inequalities in PIFU
To narrow geographical inequalities in care of patients with HVD:
- Systems should develop a cardiac network-based heart valve PIFU pathway, making it possible to standardise management across a region and potentially improve overall quality of care.
- Suitable non-severe HVD patients, where they agree, should be placed on a PIFU pathway to free up the capacity to see more patients with severe and/or complex valve disease in a timely fashion, focusing expert care and resources on those most in need.
- Heart valve services should undertake an equalities and health inequalities impact assessment (EHIA) – possibly at cardiac network level and then refined by individual local services.
7. Engagement with patients about PIFU
We recommend involving patients and patient groups in the development, implementation and communication of any local PIFU guidance, SOPs or patient information leaflets, to ensure the patient and carer voice is present and patient views are represented in these materials. NHS England has produced guidance on patient and public participation for all commissioners of health services, both within NHS England and ICBs.
Obtaining regular feedback from patients and carers about their experience of PIFU care will inform the heart valve clinic how it needs to adapt to better meet patient needs, where possible.
From listening and talking to patients and/or carers about PIFU, we know the elements of their care that matter to them the most are:
- Discussions around PIFU should start at the point of diagnosis as PIFU is part of the whole treatment and care pathway. Discussing PIFU early means that patients have plenty of time to think, ask questions and raise concerns as they move through their treatment.
- Patients can suffer with information overload if they are given too much complex information in one consultation. Having more than one conversation about PIFU and the provision of high-quality patient information gives the patient time to absorb information and reflect on what it means to them.
- Careful consideration should be given to the significant emotional impact of a HVD diagnosis on a patient. Patients will need to be given time with a clinician to ask questions about their valve condition and understand its implications; some may require multiple consultations. PIFU should only start once a patient and/or carer feels satisfied that they have been given enough time for all of their questions to be answered.
- The heart valve PIFU service needs to be well co-ordinated and clearly explained to the patient and/or carer.
8. Evaluating PIFU in heart valve services
A heart valve PIFU service is likely to be a new concept of care in many centres, and like any new service, it should be evaluated on a regular basis to ensure that it is safe, effective, and equitable.
Outcome measures for the service may include:
- audit to assess adherence of patient pathways to recommended imaging intervals
- GP feedback
- patient/carer feedback
- adverse outcomes on the PIFU pathway
- impact of PIFU on heart valve waiting lists
- number of calls to helpline and outcome of these calls
- number of patients leaving PIFU pathway and reasons for this.
9. Technology
- New technologies that allow remote monitoring, assist rapid diagnosis and support electronic communication may also support a PIFU pathway.
- Remote monitoring technologies are used to monitor for early signs of cardiac decompensation in patients with heart failure; these may also be beneficial for patients with HVD.
- Cardiac ultrasound using artificial intelligence, though not yet in widespread use, has the potential to improve the efficiency of surveillance in HVD.
- Electronic patient portals through which patients can communicate directly and virtually with the clinical team may further empower the patient and facilitate the PIFU pathway.
Case study: Leeds Teaching Hospitals NHS Trust
The service
The valve clinic at Leeds Teaching Hospitals NHS Trust operates an inclusive model, offering care for the whole range of HVDs. It cares for more than 2,000 patients with HVD, with the number of patients having increased significantly over the past 5 years; currently the clinic receives over 30 referrals a week.
The problem
In the previous clinic model patients with all types of HVD, including non-severe HVD, prosthetic valves, complex severe valve disease and severe symptomatic valve disease, were seen by a doctor in clinic. As a result clinic waits were over 10 weeks for symptomatic patients with severe HVD due to over-booked clinics and services unable to accommodate urgent assessments or manage referrals in a timely manner.
The results of a valve audit of the types of patients with HVD in the valve clinic were:
- moderate (non-severe) HVD 45%
- prosthetic valves 25%
- severe HVD 25%.
Developing PIFU to improve patient care
The HVD service restructured the clinic and introduced both a triaging system for referrals and a PIFU pathway for suitable patients with moderate (non-severe) HVD. These patients are now initially assessed by a doctor at their first visit, with the primary aim of educating them about their condition and agreeing the plan for appropriate imaging surveillance intervals. Suitable patients are then transferred to a PIFU pathway for moderate HVD. They are informed about how to contact the valve nurse helpline and given the contact number on their clinic letter and patient information sheet. Patients on the PIFU pathway are subsequently only assessed by echocardiogram at the determined intervals, with standardised outcome letters sent to the patient and their GP after each echocardiogram visit (see Appendix 2).
Administration of the PIFU pathway has been transferred from the outpatient admin team to the echo department admin team, and PIFU echoes are given a special clinic code.
The waiting list and breaches are managed using a traffic light system. If patients trigger any alerts identified in the SOP, such as progression to severe HVD or development of any valve-related symptoms, or the patient has any other clinical concerns, the valve team can be contacted via the valve nurse helpline. This helpline is staffed Monday-Friday, 9am-5pm with a full-time administrator, with the option to leave a voicemail out of hours. The helpline receives about 40 calls a week, of which 60% are clinical and 40% are admin related. Clinical queries are triaged and managed by a Band 6 or 7 heart valve specialist nurse, which takes on average 20–30 minutes (including calls, requesting tests, dictating letters, documentation). Valve clinicians are alerted via email if their input is needed, and the patient is triaged to be seen in the valve clinic according to clinical urgency. The administrator manages the administration queries.
This model has reduced the waiting time for the doctor-led valve clinic to 4 weeks and all symptomatic valve patients are being urgently assessed.
Appendix 1: Example standard operating procedure for a heart valve PIFU
This is an example standard operation procedure (SOP) for a heart valve PIFU. Imaging intervals are provided as an example only and each unit should devise its own SOP for the management of individual conditions. It is recognised that the suggested imaging intervals do not necessarily adhere to international valve guidelines but reflect an approach tailored to balance resource availability (echocardiography) with the natural history of disease progression from published studies, allowing resources to be diverted to those with more advanced and complex valve disease.
Echocardiograms should comment on the underlying rhythm in all cases. If there has been a change in rhythm this should be highlighted, and an ECG recorded if possible. Height, weight, and blood pressure should be recorded at every echocardiogram and recorded on the echocardiographic report.
Condition: Normally functioning or mildly diseased bicuspid aortic valve
Imaging follow-up interval:
2-5 years (12 months in the presence of aortic dilatation >40mm)
Imaging surveillance:
Echocardiogram
Criteria to leave PIFU +/- referral back to valve specialist:
- New or worsening symptoms
- Patient or staff concern
- New onset of dysrhythmia
- New LV/RV dysfunction
- Significant aortic dilatation
- Progression to moderate valve disease (switch to PIFU ‘moderate disease’ pathway after clinician discussion)
- PA systolic pressure >50mmHg (or high likelihood of pulmonary hypertension reported)
Comments:
Note association of aortopathy with this condition, although aortic dissection remains rare.
Consider cross sectional imaging to check for underlying aortopathy at baseline.
If echocardiogram is unable to assess for aortopathy alone, patient is not suitable for PIFU pathway.
Condition: Low moderate aortic stenosis (Vmax < 3.5mol/sec) with preserved LVEF and normal flow
Imaging follow-up interval:
1-2 years
Imaging surveillance:
Echocardiogram
Criteria to leave PIFU +/- referral back to valve specialist:
- New or worsening symptoms
- Patient or staff concern
- New onset of dysrhythmia
- New LV/RV dysfunction
- Aortic valve Vmax >3.5m/sec or AVA <1.2cm2
- PA systolic pressure >50mmHg (or high likelihood of pulmonary hypertension reported)
Comments:
Due to the natural history of moderate to severe aortic stenosis, a conservative threshold of ‘low moderate’ disease is advised for patients to enter PIFU.
Given the complexity of assessing aortic valve disease in the context of LV impairment, it is suggested that patients with low flow conditions are excluded from an PIFU pathway.
Condition: Moderate aortic regurgitation with preserved LVEF
Imaging follow-up interval:
1-2 years
Imaging surveillance:
Echocardiogram
Criteria to leave PIFU +/- referral back to valve specialist:
- New or worsening symptoms
- Patient or staff concern
- New onset of dysrhythmia
- Left ventricular dilatation
- New LV/RV dysfunction
- Progression to severe aortic regurgitation
- Aortic dimensions ≥40mm
- PA systolic pressure >50mmHg (or high likelihood of pulmonary hypertension reported)
Comments:
Note association of aortopathy with this condition.
Consider cross sectional imaging to check for underlying aortopathy at baseline.
If echocardiogram is unable to assess for aortopathy alone, patient is not suitable for PIFU pathway.
Condition: Mitral valve prolapse (no or mild regurgitation)
Imaging follow-up interval:
3-5 years
Imaging surveillance:
Echocardiogram
Criteria to leave PIFU +/- referral back to valve specialist:
- New or worsening symptoms
- Patient or staff concern
- New onset of dysrhythmia
- New LV/RV dysfunction
- Left ventricular dilatation
- Increase in mitral regurgitation to moderate (patient may transfer to ‘moderate mitral regurgitation’ PIFU pathway after clinical discussion)
- PA systolic pressure >50mmHg (or high likelihood of pulmonary hypertension reported)
Comments:
n/a
Condition: Moderate mitral regurgitation with preserved LVEF and PA pressure <50mmHg
Imaging follow-up interval:
18 months – 2 years
Imaging surveillance:
Echocardiogram
Criteria to leave PIFU +/- referral back to valve specialist:
- New or worsening symptoms
- Patient or staff concern
- New onset of dysrhythmia
- New LV/RV dysfunction or LVEF <60%
- Left ventricular dilatation
- Progression of mitral regurgitation to severe
- PA systolic pressure >50mmHg (or high likelihood of pulmonary hypertension reported)
New tricuspid regurgitation (≥moderate)
Comments:
n/a
Condition: Mild to moderate mitral stenosis (MVA > 1.5cm2)
Imaging follow-up interval:
2-3 years
Imaging surveillance:
Echocardiogram
Criteria to leave PIFU +/- referral back to valve specialist:
- New or worsening symptoms
- Patient or staff concern
- New onset of dysrhythmia
- New PA systolic pressure >50mmHg (or high likelihood of pulmonary hypertension reported)
- New tricuspid regurgitation (≥moderate)
- New RV dysfunction
- Mitral valve area <1.5cm2
Comments:
Note some patients with mitral stenosis with a mitral valve area <1.5cm2 will display symptoms and therefore need closer clinical evaluation and are not suitable for a PIFU pathway.
Condition: Normally functioning TAVI or bioprosthetic valve, mitral valve repair, transcatheter edge to edge repair
Imaging follow-up interval:
1-2 years
Imaging surveillance:
Echocardiogram
Criteria to leave PIFU +/- referral back to valve specialist:
- New or worsening symptoms
- New onset of dysrhythmia
- Patient or staff concern
- New LV/RV dysfunction
- New left ventricular dilatation
- Significant increase in mean valve gradient compared with previous echo ≥10mmHg
- New tricuspid regurgitation (≥moderate)
- New valve prosthesis regurgitation (≥moderate)
- New PA pressure >50mmHg (or high likelihood of pulmonary hypertension reported)
Comments:
Ensure routine post-operative transthoracic echocardiogram has been performed prior to entering patients onto PIFU so there is a reference scan for comparison.
Some units may wish to only start imaging surveillance of bioprosthetic valves after 5-10 years according to patient and valve characteristics.
Those with risk factors for disease progression (ie young age at implant, smokers, diabetes, renal disease) may be followed up with imaging more regularly at the discretion of the overseeing clinician.
Patients with abnormal baseline echocardiography (ie significant valve leak post-surgery, patient-prosthesis mismatch) should not be considered for PIFU.
Condition: Mechanical valve prosthesis with no associated aortopathy
Imaging follow-up interval:
n/a
Imaging surveillance:
No imaging follow up required
Criteria to leave PIFU +/- referral back to valve specialist:
n/a
Comments:
Ensure routine post-operative transthoracic echocardiogram has been performed prior to entering patients onto PIFU.
Patients with bicuspid aortic valve or co-existent untreated valve lesions may not be suitable for PIFU.
Consider TTE at 5 years in isolated mechanical MVR to assess right heart and for tricuspid regurgitation.
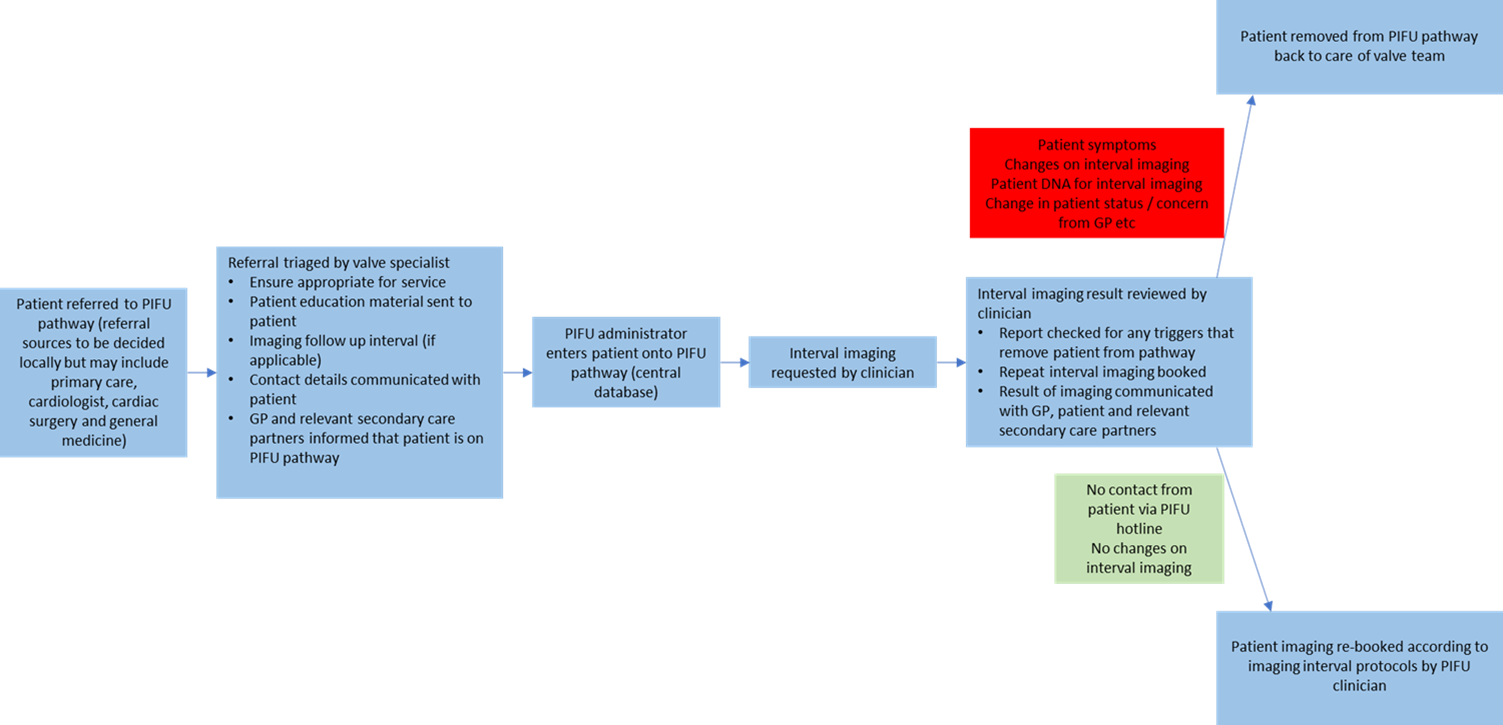
Appendix 2: Patient resources
Leaflets on individual conditions and information for patients about HVD can be downloaded free from www.bhvs.org.uk
Example patient letters
These example letters are from the valve clinic at Leeds Teaching Hospitals NHS Trust.
Stable moderate asymptomatic AS (< 3.5mol/sec)
Dear XXX
Your echocardiogram completed today as part of the valve surveillance clinic showed stable moderate aortic stenosis. This is unlikely to cause you any symptoms or strain on your heart; however, this does require ongoing surveillance with repeat echocardiograms as over time this may progress.
It is important that you maintain good dental hygiene and attend your dentist once or twice a year. The reason for this is that occasionally bacteria can enter through the teeth and affect your valve causing an infection called endocarditis. The risk of this can be reduced by maintaining good dental hygiene.
If you have any queries or concerns, or if you start to develop any symptoms (such as new or worsening shortness of breath, pains in your chest or any other symptoms during exertion), please contact one of our heart valve specialists on xxxxxxxx. They will assess your symptoms and decide if a sooner appointment or any other investigations are required.
You will receive a letter in the post for a repeat appointment in the valve surveillance clinic in around 18 months’ time. Please find enclosed a leaflet on your condition for you to read.
We have copied this letter to your GP, so they are aware that you are under valve surveillance with our Valve PIFU team and that they do not need to arrange any further echocardiograms as this will be co-ordinated by our team. If your GP has any concerns that your valve disease may have progressed in between your surveillance echocardiograms, or that you are developing symptoms related to your heart valve condition, they can also contact us on the phone number above or by letter.
Stable moderate asymptomatic MR
Dear XXX,
Your echocardiogram completed today as part of the valve surveillance clinic showed stable moderate mitral regurgitation. This is unlikely to cause you any symptoms or strain on your heart; however, this does require ongoing surveillance with repeat echocardiograms as over time this may progress.
It is important that you maintain good dental hygiene and attend your dentist once or twice a year. The reason for this is that occasionally bacteria can enter through the teeth and affect your valve causing an infection called endocarditis. The risk of this can be reduced by maintaining good dental hygiene.
If you have any queries or concerns, or if you start to develop any symptoms (such as new or worsening shortness of breath, pains in your chest or any other symptoms during exertion), please contact one of our heart valve nurse specialists on xxxxxxxx. They will assess your symptoms and decide if a sooner appointment or any other investigations are required.
You will receive a letter in the post for a repeat appointment in the valve surveillance clinic in around 2 years’ time. Please find enclosed a leaflet on your condition for you to read.
We have copied this letter to your GP, so they are aware that you are under valve surveillance with our Valve PIFU team and that they do not need to arrange any further echocardiograms as this will be co-ordinated by our team. If your GP has any concerns that your valve disease may have progressed in between your surveillance echocardiograms, or that you are developing symptoms related to your heart valve condition, they can also contact us on the phone number above or by letter.
Appendix 3: Acknowledgements
We are grateful to the following stakeholders for their involvement in the development of this document.
- British Cardiovascular Society
- Dr Laura Dobson, Consultant Cardiologist, Manchester University NHS Foundation Trust
- Heart Valve Voice the UK’s dedicated heart valve charity
- Mr Alan Keys, Patient Representative, Healthwatch East Sussex, British Cardiovascular Society
- Professor Simon Ray, Consultant Cardiologist, Manchester University NHS Foundation Trust Joint National Lead for Cardiology GIRFT
- Dr Dominik Schlosshan, Consultant Cardiologist, Leeds Teaching Hospitals NHS Trust
Publication reference: PRN00481

