Classification: Official
Publication reference: PRN02394
To:
- Trust chief operating officers (COOs)
- Trust medical directors
- Trust heads of procurement
- Trust heads of emergency preparedness, resilience and response (EPRR)
- Trust directors of communication
- Trust materials management teams
- Independent sector (via trusts)
cc.
- Regional COOs
- Regional directors of communication
- Regional deputy directors of EPRR
- Regional medical directors
- Integrated care board (ICB) EPRR leads
- ICB medical directors
- ICB procurement leads
- ICB communication leads
Dear colleagues,
Heraeus Medical – bone cement products
We are writing to update you on a significant disruption that has emerged in relation to the supply of bone cement products sold by Heraeus Medical.
Stock position and alternatives
A packaging fault temporarily halted production at Heraeus’ main production site. Whilst production has now restarted, product availability will be impacted for at least two months. This is a global issue.
Stock already in the UK supply chain may be sufficient for ~two weeks’ supply, at normal ordering volumes, beyond this there will be a period of six-eight weeks’ gap in supply.
Please note, existing stock already in trust cold chain storage is not affected, and no stock recall is in place. Independent provider partners will also be impacted.
Work is underway to secure additional stock from alternative suppliers where possible
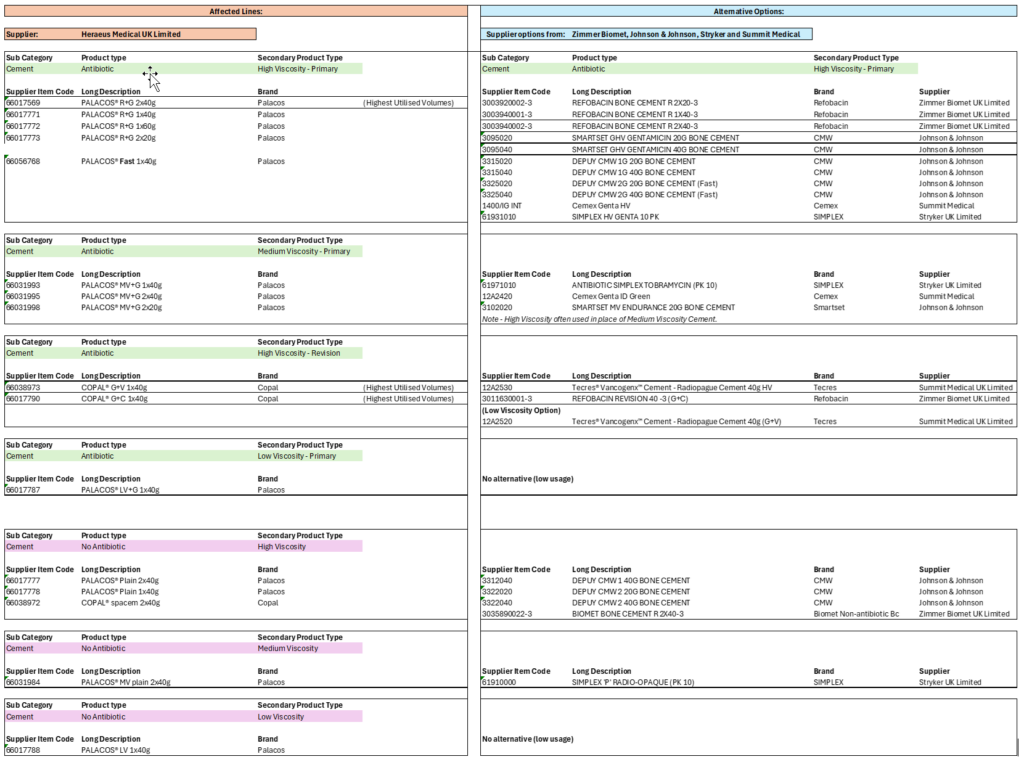
A list of the affected products, and potential alternatives is included in Annex A.
Further information on supply and alternatives can be found on NHS Supply Chain’s dedicated webpage, which will be regularly updated: Supply Issues Heraeus Medical Bone Cement Products » NHS Supply Chain
Implications for care
These products are used in both trauma and elective orthopaedic procedures, usually in arthroplasty.
An indicative clinical prioritisation is as follows:
- Trauma and urgent patients (e.g. fractured neck of femur, those requiring a cemented stem, or other urgent cases requiring the use of quick-setting bone cement).
- Urgent elective arthroplasty – primary treatment for patients with significant pain/disability which are not suitable for uncemented implants.
- Patients undergoing complex revision hip/knee revision cases which cannot wait 6-8 weeks – for example patients with infection who require antibiotic cement.
Use of alternatives and changes to clinical practice
For some trusts and units switching to alternative suppliers should be comparatively straightforward and achievable within weeks. Other switches may not be ‘like-for-like’ and will require staff re-training and changes to process, given the different technical specifications of the products and use cases.
Business continuity and maximising use of available capacity
This could include focusing on procedures other than arthroplasty if cement is required but not available. Alternatives would be to allow other specialities to use the theatre time and staff whilst this issue persists. It will be important to find alternative clinical activity for affected surgeons who are not able to use those theatre lists.
If it is unavoidable to cancel orthopaedic cases during this time, the freed up clinical time should be refocused into additional outpatient activities, focussing on new patients, which is where the largest part of the orthopaedic waiting lists resides. Time should also be reprioritised for clinical triage for patients waiting more than 18 weeks. Every effort must be made to continue to improve RTT performance during this time. Trusts are also urged to revisit their business continuity and resilience plans where appropriate.
Next steps
The Department of Health and Social Care (DHSC), NHS England and NHS Supply Chain will continue to work with suppliers to minimise the length of disruption and maximise availability of clinically acceptable alternatives, with daily incident coordination meetings. We will also work with suppliers to explore how to ensure available stock is allocated as equitably as possible across trusts.
Bone cement is distributed via temperature controlled cold chain logistics, which is required to ensure stability of the product. NHS Supply Chain are not commissioned to provide cold chain services and therefore it is not possible for stock to be brought into the NHS Supply Chain network or apply conventional demand management controls.
NHS Supply Chain are working closely with the affected supplier and alternative suppliers to secure available stock, and to support suppliers’ demand management processes to prioritise need in line with NHSE prioritisation guidelines.
Further communications and contact details for queries
If trusts have concerns on products and supply following the actions set out above, they should contact their NHS Supply Chain Customer Services Advisor and their ICS Manager in the first instance or escalate via EPRR routes to ICB and regional tiers.
Updates will be provided in due course via further NHS England communications, the Important Customer Notice (ICN), and through existing meetings and networks such as the following: the ICB procurement leads network, Independent Healthcare Provider Network, Clinical Procurement Specialists Network (CPSN) and British Orthopaedic Association.
Your cooperation and proactive engagement are vital to the success of this strategy, ensuring we continue to provide safe and effective care across all settings.
Yours sincerely,
Prof Tim Briggs, National Director for Clinical Improvement and Elective Recovery, Chair of GIRFT, NHS England
Ian Eardley, National Clinical Director for Elective Care, NHS England
Fergal Monsell, President, British Orthopaedic Association
Annex A – affected product lines and potential alternative products
As of 18 February 2026 – see ICN for latest information.