The Medicines Repurposing Programme identifies and progresses opportunities to use existing medicines in new ways, outside of the current marketing authorisation. Our multi-agency steering group uses eligibility and prioritisation criteria to decide which medicines enter the programme.
Medicines within the programme are provided with a tailored package of support; for example, providing help with evidence generation, applying for a licence variation, and/or support for equitable patient access. The programme was established in March 2021.
Successful first licence variation
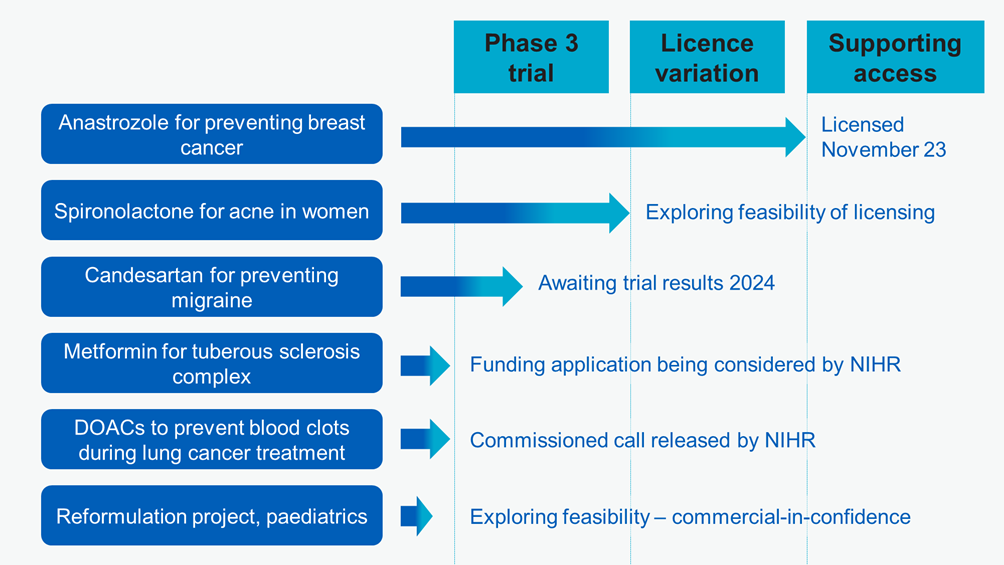
We are delighted that the first licence variation application supported by our programme was approved by the Medicines and Healthcare products Regulatory Agency (MHRA) in November 2023. The drug, anastrozole, has been licensed for several years to treat breast cancer. The recent MHRA decision means anastrozole is now also licensed to help prevent breast cancer in post-menopausal women at increased risk.
Anastrozole was selected as our first project because its use as a preventive treatment for breast cancer is supported by high-quality evidence (the IBIS-II trial) and recommended by NICE. Previously, there has been very low uptake of anastrozole for prevention. Partly this was because the preventive use was ‘off-label’ (meaning it was not previously included in anastrozole’s marketing authorisation) and some prescribing is by non-specialists, who may not have been familiar with the evidence supporting preventive use of anastrozole.
NHS England conducted a formal competitive selection process and contracted the pharmaceutical company Accord Healthcare to apply for a licence variation. We have agreed a process with the MHRA and the British Generic Manufacturers Association (BGMA) to facilitate other companies also applying for the licence variation.
Among post-menopausal women at increased risk of breast cancer, taking anastrozole can reduce the occurrence of the disease by about 50% on average. An increased use of anastrozole could prevent about 2,000 cases of breast cancer and save the NHS about £15 million in total by avoiding the costs of cancer treatment. Accord Healthcare’s licensing work was done on a not-for-profit basis and cost the NHS under £30,000. NHS England will communicate with Integrated Care Boards (via regional medical directors), to signpost to information and materials that support patient access to anastrozole.
Building a repurposing pipeline
Spironolactone for women with acne
Spironolactone is used for heart conditions and high blood pressure, but it also reduces a hormone that can cause acne. The recently published SAFA trial found higher acne-specific quality of life ratings in adult women who took spironolactone than those who took placebo.
Although spironolactone has been used to treat acne in women for a while, until now most prescribing has been by skin specialists (dermatologists). We are exploring options to support wider prescribing, including by GPs. We are assessing the feasibility of applying to the MHRA for a licence variation.
Candesartan to prevent migraine
Candesartan is licensed to treat high blood pressure and heart failure, and there is some evidence it may also help to prevent migraines. Some hospital doctors currently prescribe candesartan for migraine, but it is not widely used by GPs in primary care.
While there have been several medicines licensed recently for preventing migraine, those new treatments can only be given by hospital doctors and are used later in the treatment pathway, after several previous treatments have failed.
Candesartan may offer an earlier treatment option for use in primary care. We are awaiting the results of an ongoing Norwegian study. When the results are available, we will consider whether to take actions to support wider patient access, potentially including pursuing a licence variation.
Metformin for a rare genetic condition
Metformin has been used for decades to treat type 2 diabetes. There is early evidence it may also be effective in reducing seizures (fits) in people with tuberous sclerosis complex, a rare genetic condition that causes non-cancerous tumours and epilepsy.
Following a request from the repurposing programme, the National Institute for Health and Care Research (NIHR) released a commissioned call. That is, an invitation for researchers to apply for funding to address a research question. NIHR is now considering a funding application.
Oral anticoagulants to prevent blood clots during lung cancer treatment
Direct oral anticoagulants (DOACs) are currently used to prevent venous thromboembolism (a blood clot in a vein) after hip or knee replacement surgery.
People with cancer also have an increased risk of venous thromboembolism but are not usually offered preventive anticoagulation by the NHS. This is because anticoagulation can increase the risk of bleeding in people with cancer.
NIHR has released a commissioned call that invites applications for research to find out whether, for people having lung cancer treatment, the benefits of DOACs outweigh the risks.
Identifying further candidate medicines
We are working to increase the number of medicines supported by the programme. We collaborate with the NIHR Innovation Observatory to identify trials of repurposed medicines from international trial registers. Voluntary-sector organisations, healthcare professionals and companies are also welcome to continue to propose candidate medicines.
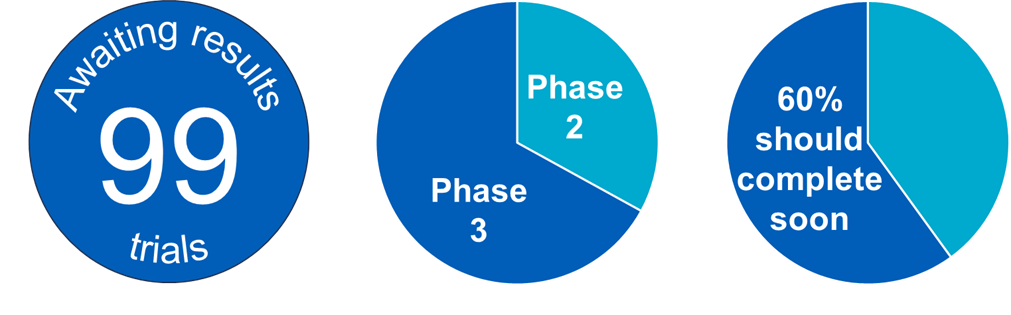
The programme is awaiting the results of 99 clinical trials. While several will prove ineligible for the programme or have negative results, it is encouraging to see a pipeline of candidate medicines potentially suitable for the programme.
For priority projects that are not yet ready to join the programme, we are also supporting researchers to seek early scientific advice from regulators – so that opportunities to contribute to a future licence variation are not missed.
Partnership working in England and internationally
Engagement with stakeholders, patients, and the public is embedded into our work, creating a partnership approach that shapes, strengthens, and delivers the programme’s aims. Our valued patient and public voice (PPV) partners have a decision-making role in the programme steering group. We also involve patient groups and PPV partners in work on specific medicines.
Repurposing initiatives have recently been established elsewhere. We have led in establishing the Medicines Repurposing International Network (MERIT), which supports collaboration and communication between publicly funded repurposing initiatives. Fourteen organisations have joined the network, spanning eight countries plus the European Union.
Conclusion
Our innovative multi-agency programme is maturing, expanding, and starting to deliver new treatment options for NHS patients. In the coming year, we plan to:
- adopt 3-6 additional medicines into the programme
- explore a more complex repurposing project requiring a reformulation (that is, developing a medicine to be taken in a new form)
- support prioritised research projects to obtain MHRA scientific advice.
Join our mailing list, email england.repurposing@nhs.net
Web: NHS England » Repurposing medicines in the NHS in England

Publication reference number: PRN00729

