1. Executive summary
1.1 Current CWT standards. CANCER CARE SPELL DELAY REASON (DECISION TO TREAT)
Maximum 28 days from:
Receipt of urgent referral for suspected cancer, receipt of urgent referral from a cancer screening programme (breast, bowel, cervical, lung), or receipt of urgent referral of any patient with breast symptoms (where cancer not suspected), to the date the patient is informed of a diagnosis or ruling out of cancer – 75% (raising to 80% in March 2026)
Maximum one month (31 days) from:
From Decision To Treat/Earliest Clinically Appropriate Date to Treatment of cancer – 96%
Maximum two months (62 days) from:
From receipt of an urgent GP (or other referrer) referral for urgent suspected cancer or breast symptomatic referral, or urgent screening referral or consultant upgrade to First Definitive Treatment of cancer – 85%
1.2. Aim of this document
This guidance provides a set of rules to ensure that Cancer Waiting Times data are recorded consistently, and in a way which allows transparent and accurate reporting. These take into account as far as possible the appropriate clinical management of patients, recognising that specific rules cannot be created for every situation, and that there will be some instances in which appropriate clinical care results in a breach. The thresholds set make allowance for such instances, and this guidance does not prohibit or discourage appropriate clinical practice.
It aims to ensure that staff from both informatics and clinical teams understand which patients should be reported on, and how to record data to monitor the Cancer Waiting Times standards.
This guidance has been written based on the following principles:
- The thresholds have been set taking into account that there will be patients who choose to delay their pathway, pathway delays for clinical reasons or pathways which are clinically complex.
- Patients must have confidence that any advice or decisions affecting their treatment are based solely on clinical grounds, rather than the need to meet these standards.
This document should be read in conjunction with the Cancer Waiting Times User Manual providing further detail on the functionality of the Cancer Waiting Times System.
1.3. Updating the CWT Guidance (v.12.1)
We are updating the guidance to version 12.1 to apply to activity that ends on or beyond the 1st of July 2025.
1.4. Summary of changes from vs. 12.0 to vs.12.1
Faster Diagnosis Standard specifics
- Section 2.2.1 – Additional requirement added in, that systems should have processes in place to accept direct referrals from the Independent Sector where a patient meets criteria for an Urgent Suspected Cancer referral. Implemented from 1 July 2025.
Section 2.2.7 – Guidance added on how to manage Urgent Suspected Cancer referrals where the patient is initially seen in a community hub, confirming that the patient’s clock has started, and that data needs to be submitted for this activity. Implemented: Immediately – clarification on existing guidance. - Section 2.2.12, 2.2.13, and 2.2.17 – Incorporation of existing annex to guidance around recording of Lung Screening (previously known as Targeted Lung Health Checks) as Screening referrals added into main guidance. Implemented: Immediately – Annex already in place.
Section 2.2.16 – Additional guidance added in to record patients who decline a diagnostic on a Bowel Screening pathway but subsequently opt back in to pathway.
(Implemented: Immediately – clarification on existing guidance). - Section 2.3.1 – Adaption of national rules around referral management. More flexibility for Breast Symptomatic referrals to allow for more clinically appropriate alternatives, whilst patients remaining on the pathway continue to have the standards applied to them. Implement from 1 July 2025.
- Section 2.3.5 – Rewording of Advice and Guidance section, to make clearer that this service should not be used for Urgent Suspected Cancer referrals given the clinical urgency. Removal of this requirement for Breast Symptomatic referrals, to allow more flexibility in development in local services for these patients. Implement from 1 July 2025.
- Section 2.3.6 – DNA adjustment guidance updated, so that a patient who has not taken the required preparation is not recorded as a DNA. In addition, this adjustment can now only be applied where a patient has either booked their appointment through the E-RS or an alternative booking system directly, agreed an appointment by phone or where several unsuccessful attempts have been made by phone, by post where at least a week’s notice is given. Implement from 1 July 2025.
- Section 2.4.2 – Additional guidance added on application of interval scanning, including the specific example covering Prostate PSA monitoring. Implemented: Immediately – clarification on existing guidance.
Treatment standard specifics
- Section 3.8 – Addition of Rectal Spacer prior to radiotherapy as an enabling treatment, and additional wording for existing colostomy guidance. Implement from 1 July 2025.
- Section 3.10.5 – Clarification on active monitoring guidance, making it clearer that it cannot apply where a pathway is progressing with the intention of treatment being delivered. Implement from 1 July 2025.
- Section 3.10.6 – Updated Prostate cancer guidance around active monitoring to reflect Cambridge Prognosis Classification. Implement from 1 July 2025.
- Section 3.19 – Update to patient choice adjustment, to apply to when patients choose to have treatment after a specific date or where they are offered a date within 31 days and decline this. Implement from 1 July 2025.
Referral/upgrade to first treatment standard specifics
- Section 4.3.4 – Additional scenarios where consultant update would apply automatically, to where a trust radiology or pathology system flags a patient as suspicious of or with confirmed cancer, where an interval scan is abnormal or where a patient through bowel screening who initially declines all diagnostics opts at a later point to progress their pathway. Implement from 1 July 2025.
- Section 4.3.5 – Additional guidance added on how to record 62 day and 31-day pathways where a patient is diagnosed with multiple primaries on the same pathway. Implement from 1 July 2025.
- Section 4.6.2 – Additional guidance around inter-provider transfer recording, including the scenarios where a provider is not notified a patient is on an active 62-day pathway. Implement from 1 July 2025.
Tumour specific guidance
- Section 5.10.1 – Additional wording added for what would count as a first attendance for tele-dermatology, including an appointment in secondary care where a dermoscopic image/photo is taken by a trained healthcare professional. Implemented: Immediately – clarification on existing guidance.
- Section 5.12.4 – Guidance around bladder cancer updated, so the Mitomycin now never counts as a First Definitive Treatment. Implement from 1 July 2025.
2. Faster Diagnosis Standard specifics
2.1. Faster Diagnosis Standard overview
The cancer waiting times service standard is:
Maximum four weeks (28 days) from receipt of urgent GP (or other referrer) referral for suspected cancer, breast symptomatic referral or urgent screening referral, to point at which patient is told they have cancer, or cancer is definitively excluded. [Operational Standard – 75.0% – raising to 80% in March 2026]
2.2. Starting the clock and inclusion of Faster Diagnosis Standard and recording of first seen date.
2.2.1 Inclusion of patients referred as an urgent suspected cancer or breast symptomatic referral.
The Faster Diagnosis standard apply to patients referred with suspected cancer or breast symptoms from one of the following referrers:
- General Medical Practitioner (GMP)
- General Dental Practitioner (GDP)
- Optometrist
- Any other referral source as agreed locally by commissioners and providers
The full list of the possible referral sources are listed on the NHS data dictionary website.
Systems should be in place as a minimum to allow GPs, GDPs, and Optometrists to make urgent suspected cancer referrals, and symptomatic breast referrals to be made from any route. Referrals can be made and recorded via other sources for urgent referrals where this is locally agreed jointly by commissioners and providers. Examples of where this could be expanded include referrals from other healthcare professionals such as an Advanced Nurse Practitioner (ANP) or referral direct from an A&E attendance into a suspected cancer referral clinic. Where these models are agreed these referrals should be recorded as an Urgent Suspected Cancer referral.
Systems should also have processes in place to enable direct referrals from the Independent Sector, where the patient meets normal referral criteria for an Urgent Suspected Cancer referral and is eligible for NHS care. This includes scenarios where a patient is being seen by a private GP or consultant and investigations are suspicious of cancer.
The standards apply to all NHS providers and private providers either where the activity is directly commissioned by an NHS England commissioner or subcontracted by an NHS provider.
2.2.2 Referrals for suspected recurrence of cancer.
A GP (or other referrer) can make an urgent referral for a suspected recurrence or a suspected second new primary cancer.
- the Faster Diagnosis Standard would apply.
- if the patient is diagnosed with a recurrence they are covered by the 31-day treatment standard as a subsequent treatment. They would not be covered by the 62-day standard, as the patient will have already received their first treatment for cancer.
- if the patient is diagnosed as a new primary, they will be covered by both the 31-day treatment standard and the 62-day standards.
2.2.3 Clock start date urgent suspected cancer or breast symptomatic referrals.
The Faster Diagnosis Standard start point is the receipt of the referral by the provider who will first see the patient (recorded as the CANCER REFERRAL TO TREATMENT PERIOD START DATE). Receipt of referral is day zero.
Referrals received after a working day has finished should have the CANCER REFERRAL TO TREATMENT PERIOD START DATE set as the date that the referral was received and not the next working day.
If further information is required to manage the referral the receipt of initial referral would still be recorded as day zero. Commissioners, referrers, and providers should work together to ensure processes are in place to ensure all necessary information is sent with a referral. It would be inappropriate to pause the clock given the patients expectation will be that a referral has been made.
Patients referred on a Faster Diagnosis Standard pathway should still be recorded in the dataset as:
- PRIORITY TYPE CODE – 3 (Two Week Wait)
2.2.4 First seen date for urgent suspected cancer or breast symptomatic referrals.
Although the Two Week Wait performance standard no longer applies, it is still necessary to record DATE FIRST SEEN as one of the following: –
- the patient is seen either in person or virtually for the first time by a consultant (or member of their team) following the referral receipt.
- the patient is seen at a diagnostic clinic or goes ‘straight to test’ in a consultant-led service (unless that test is a blood test).
- the only exception to this is where patients with suspected skin cancer are being managed through a tele-dermatology pathway, as detailed in section 5.10.1.
A virtual consultation can only count as a first seen date, where it is a consultant led clinic (including a nurse acting on behalf of a consultant), and a patient’s full symptoms are considered. The patient would have to be present for this consultation.
2.2.5 Patient seen as an emergency prior to being seen following a referral.
Where a Faster Diagnosis Standard patient is admitted as an emergency for the same condition (i.e. related to the suspected cancer) before they are seen they should no longer be recorded against the 28-day Faster Diagnosis Standard. The emergency admission is the referral into the system and supersedes the original referral. However, the patient should be upgraded to the 62-day pathway by a consultant or authorised member of their team from the emergency admission if cancer is suspected, and this is the cause of the admission, for example, where a patient is awaiting an appointment for a Lower GI investigation on a cancer pathway but is admitted with a bowel obstruction before they can be first seen.
This would not apply where a patient attends an accident and emergency (A&E) department and is not admitted. In such a scenario the original clock start would apply.
2.2.6 Symptomatic Breast Referrals.
The difference between the urgent GP suspected cancer referral and the symptomatic breast referral depends on whether the referrer suspects cancer or not. Breast symptoms are defined as any breast symptoms (covered in the National Institute for Health and Care Excellence (NICE) Suspected cancer: recognition and referral NG12 guidelines) that a healthcare professional believes need to be seen by a specialist, excluding referrals from family history clinics (unless a patient is symptomatic) or for cosmetic breast surgery.
These referrals can be distinguished from suspected cancer referrals through the data item TWO WEEK WAIT CANCER OR SYMPTOMATIC BREAST REFERRAL TYPE where the breast symptomatic patients are given the code ‘16’.
2.2.7 Community provider referrals.
Where a GP refers to a community provider as the first step in a Suspected Cancer pathway, we would expect this to start the Faster Diagnosis and, if applicable, 62-day standard clock, where this decision is based NG12 NICE guidance threshold for Suspected Cancer referral. It is the responsibility of the provider commissioned to provide this service to submit data related to Cancer Waiting Times standards. This would include community models where patients are either referred for review or completion and review of tele-dermatology image, where the GP considers the patient already meets the criteria for an Urgent Suspected Cancer referral.
2.2.8 Non-specific symptom referrals.
Referrals into non-specific symptoms pathways should be recorded in the same way as urgent suspected cancer referrals as follows:
- PRIORITY TYPE CODE – 3 (Two Week Wait)
- TWO WEEK WAIT CANCER OR SYMPTOMATIC BREAST REFERRAL TYPE – 17 (Suspected – non-specific symptoms)
The same set of standards and principles apply to these referrals as those set out for the site specific urgent suspected cancer referrals.
2.2.9 Triage from an abnormal direct access diagnostic.
Where a pathway has been implemented and agreed locally where a patient is directly triaged from an abnormal direct access diagnostic scan with a suspicion of cancer then the decision to triage directly would act as the start of the pathway and be counted as an urgent suspected cancer referral, and not an upgrade.
The CANCER REFERRAL TO TREATMENT START DATE (clock start) should be recorded as the date of triage into secondary care management.
The SOURCE OF REFERRAL FOR OUT PATIENTS should be recorded as 03 – referral from a GENERAL MEDICAL PRACTITIONER.
The DATE FIRST SEEN would then be recorded as the next appointment or diagnostic as outlined in section 2.2.4.
If local arrangements mean the urgent referral is received prior to the direct access diagnostic, then the date the urgent referrals is received by the provider should be used as the CANCER REFERRAL TO TREATMENT START DATE.
This can apply to any pathway. Examples of its application are included below for lung and oesophago-gastric cancers as detailed in the rapid cancer diagnostic and assessment pathways on the NHS England website.
Where this is applied the CANCER DIAGNOSTIC REFERRAL ROUTE should be recorded as 01 (Abnormal diagnostics results following a NICE guidance NG12 referral to a direct access diagnostic service)
2.2.10 Timed diagnostic pathway for lung cancer.
This pathway sets out how a patient could be transferred to secondary care without an additional referral from their GP, if the patient has had an abnormal result for a direct access chest x-ray followed by an abnormal CT result, and a locally agreed escalation process to secondary care is followed.
Where a patient is transferred to secondary care this should be recorded as an Urgent Suspected Cancer referral, following triage of the CT, resulting in follow-up being required in secondary care. The CANCER REFERRAL TO TREATMENT START DATE (clock start) should be recorded as the date of this triage.
2.2.11 Timed diagnostic pathway for oesophago-gastric cancer.
This pathway outlines a diagnosis pathway for patients referred with oesophageal or gastric cancer symptoms. It is acknowledged that this pathway could start with an urgent direct access upper gastrointestinal endoscopy ordered by the GP (satisfying relevant NG12 risk criteria).
Where the upper gastrointestinal endoscopy is abnormal and suspicious of cancer, patients could be followed up by secondary care directly from endoscopy without the need for an additional referral from GP. In this case, the decision to escalate the patient (i.e. decision to follow up the patient in a secondary care provider) should be counted as an Urgent Suspected Cancer referral. The CANCER REFERRAL TREATMENT START DATE (clock start) should be recorded as the date of this decision, which would usually be the date of the endoscopy itself where an abnormality is seen.
2.2.12 When does the 28-day FDS and 62-day standard start for the NHS cancer screening programmes?
The clock start (day 0) is when a referral is received by a provider in the screening pathway for further investigation after an initial screening test. Each individual screening programme is as follows:
- Breast – receipt of referral for breast screening assessment (i.e. not back to routine recall)
- Bowel (FIT) – receipt of referral for an appointment to discuss suitability for colonoscopy with a specialist screening practitioner (SSP)
- Cervical – receipt of referral for an appointment at colposcopy clinic.
For the purposes of the Cancer Waiting Times standards Lung Screening (previously known as Targeted Lung Health Checks) are also recorded as a screening referral in the dataset. For this programme, the clock start would be recorded as:
- Lung Screening – referral received by a provider for further investigation for Suspected Cancer after an initial screening test. For a Lung Screening referral this would be the receipt of referral to the multidisciplinary team to review the low dose CT scan results.
2.2.13 What is recorded as the Date First Seen for screening cases?
The DATE FIRST SEEN for the individual screening programmes are as follows:
- Breast Screening – first attendance for breast screening assessment
- Bowel Screening – first attended appointment with specialist screening practitioner (SSP) to discuss suitability for colonoscopy
- Cervical Screening – first attended colposcopy appointment
- Lung Screening – The next activity in the pathway following referral, which could include a consultation (Virtual or In-Person) or a further diagnostic test.
It is the responsibility of the provider commissioned for this first attendance to upload this information onto the National CWT system.
The SOURCE OF REFERRAL FOR OUTPATIENTS should be recorded as 17- Referral from National Screening Programme.
Where a screening provider also communicates the diagnosis of cancer or ruling out of cancer to a patient, this provider also needs to complete the 28-day FDS data items.
2.2.14 Cervical Screening specifics.
Referrals from the cervical screening programme should be those identified with a PRIORITY TYPE CODE 2 (urgent). Referrals to be counted on the Faster Diagnosis Standard, and if cancer, the 62-day screening standards are as follows:
- Cytology showing borderline changes in endocervical cells or high grade (moderate or severe) or worse (i.e. abnormalities within scope of the standard) This includes patients with possible invasive cancer, possible glandular neoplasia, severe dyskaryosis and moderate dyskaryosis.
Referrals with a PRIORITY TYPE CODE 1 (routine) and patients covered by the Referral To Treatment (RTT) pathway are as follows:
- All cervical screening programme referrals not included in priority 2 (i.e. abnormalities not covered by this standard – cancer not suspected/likely)
2.2.15 Breast Cancer Screening start date specifics.
Local protocol dictates whether it is based on one reader’s recommendation or following consensus/arbitration of the mammogram results. The referral is triggered when the reader(s) decides to recall the patient for further assessment and then the referral is received
2.2.16 Specific guidance around application of Cancer Waiting Times guidance to Bowel Screening.
If a person is medically fit for colonoscopy and decides not to book their colonoscopy date but take time to consider if they wish to progress with the procedure – screening centres are advised to give a maximum of 2 weeks before making contact for the patient’s decision.
Usually, if they are still undecided after 2 weeks, the patient should be informed that they have an open invitation which means they can request a test over the next two years if they recontact the service. In this scenario their episode should be closed, recorded as an exclusion – 02- Patient declined all diagnostics.
If the patient then contacts the service to book their colonoscopy this should then be recorded as a new pathway, as a consultant upgrade from the point at which the patient contacts the service asking for a colonoscopy.
2.2.17 Lung Screening start date specifics.
A pathway would also apply where another type of Cancer is suspected where this has been identified by the low dose CT. Where this is the case, the referral would still be recorded as a Suspected Lung Cancer referral, so all activity from the programme can be identified, but if diagnosed with cancer, the relevant diagnosis fields should reflect the primary cancer site.
2.3. Principles around management of urgent suspected cancer referrals
2.3.1 Referral policy and guidance.
The following are national requirements for management of urgent suspected cancer (including non-specific symptoms) and breast symptomatic referrals:
- Standardised referral forms for urgent suspected cancers and breast symptomatic referrals should be used to ensure consistency and completeness of referral information.
- In addition, for Urgent Suspected Cancer referrals, if a consultant thinks a referral is inappropriate this should be discussed with the referrer. Only the referrer can downgrade or withdraw a referral.
This national requirement does not cover breast symptomatic referrals, recognising that it may be appropriate to either offer advice or divert patients to community care led service.
- The date of receipt of initial referral or the conversion of the UBRN into a booking should always count as the start of the pathway and be recorded as CANCER REFFERAL TO TREATMENT PERIOD START DATE. This includes scenarios where additional information is requested from the referrer and where a patient is unavailable for a period of time.
- A patient should not be discharged because they are unavailable within a specified timeframe, and processes should be in place to ensure patients have the choice to book outside of a fixed timeframe.
In addition, a local protocol/policy should be agreed between commissioners, referrers, and providers with the following suggested overarching principles, though these do not override the national requirements above.
- the best interest of the patient should be at the forefront of the local policy
- referrals between primary and secondary care organisations should be monitored locally
- providers should run daily checks for missing referral letters following an e-Referral Service (e-RS) referral, and follow these up with the relevant GP practices
- the duty of care is with the referring practice. The practice will therefore need to have systems in place to ensure that referral letters are sent promptly and to ensure that patients they have referred/convert their UBRNs in a timely way, where patients book their appointments directly through the e-referrals system
- for referrals, the required information should be sent to the receiving provider within one working day of the GP referral.
The patient should be encouraged to accept the earliest appointment. NICE Suspected cancer: recognition and referral NG12 guidelines explain the information that should be provided to the patient to encourage patients to accept the earliest appointment where possible. It would also be helpful for the referrer to reiterate the importance of keeping an appointment once it has been made. Providers and commissioners should consider how communication should be tailored to the needs of local communities to best encourage attendance.
2.3.2 NHS E-Referrals Service (e-RS).
For patients booking an appointment through the e-RS it is good practice to ensure the patient has booked an appointment before leaving the practice. It is also good practice to ensure that someone at the practice monitors e-RS bookings on a daily basis to check that all UBRNs have been converted into a booking.
The e-RS will only offer patients an appointment within a fixed period depending on the polling range set for the service and urgency. If a patient cannot make themselves available for an appointment within a fixed period, despite having been given appropriate information, it is technically possible for a GP or other referrer to defer making the referral until the patient is available for referral. However, trusts should develop systems which enable a patient to choose to be seen beyond the polling length set by the trust if they are not available. It is important in these scenarios that referrers have fully informed the patient of the clinical urgency of their appointment, to ensure the patient can make an informed choice.
Patients that choose to delay an appointment even if beyond 28 days do not exempt themselves from the standards. The operational standards take account of the volume of patients likely to exceed the standards due to patient choice.
2.3.3 Referrals not made via e-RS.
For urgent suspected cancer referrals received by a route other than e-RS, referrals should not be rejected in the interests of patient safety. A patient should be offered an appointment.
A recommended process for this is included below. In the interest of patient safety, if there is no response from the GP practice within the next working day, the provider will contact the patient to make an appointment, regardless of whether they have received the e-RS referral from the GP practice or not.
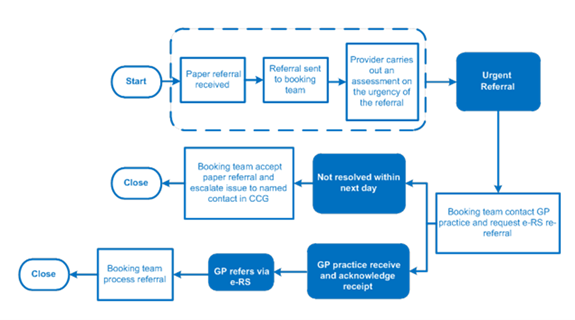
2.3.4 Referral sent to wrong trust.
There should be agreed local referral protocols in place between primary and secondary care so that the referrer knows where to send patients. If they have sent a referral to a wrong provider, that provider could liaise with the referrer and ask them to withdraw the referral and re-refer to a correct provider. This new referral would be recorded as the start of the pathway. Alternatively, the wrong provider could forward the referral on to the correct provider if this is faster and in the patient’s interest. In this case the clock start would still be the original from the referrer.
2.3.5 NHS E-RS Advice and Guidance (A&G) for cancer pathways.
The A&G function should not be used in place of an Urgent Suspected Cancer referral. For example, where a patient clearly meets NG12 criteria for Urgent Suspected Cancer, A&G should not be used, a direct referral should be made at this stage.
For other referrals A&G can be used locally were agreed at a system level. This may vary by pathway, depending on what is clinically appropriate, and must follow engagement with referrers and providers to develop any new processes.
Prior to any implementation, systems/commissioners should undertake a local training needs analysis and carry out any training, as necessary. There should also be ongoing support available to referrers and providers.
A&G requests can be converted into an urgent suspected cancer referral in line with the local referral and commissioning guidelines and where this happens must be classed as an urgent suspected cancer referral, not a consultant upgrade.
Where an A&G referral is converted the e-RS pathway start will capture the date on which the provider converts the referral. When making the decision on if to convert A&G directly into a referral and appointment, the clinician reviewing should take into consideration whether they have the required information, and whether the patient is likely to know there is a suspicion of cancer.
Systems or commissioners should regularly review A&G services and conduct quality assurance analysis to ensure they meet local requirements. Lessons learned should also be reviewed and findings shared across the system.
2.3.6 First seen adjustment.
Although the first seen standard has been retired, it is important we still record this data item correctly, as doing so drives the calculation of breach allocation and other elements of the cancer pathway. An adjustment is allowed if a patient does not attend (DNAs) for the allocated appointment time and gives no notice for their initial out-patient appointment / diagnostic clinic that would have been recorded as DATE FIRST SEEN. If the patient arrives after the scheduled appointment time, and it is not possible to fit them in (e.g. fully booked) or there is not enough time left to carry out the planned procedure/tests in the remainder of the session then this is classed as a DNA.
Under this adjustment, the clock can be reset from the receipt of the referral (recorded as the CANCER REFERRAL TO TREATMENT PERIOD START DATE) to the date upon which either the patient makes contact to re-book their appointment or the date the appointment is re-booked should the patient not directly contact the provider to do so. This period is called the WAITING TIME ADJUSTMENT (FIRST SEEN) and is effectively deducted from the total waiting time.
An adjustment is not possible if a patient cancels or reschedules an appointment or is not available to be seen for a period of time.
For an adjustment to be counted, there should be assurance that reasonable steps have been taken to ensure the patient has been given notice of their appointment. For this to apply the appointment needs have been either:
- Been booked directly by the patient through the electronic booking system (E-RS)
- Be agreed with patient on the phone.
- If booked via post, that several attempts are made to contact the patient via phone, and the patient is given at least a weeks’ notice of their appointment (measured from the date the appointment letter is sent)
Where an alternative electronic booking system is used for the patient to either directly book the appointment, or confirm they accept an appointment offer, an adjustment can also be made where the patient does not attend their appointment.
Where a patient does not provide this confirmation, or the System itself does not provide this functionality, the rules would revert back to initial booking mode. E.g. if a patient was notified of an appointment by post with less than a week’s notice and then receives a text reminder (without confirmation from patient they will attend) and then does not attend, an adjustment would not be recorded.
It is not possible to apply this adjustment if a patient turns up in a condition where a procedure is not possible (e.g. if they have not taken a preparation they needed to take prior to the appointment) – It is important that patients are fully supported, in these situations.
2.3.7 If a patient DNAs their initial out-patient appointment (OPA), how should the process of re-booking be managed?
If a patient DNAs their initial OPA, the provider should proactively contact the patient (e.g. by phone) to start the process of re-booking.
However, if a patient cancels their first out-patient appointment and then DNAs the rearranged date, the clock can be reset to the date the appointment is rebooked by using the WAITING TIME ADJUSTMENT (FIRST SEEN).
2.3.8 Can an adjustment be made if a patient DNAs the first out-patient appointment after a consultant upgrade onto a 62-day standard?
The adjustment for when patients DNA the first out-patient appointment can be used for the consultant upgrade route up to and including the DATE FIRST SEEN. As most referrals along this route occur at the same time as the DATE FIRST SEEN, this adjustment will be rare but should occur if a consultant upgrades a patient after reading a referral letter but then the patient DNAs.
2.3.9 Can adjustments be made if a patient DNAs a diagnostic appointment?
An adjustment is only possible if a patient does not attend their first attendance. So, where the diagnostic appointment is also the first attendance an adjustment should be applied.
A DNA for a diagnostic appointment cannot be used as an adjustment if it occurs after the first attendance.
2.3.10 What is the position on adjustments if a patient wishes to wait for a specific diagnostic option?
No adjustment would be possible in this scenario. Adjustments are only possible if a patient DNAs their first attendance or after a Decision To Treat has been made.
2.3.11 Management of DNAs and cancellations.
Patients should not be referred back to their GP after a single Did Not Attend (DNA) or cancellation. Patients should only be referred back to their GP after multiple DNAs following a clinical decision to do so.
Patients should never be referred back to their GP after an appointment cancellation unless this has been agreed with the patient – by cancelling an appointment a patient has shown a willingness to engage with the NHS.
2.3.12 Reasonable offer of appointment.
A ‘reasonable’ offer of an appointment is defined by locally agreed access policies. Providers should refer to Elective care Improvement Support Team: model access policy and should make a reasonable offer for diagnosis or treatment in a cancer pathway as has been agreed locally.
Part of being reasonable means that the patient has been consulted and listened to, considering what the patient would find reasonable.
In cases of contention, such as treatments offered on the same day, the commissioner should decide whether the offered appointment was reasonable.
2.3.13 First seen adjustment for screening.
A first seen adjustment can be applied using the same rules as first appointment for urgent suspected cancer or breast symptomatic referrals.
2.4 Ending the Faster Diagnosis Standard pathway
The 28-day FDS pathway ends only at the point of communication with the patient, whether that is to inform them of a diagnosis of cancer, a ruling out, or if they are going to have treatment before a clinical diagnosis of cancer can be made.
No diagnostic test is 100% accurate. Where all reasonable diagnostics to exclude cancer have been completed and the patient is discharged back to their GP or moved onto a benign pathway within secondary care, the point at which this is communicated to the patient should be recorded as the end of the 28-day FDS pathway. In such scenarios this should be recorded as a ruling out of cancer. Practically speaking, therefore a “ruling out” or “definitive exclusion” of cancer for the purposes of the FDS requires that a patient has received reasonable diagnostics and been told that they are no longer being investigated for a suspected cancer.
Providers who are commissioned to deliver activities that lead to a patient’s diagnosis are responsible for recording, submitting, and meeting the 28-day FDS. Where the 28-day FDS pathway is a shared pathway, only the provider communicating the diagnosis to the patient will be expected to record and submit the end of the pathway FDS data items. This provider should also feedback this information to other providers involved in the patient’s pathway.
2.4.1 Communicating the diagnosis to a patient.
All diagnoses of cancers should be made through direct face-to-face communication with the patient, unless otherwise explicitly agreed with the patient.
Reasonable forms of communication with patients to confirm cancer has been ruled out include:
- direct communication with the patient, over phone, video conferencing or similar.
- written communication by letter, or by email (this can include an endoscopy report as a patient facing communication with any medical terminology explained in full).
- face to face communication at an outpatient appointment.
Where direct communication is not possible due to the patient not having the mental capacity to understand a diagnosis either temporarily or permanently, communication to the patient’s recognised carer or a parent/guardian should be recorded in the same way as if the patient were told directly.
Example where this could apply are:
- Patients with advanced dementia
- Patient who is unconscious
- A child where they are too young to understand the diagnosis.
This would not be appropriate where it is not possible to contact a patient.
Providers should ensure that communication is easy to understand, and that support is available to patients who would like further information. Providers should undertake audits of their communication practice to ensure that letters/emails are being received and understood by patients. An accurate record of all communication as confirmed by the patient must be maintained in the patient record.
In the case of direct communication of the diagnosis with the patient either face to face or via phone, video conferencing or similar the date of the conversation should be recorded as the CANCER FASTER DIAGNOSIS PATHWAY END DATE.
Where a patient has expressed a preference for telephone communication, calls to confirm test results should be booked in the same way as triage appointments or outpatient appointments. Where a patient does not respond to a call, every effort should be made to contact the patient and book a new call at a different time or another date. In such a scenario the pathway should continue until the communication is made.
Where an e-mail is sent the CANCER FASTER DIAGNOSIS PATHWAY END DATE should be recorded as the email sent date.
Where a letter is sent the CANCER FASTER DIAGNOSIS PATHWAY END DATE should be recorded as the letter sent date.
Where an arrangement is made for a health care professional in primary care for example the patient’s General Practitioner to inform the patient of their diagnosis the date of this communication can only be recorded as the CANCER FASTER DIAGNOSIS PATHWAY END DATE where the secondary care provider has a clear record of this communication. In such cases ORGANSATION SITE IDENTIFIER (OF CANCER FASTER DIAGNOSIS PATHWAY END DATE) should be recorded as the secondary care organisation responsible for communication of diagnosis.
2.4.2 Patients having interval scans/test.
In a case where a patient is ordered an interval scan or test, the 28-day FDS clock will stop.
The CANCER FASTER DIAGNOSIS STANDARD PATHWAY END DATE should be recorded as the date the patient is told that this is the plan. The CANCER FASTER DIAGNOSIS STANDARD PATHWAY END REASON should be recorded as ’04 – Interval Scanning’.
This should only be applied where:
- This is in line with clinical guidance (e.g. pulmonary nodules for lung); or
- Where explicit clinical guidance does not exist, it should be made clear to the patient that the reason for this is that the risk of malignancy is too low to justify further diagnostics at this stage. The duration of the interval must be clearly recorded in this scenario.
Examples where this would not apply are:
- repeat or further diagnostics are required due to inconclusive results of previous diagnostics;
- the clinical recommendation is that the scan or test is done as soon as possible;
- a patient chooses to delay their scan or test against clinical recommendation;
- a patient is medically unfit for diagnostics; or
- a patient has another more clinically urgent condition which needs to be treated first.
The key distinction on if interval scanning can be recorded, is whether a diagnostic has been arranged at a fixed interval based on the clinical recommendation. It cannot be applied where; a scan would be completed sooner if there was available capacity, or the patient opts for an earlier date.
In the case of prostate specific antigen (PSA) monitoring on the Prostate pathway, this can be applied where a clinical decision is made following a borderline result, to repeat the PSA test after a fixed interval. It would not be applied if the clinical decision was made to repeat the PSA test as soon as possible.
It is important that patients having interval scans are tracked and monitored to ensure the scan or test is completed when planned.
Where a patient is subsequently diagnosed with cancer following an interval scan, a new pathway should be recorded from the date that an abnormal scan is reported. This would be recorded as a new Consultant Upgrade pathway.
2.4.3 Diagnostic Uncertainty.
If a patient on the 28-day FDS pathway cannot be given a formal non-malignant diagnosis and is followed up due to diagnostic uncertainty the patient remains on the 28-day FDS tracking until either a cancer diagnosis is made or a non-malignant diagnosis is confirmed, and this is communicated to the patient. The one exception to this are where interval scanning is arranged as detailed in section 2.4.2 above.
2.4.4 Diagnoses of a different type of cancer than initially referred.
For a patient where a specific cancer is ruled out but is still considered high risk and requiring further urgent investigation, an inter-specialism referral should be considered the normal course of action. The 28-day FDS clock continues to run until suspicion of cancer has been reasonably ruled out.
If a patient is referred for a suspected cancer and a different cancer is incidentally found that is unrelated to the referral, the 28-day FDS pathway will end when the patient is told their diagnosis or, where it comes first, the Decision To Treat the incidental cancer.
2.4.5 Cancers of unknown primary.
The 28-day FDS is to the point at which a patient is either told they have cancer or cancer is excluded. For the purposes of the 28-day FDS, if a patient is told they have cancer, but the primary site is currently unknown then this would act as a clock stop and CANCER PRIMARY SITE (CANCER FASTER DIAGNOSIS PATHWAY) recorded as 15 – Metastatic disease of unknown primary.
2.4.6 Diagnosis of cancer prior to referral.
Where a patient is told they have cancer prior to referral, this communication should be confirmed when the patient is first seen and the CANCER FASTER DIAGNOSIS PATHWAY END DATE should be recorded as the FIRST SEEN DATE.
2.4.7 Diagnosis of recurrence of previously treated cancer.
Where a patient has been previously diagnosed and treated for cancer and is referred via an Urgent Suspected Cancer, Breast Symptomatic or Urgent Screening referral then the 28-day FDS will apply.
Where the patient is told that they have cancer, it may be unclear if the tumour is a new primary cancer or a secondary site from the previously diagnosed cancer. The 28-day FDS would still stop at this point with the CANCER FASTER DIAGNOSIS END REASON being recorded as 01-Diagnosis of Cancer and the CANCER PRIMARY SITE (CANCER FASTER DIAGNOSIS PATHWAY) recorded as the site of the tumour.
The relevant first definitive treatment standard would only apply if this tumour was then shown to be a new primary.
2.4.8 Ending the Faster Diagnosis Standard pathway before diagnosis.
In most cases, the pathway will end when the patient is informed that the possibility of cancer has been ruled out or that they have been diagnosed with cancer.
However, there may be cases where a Decision To Treat is made before a diagnosis is made and communicated to the patient, for example, skin or ovarian cancers.
This would apply to cases where a patient is diagnosed with a reportable cancer AND those who are diagnosed with another condition or where cancer is excluded.
In such cases the CANCER TREATMENT PERIOD START DATE should be recorded as the Decision To Treat date, as normal. Once the patient is told their diagnosis, the CANCER FASTER DIAGNOSIS PATHWAY END DATE should be recorded as the date of communication with the patient as normal, even where this falls after the treatment date.
For reporting purposes, the CANCER FASTER DIAGNOSIS END DATE will always be used to derive the reporting month and the provider to which the activity will be allocated. However, where the CANCER TREATMENT PERIOD START DATE falls before this date, it will automatically be used to calculate the overall FDS waiting time.
2.4.9 Histological diagnosis.
In some instances, the clinical team will want to wait until they have the histology results before deciding as to whether the patient has cancer. In these circumstances the Faster Diagnosis pathway would end after a histological diagnosis, at the point the outcome is communicated with the patient. This does not need to happen for all patients, as the clinical team can consider they have enough information to communicate a diagnosis or ruling out of cancer before having the histological result.
2.4.10 Cancer outside the scope of treatment standards.
Where a cancer is diagnosed but is outside the scope of the treatment CWT standards, for the purposes of the 28-day FDS the CANCER FASTER DIAGNOSIS PATHWAY END REASON should be recorded as 02- Ruling out of Cancer.
Details of inclusions and exclusions for the treatment standards are included in tumour specific section of this guidance. (Section 6)
2.5 Exclusions from Faster Diagnosis Standard
Where a patient is excluded from the 28-day FDS the following fields should be completed:
- CANCER FASTER DIAGNOSIS PATHWAY END REASON – Option 03 – Excluded from the cancer Faster Diagnosis Standard
- CANCER FASTER DIAGNOSIS PATHWAY END DATE – Recorded as the date the patient is discharged back to the GPs care, date of death or otherwise excluded depending on exclusion reason
- CANCER FASTER DIAGNOSIS PATHWAY EXCLUSION REASON – With reason selected
The 28-day FDS will not apply to these patients, with the exception of when 01- Patient died before communication of diagnosis is selected as the CANCER FASTER DIAGNOSIS PATHWAY EXCLUSION REASON in which case the patient would be included if the date of death is more than 28 days after the clock start.
The below sets out the exclusion reasons and the scenarios where they can apply:
2.5.1 Patient died before a communication of diagnosis (01).
This is to be used where a patient dies before a communication of cancer diagnosis or exclusion of cancer. In such cases the CANCER FASTER DIAGNOSIS PATHWAY END DATE should be recorded as the date the patient died.
For the purposes of the Faster Diagnosis Standard, if this is within the target timeframe (28 days), the patient would be excluded, but if it is after 28 days then the patient would be included as a breach of the standard.
2.5.2 Patient declined all diagnostic appointments (02).
This can only be used where a patient declines all diagnostics appointments and is therefore discharged back to the GPs care.
This cannot be used in the scenario where a patient declines or delays one or more diagnostics and the patient, is still followed up urgently in hospital, for example with alternative diagnostics, or the patient asks for time to consider if they would like a diagnostic.
2.5.3 Patient declined all appointments (03).
This can only be used where a patient declines all appointments and is therefore discharged back to the GPs care. In this scenario this should be clearly communicated to the GP.
2.5.4 Patient opted for private diagnostics (Patient may come back for NHS funded treatment) (04).
This can be applied where a patient has opted to have their diagnostics through private funding.
This should not be applied if NHS diagnostics are sub-contracted to a private provider as the activity would still be NHS funded, and an NHS provider would still be commissioned to provide the diagnostics.
2.5.5 Repeated Did Not Attends (DNAs)/Patient triggered cancellations (05).
This can only be applied following multiple DNAs and patient cancellations where a clinical decision is made to discharge the patient back to the GPs care.
The exact protocol for this should be agreed locally with the following principles:
- You would not discharge a patient after a single DNA.
- You cannot apply this exclusion reason where a patient is continuing to be followed up by the provider.
- You cannot apply this where a patient has rescheduled an appointment even multiple times, as this shows the patient has engaged and still requires follow up.
2.5.6 Patient ineligible for NHS funded care (06).
This can be applied if a patient is found to be ineligible for NHS funded care, and as a result is discharged by the provider. This cannot be applied if a patient continues the pathway under NHS care.
Providers should also be aware of relevant responsibilities for providing treatment which clinicians consider to be immediately necessary or urgent to any patient who is not exempt from charges, even if they have not paid in advance. Further information is available at on the GOV.UK website: NHS visitor and migrant cost recovery programme – GOV.UK
This document confirms that refugees and asylum seekers are eligible for NHS care. In addition, undocumented migrants with cancer can be treated if their case is deemed urgent by a clinician.
3. Treatment standard specifics
3.1 Treatment standard overview
The CWT service standard is:
- Maximum 31 days from Decision To Treat/Earliest Clinically Appropriate Date to Treatment of cancer [Operational Standard -96%]
3.2 Coverage of treatment standards
Treatment CWT service standards are applicable to patients cared for under the NHS in England with ICD codes C00-C97 (excluding basal cell carcinoma of Skin) and D05 (carcinoma in situ – breast). This includes those patients:
- being treated within a clinical trial;
- whose cancer care is undertaken by a private provider on behalf of the NHS, i.e. directly commissioned by an English NHS commissioner;
- whose care is sub-contracted to another provider – including a private provider – (and hence paid for) by an English NHS provider, i.e. commissioned by an English NHS commissioner but subcontracted out by the commissioned provider;
- patients who choose initially to be seen privately but are then referred for first and/or subsequent treatments in the NHS.
- diagnosed with a second new cancer;
- without microscopic verification of the tumour (i.e. histology or cytology) if the patient has been told they have cancer and/or have received treatment for cancer; or
- with any skin squamous cell carcinoma (SCC).
The one-month (31 days) treatment standard applies to:
- All First Definitive Treatment for Cancer
- All subsequent treatment modalities with exception of Active Monitoring and Palliative care treatments where CANCER TREATMENT MODALITY is recorded as 07 -Specialist Palliative Care ,08 – Active Monitoring (excluding Non-specialist Palliative Care) or 09 – Non-specialist Palliative Care (excluding Active Monitoring)
3.3 What is a definitive treatment?
A treatment is an intervention intended to manage the patient’s disease, condition, or injury and to avoid further intervention. It is a matter of clinical judgement, in consultation with the patient.
For cancer waits a First Definitive Treatment (FDT) is defined as the start of the treatment aimed at removing or eradicating the cancer completely or at reducing tumour bulk.
The tumour specific guidance in section 5 should be referenced when making a judgement on if a particular procedure should be recorded as an FDT. Examples include the guidance around Trans Urethral Resection of Bladder Tumour (TURBT) for bladder cancer (section 5.12.2) and polyp removal for colorectal cancer (section 5.7.1)
3.4 Decision To Treat
The Decision To Treat (DTT) date is the date the patient agrees a treatment plan, i.e. the date that a consultation between the patient and the clinician took place and a Planned Cancer Treatment was agreed. This is recorded as the CANCER TREATMENT PERIOD START DATE.
The date the patient signs the consent form may, depending on administrative procedures locally, take place some days after the DTT. It is advised that the meeting at which the treatment plan is agreed is classed as the DTT, not the date the consent form is signed. If a patient is having a joint procedure (e.g. Breast mastectomy with immediate reconstruction) the DTT date would be the first consultation where the intent to do a joint treatment was agreed, regardless of which clinical team the agreement was with.
3.4.1 Can a DTT date be changed?
Yes; if a patient decides they do not want the treatment originally agreed to (e.g. if a patient is offered surgery and is given a To Come In (TCI) date then decides they would rather have chemotherapy then the DTT is reset to when the chemotherapy is agreed); or due to clinical considerations after the agreement it is decided that the agreed treatment is no longer appropriate (e.g. pre-operative tests find complications); and a different treatment is discussed and agreed to, then the date of agreement for the treatment the patient goes on to have would be the new DTT and the 31-day clock is reset.
If the patient is on a 62-day pathway, the clock continues.
3.4.2 If a patient’s DTT is in the private sector but treatment is in the NHS (By the same clinician they were seeing privately), how do we record the patient?
As the clinician seeing the patient in private practice is the same one that will be treating the patient in the NHS it would not appear a good use of NHS time to have an additional consultation to agree the treatment again. However, as the DTT should be reached somewhere along the pathway of care the patient is following whilst in NHS commissioned care, the DTT in this scenario should be the point at which the English NHS provider commissioned to provide the treatment is notified that the patient is being transferred back into the NHS and that the clinician has already agreed with the patient the course of action.
3.4.3 When does the 31-day period start for a treatment that can only be provided following an application for funding to the commissioner?
The clock would start at the DTT for the treatment in question (recorded as the CANCER TREATMENT PERIOD START DATE). If an application then has to be made to the commissioner to approve funding of the treatment the 31-day clock has started and would not stop for the commissioner’s decision-making process, i.e. the commissioner would need to ensure their processes are streamlined to manage the pathway for patients effectively, including hearing appeals etc.
3.4.4 If a commissioner declines to fund a specific treatment (and there is no appeal) would a new Decision To Treat for an alternative treatment and hence new 31-day period be started?
The change of treatment would not be counted as part of the same 31-day period as that period did not end with a treatment. The 31-day clock would re-start once a new DTT date for an alternative treatment is made (this would be recorded as the CANCER TREATMENT PERIOD START DATE).
The clock for the 62-day period (if applicable) would continue until a treatment takes place (i.e. until there is a TREATMENT START DATE (CANCER)).
3.5 First definitive treatment
The FDT is normally the first intervention which is intended to remove, debulk or shrink the tumour.
Where no definitive anti-cancer treatment is planned almost all patients will be offered a palliative intervention (e.g. stenting) or palliative care (e.g. pain relief), which should be recorded for these purposes.
3.5.1 If a cancer treatment is unsuccessful is this still classed as an FDT?
If a procedure is intended to be ‘anti-cancer’ but is unsuccessful, such as an open and close surgery where the tumour is not removed, then this is still classed as an FDT.
3.5.2 If a patient is treated for a suspected cancer but during the treatment it is found that the patient has an entirely different cancer which has not been treated by the treatment, does the treatment count as an FDT?
No, if a patient is treated for one suspected cancer but found to have a different cancer to that which was suspected (i.e. the original diagnosis was incorrect) and the original treatment was not able to treat the newly identified cancer, then the original treatment is not classed as a FDT. Even though the treatment had an anti-cancer intention it is more important that the patient is continued to be monitored to ensure they are re-diagnosed and treated as quickly as possible.
3.5.3 Can diagnostic procedures be counted as an FDT?
A purely diagnostic procedure (including biopsy) does not count as an FDT unless the tumour is effectively removed by the procedure. If the intention was diagnostic and the excised tissue was found to be malignant, the procedure could count as an FDT if the tumour has effectively been completely removed by the excision.
The tumour specific guidance (section 5) provides further clarification on specific procedures.
3.6 Incidental findings
Some patients may be diagnosed with cancer during routine investigations where cancer has not been suspected or while being treated for another condition, i.e. incidental findings.
These patients should be monitored under the 31-day Decision To Treat (DTT) to treatment standard. Where the patient is treated immediately at the point of diagnosis, the DTT (recorded as CANCER TREATMENT PERIOD START DATE) will be the same date as the date of the admission (e.g. when a patient is incidentally found to have a cancer during surgery for a suspected benign condition).
Where the incidental finding is made prior to a Decision To Treat, the patient should be placed on the 62-day consultant upgrade pathway, with this mandated on the point as either when a radiology or pathology report is reported and triggers a trust cancer flagging system or the first referral is made to a Cancer MDT meeting, whichever is the earliest.
If a patient is referred as an urgent suspected cancer referral and a different cancer is incidentally found that is unrelated to the referral, the Faster Diagnosis Pathway period would end with the communication of diagnosis, or (where it comes first) DTT, for the incidental cancer. Although the cancer diagnosis was incidental, it was found during investigations as part of the suspected cancer referral.
If a patient is referred as an urgent suspected cancer referral and a cancer is incidentally found that is unrelated to the referral, the 62-day period would end with the First Definitive Treatment (FDT) for the incidental cancer. Although the cancer diagnosis was incidental, it was found during investigations as part of the initial referral.
3.7 Multiple diagnosis
Patients diagnosed with two or more primary cancers as a result of one referral from primary care would be recorded using two or more PATIENT PATHWAY IDENTIFIERs.
One would be generated at the point of referral and the other(s) when the other primary is first suspected, and a new (parallel) pathway starts.
There would be one 28-day FDS/62-day pathway linked to this referral, relating to the initial referral and diagnosis. The other cancer(s) would be considered a 31-day pathway only, unless a consultant upgrade takes place, in which case the second primary would also be covered by the 62-day consultant upgrade pathway.
3.8 Enabling treatments
The enabling treatments that can be classed as FDTs (regardless of setting) are:
- colostomy for bowel obstruction or to prevent bowel obstruction where this is necessary prior to definitive treatment unless this is necessary due to the length of wait for definitive treatment
- stenting where this is necessary prior to definitive treatment unless this is necessary due to the length of wait for definitive treatment (e.g. oesophageal stent, uterine stenting for advanced cervical cancer, pancreatic or biliary stent to relieve jaundice & colonic stent to relieve an obstruction)
- Gastrojejunostomy
- Portal vein embolization prior to surgery for liver cancer (primary or secondary) to allow liver growth prior to surgery
- Dental extractions to enable radiotherapy
- Percutaneous gastrostomy line insertions and radiologically inserted gastrostomy lines
- Vaccinations prior to removal of spleen
- Trans-positioning of ovaries (for preserving fertility/side effects)
- Drugs forming part of chemotherapy regimens which need to be administered prior to the first administration of chemotherapy, for example vitamin B12 injections for lung cancer regimens. (In this scenario the CANCER TREATMENT MODALITY should be recorded as 97- Other treatment (not listed)).
- Patients given Rectal Spacers ahead of radiotherapy
The following are procedures which would not count as an enabling treatment based on previous advice:
- Iron tablets
- Monofer or ferinject iron infusion
- Peripherally inserted central catheter line insertions
- Cystodiathermy
Enabling treatments have been developed / reviewed against the following principles:
- The enabling treatment is clinically necessary prior to cancer treatment
- The enabling treatment is not necessary because of a delay in cancer treatment
- A clinically significant delay of more than one week is required between enabling treatment and commencement of cancer treatment.
- The enabling treatment is targeted towards a specific group of patients and wouldn’t also include a large number of patients who wouldn’t meet the other principles.
Where a surgical enabling treatment is required the CANCER TREATMENT MODALITY should be recorded as 24- Surgery (enabling treatment).
Treatments following an enabling treatment should be completed as soon as is clinically appropriate. The 31-day Decision To Treat (or Earliest Clinically Appropriate Date) to Treatment Standard would apply to these treatments.
The NHS Cancer Programme is aware that pathways and treatments are likely to evolve over time, and it may therefore be appropriate to update this list in future versions of the guidance. If there are treatments which you feel should be included as an enabling treatment, please contact england.cancerwaitsdata@nhs.net, setting out details of the treatment and also how this treatment is applicable to the principles set out above. These can then be clinically reviewed for consideration in future versions of the guidance.
3.9 Anti-Cancer Drug Regimen
Cancer Treatment Modality
- 02 Anti-Cancer Drug Regimen (Cytotoxic Chemotherapy)
- 03 Anti-Cancer Drug Regimen (Hormone Therapy)
- 14 Anti-Cancer Drug Regimen (Other)
- 15 Anti-Cancer Drug Regimen (Immunotherapy)
3.9.1 Under what circumstances are these First Definitive Treatments (FDT)?
- chemotherapy (including prior to planned surgery/radiotherapy)
- biological therapy including treatments targeted against a specific molecular abnormality in the cancer cell (e.g. rituximab, trastuzumab, imatinib) and treatments which target the immune system (e.g. interferon, interleukin 2, BCG)
- Hormone treatments when either:
- given as the sole treatment modality
- the treatment plan specifies that a second treatment modality should only be given after a planned interval.
3.9.2 Is each dose of chemotherapy classed as a different treatment?
A course of chemotherapy is counted as a single treatment. A course could be comprised of a single dose or many cycles of doses.
3.9.3 Would a change in drug type within chemotherapy be classed as a subsequent treatment?
If you are modifying a regimen during the course of the chemotherapy, then the same 31-day standard could apply if it was decided to make a change to a drug mix, but the treatment was carrying on uninterrupted.
A change in drugs would be classed as a subsequent treatment if it was classed as a different course of chemotherapy.
The key should be whether a new consent form has been signed or not, i.e. if it has then this should be classed as a new treatment and therefore a new 31-day period started.
3.9.4 What is the date of the FDT if treatment is self-administered?
The TREATMENT START DATE (CANCER) should be recorded as the date of the outpatient appointment where the patient is given the prescription.
3.9.5 How should we record a treatment which is self-administered and prescribed by a GP on behalf of a secondary care provider?
Where a GP is asked to provide a prescription to the patient by a secondary care MDT, then the provider who hosts the MDT should record the TREATMENT START DATE (CANCER), under their organisation code as the date the GP makes the prescription. It is the responsibility of the GP to carry this out, but the MDT and secondary care provider retain a responsibility to ensure that the patient under their care receives the treatment recommended by the MDT.
3.9.6 How should we record the use of supportive care drugs on the CWT system?
Supportive care drugs alone are not considered a FDT unless a patient is receiving palliative care only (of which these drugs are part) and no active treatment is planned.
3.9.7 Is hormone therapy recorded as an FDT when another treatment is planned?
Hormone treatment can only be classed as FDT if it is to be the sole treatment modality, or the treatment plan specifies that a second treatment modality should only be given after a planned interval.
Hormone treatment conducted at the same time as another modality would either be:
- neoadjuvant therapy – (therapy is necessary prior to treatment as specified by the care plan) in which case this would be counted as an FDT
- part of a combined treatment in which case a single treatment package is recorded.
- counted as a subsequent treatment, including adjuvant therapies (where the hormone is given after surgery to prevent recurrence).
The following illustrate the interpretation of this in common scenarios:
- Breast cancer, estrogen receptor positive (ER+) suitable for surgery but delayed due to capacity/risk to patient and started on hormones, would not stop the clock.
- Breast cancer, ER+ not suitable for surgery and operation not planned, hormones would count as a clock stop.
- Prostate cancer, plan for hormones is for a specified period of time, followed by radiotherapy – hormones would stop the clock.
- Prostate cancer, surgery planned but delayed due to capacity/risk to patient and started on hormones, would not stop the clock.
- Prostate cancer, started on hormones, no current plans for other treatments but could be reviewed in the future. Would count as a clock stop.
Where a patient is given hormones treatment because they are currently not fit for another treatment but may become fit for treatment in the future then hormones can count as first definitive treatment.
3.10 Palliative Care and Active Monitoring
Cancer Treatment Modality
- 07 Specialist Palliative Care
- 08 Active Monitoring (Excluding Non-Specialist Palliative Care)
- 09 Non-Specialist Palliative Care (Excluding Active Monitoring)
Specialist Palliative Care (SPC) can be counted as a First Definitive Treatment if provided when no active treatment is planned, delivered via:
- hospital SPC teams, including in dedicated inpatient, outpatient, and day palliative care units
- community SPC teams, including in hospice settings.
3.10.1 What is the difference between Specialist Palliative Care (Code ‘07’) and Non-Specialist Palliative Care (Code ‘09’)?
Specialist palliative care is delivered under the management of a team led by a consultant or nurse in palliative medicine.
Non-Specialist Palliative Care is any palliative care (excluding active monitoring) that is not given under the management of a consultant or nurse in palliative medicine.
3.10.2 Are palliative treatments (surgery, radiotherapy, or anti-cancer drug regimens) classed as palliative care?
For CWT, palliative treatments (surgery, radiotherapy, or anti-cancer drug regimens) should not be classed as palliative care generally. This should be classed as the relevant treatment (surgery, radiotherapy, or anti-cancer drug regimens).
3.10.3 How is care at a hospice recorded?
For the purposes of CWT if a patient is transferred to a local voluntary hospice for palliative treatment and no active treatment is planned, then the date of the referral to the hospice would count as the start date of the treatment. This would be recorded by the NHS organisation that made the decision to transfer the patient to the independent palliative care provider.
3.10.4 Is specialist palliative care (SPC) in hospices excluded from CWT if carried out by a non-NHS provider?
The CWT system is not able to capture data from non-NHS hospices as they do not have an N3 connection or an ODS (Organisation Data Service) and (most importantly) are not subject to the data standard. We know some patients diagnosed do not receive first treatment within an NHS provider for various reasons which include:
- the local SPC service being non-NHS
- the patient electing to follow private treatment options; or
- the patient passing away before treatment can be administered.
However, there are some instances where these services should be recorded:
- if the voluntary provider is sub-contracted to provide the service by an English NHS organisation that has been commissioned to provide the care. In this case the commissioned organisation should report the activity, with the start date being the initial consultation (if available), or the referral to the voluntary service (if the consultation date is not available)
- if the service commissioned is a joint venture between an English NHS provider and a voluntary provider the activity should be recorded by the NHS provider with the start date being the initial consultation
- If the activity is commissioned from the voluntary sector by the NHS and the contract includes the requirement for the voluntary provider to provide the regular NHS datasets. In these instances, we would have expected the voluntary organisation to have made arrangements to pass these data back to the commissioning authority for processing with the start date being the initial consultation
- if the consultant caring for the patient in the voluntary service is providing the service as outreach, the employing NHS organisation would record these statistics as per their normal practices.
Patients treated under these scenarios will be in the minority and most care in voluntary organisations remains outside the scope of this data collection as it is not commissioned by the NHS.
What is Active Monitoring and when can it be used?
This is where a cancer diagnosis has been reached, but it is not appropriate to give any active anti-cancer treatment at that point in time.
The decision to whether it is appropriate to give a treatment should only consider the diagnosed cancer and not patient thinking time or other medical conditions that the patient has.
The patient is therefore monitored until a point in time when it is appropriate to give an active treatment for the diagnosed cancer. A patient would have to agree that they are choosing to be actively monitored for a period of time rather than receiving active cancer treatment.
Active monitoring may be used for any tumour site if appropriate and it would start on the date of the consultation where this plan of care was agreed with the patient. The one exception to this is a patient who is diagnosed with low or low-intermediate prostate cancer, who would be recorded as active monitoring at the point the diagnosis is communicated to the patient, even if the patient is considering their treatment options.
Whilst a patient is being actively monitored, they may receive symptomatic support.
Active Monitoring can be recorded where a patient is currently considered clinically unfit for cancer treatment but receiving support to improve their overall fitness. Examples include:
- dietetics support for malnourished patients
- respiratory support for those with breathing difficulties
- haematology input where patients are anaemic
However Active monitoring cannot be used where an active anti-cancer treatment has been planned (i.e. a Decision To Treat has been made) or is likely to be planned and the pathway is progressing, but comorbidities need to be addressed before treatment.
It is also not possible to record Active Monitoring where a patient is advised to undertaken life-style changes or undergo a Prehabilitation Programme prior to planned treatment.
3.10.5 Active monitoring in low and low-intermediate prostate cancer.
Prostate cancer diagnoses should be classified using the Cambridge Prognosis Group Classification as detailed in NICE guidance (NG131).
| Cambridge Prognosis Group Category | Cancer Waiting Times Classification | Criteria |
|---|---|---|
| 1 | Low | Gleason score 6 AND PSA <10 ng/ml AND Stages T1-T2 |
| 2 | Low – Intermediate | Gleason score 3+4 = 7 OR both PSA 10-20 AND stages T1-T2 |
| 3 | Intermediate – High | Gleason 3+4 = 7 AND PSA 10-20 ng/ml AND Stages T1-T2 OR Gleason 4+3 = 7 AND Stages T1-T2 |
| 4 | High | Gleason 8 OR PSA >20 ng/ml OR Stage T3 |
| 5 | High | Gleason 9-10 OR Stage T4 |
This patient’s risk classification should be recorded using the data item PROSTATE CANCER CLINICAL RISK CATEGORY, as per the above table.
It is recognised that patients with Cambridge Prognosis Group categories 1 or 2, do not clinically require rapid treatment, and will often benefit from time to consider their future treatment options which can have considerable debilitating side effects. Therefore, these patients should be recorded as being on active monitoring from the date the diagnosis is communicated with them, even if they were still considering their treatment options.
This would only apply where sufficient diagnostics have been undertaken to establish a patients prognosis category. If further staging investigations are outstanding, for example a bone scan, active monitoring would not apply at that stage, given the Cambridge Prognosis Category would not be known before these diagnostics are undertaken.
Although the 62-day clock will have stopped, it is still important that trusts have systems in place to robustly track these patients to ensure they are followed up appropriately. In this cohort of patients any future treatment would then be classified as a subsequent treatment and monitored using the 31-day standard.
It is essential that where patients are automatically placed on an active monitoring pathway, the risk classification system used is clearly explained to them. It is also essential that their right to rapid treatment should they wish to proceed immediately is not infringed, and that as soon as a decision is made to proceed with treatment, the 31-day subsequent treatment clock starts immediately.
3.10.6 Can active monitoring be used to allow a patient time to consider treatment options?
Active monitoring is not a substitute for patient ‘thinking time’ with the exception of low and low-intermediate risk prostate cancer patients.
3.10.7 If a decision is made to observe the progress of a patient for a few months as cancer is suspected but still not confirmed, can active monitoring be used as a clock stop?
No. Active monitoring is only a legitimate treatment option for confirmed cancers. In this scenario the patient has not received a confirmed diagnosis of cancer, therefore CANCER TREATMENT MODALITY cannot be recorded as ‘Active Monitoring.’ This scenario is one of diagnostic uncertainty. The 28-day and 62-day period remains open, and the patient will breach if cancer goes on to be confirmed. The operational standard for the 28-day and 62-day standard has been set to allow for breaches due to clinical reasons.
3.10.8 A patient is on the 62-day pathway and is diagnosed with another medical condition, unrelated to the cancer, which needs treating/resolving before cancer treatment can be given – can active monitoring be used for the cancer?
No, in this scenario active monitoring is not appropriate, but the guidance around clinically urgent treatment of another condition should be reviewed to see if a treatment adjustment could be applied.
3.10.9 Can Active Monitoring be used as a subsequent treatment?
Active monitoring can be a subsequent treatment, but you would only want to use it where the intention was for long term surveillance where the decision had been taken to monitor the progress of a specific condition.
This category of treatment would exclude any ongoing assessments to determine fitness for a subsequent treatment (as this would be prior to the setting of an Earliest Clinically Appropriate Date). It would also exclude routine follow-up, as this is not intended as a treatment. Active monitoring as a subsequent treatment will not count towards performance against the 31-day standard.
3.11 Radiotherapy
Cancer Treatment Modality
- 04 Chemoradiotherapy
- 05 Teletherapy (Beam radiation excluding proton therapy)
- 06 Brachytherapy
- 13 Proton Therapy
3.11.1 Under what circumstances is radiotherapy a First Definitive Treatment (FDT)?
When used to treat either the primary site or to treat metastatic disease of a known or unknown primary.
3.11.2 What is recorded as the start of treatment for Chemoradiotherapy?
When the treatment is Chemoradiotherapy, the start of treatment would be the first delivery of either Chemotherapy or Radiotherapy, whichever comes first.
3.12 Surgery
Cancer Treatment Modality
- 23 Surgery (excluding enabling treatment)
- 24 Surgery (enabling treatment)
3.12.1 Under what circumstances is this a FDT?
- complete excision of a tumour
- partial excision/debulking of a tumour (but not a biopsy for diagnostic or staging purposes unless it effectively removes the tumour even if margins are not clear)
- palliative surgical interventions where no active treatment is planned to follow (Unless specified as an enabling treatment in section 3.8)
The tumour specific guidance in section 5 should be referenced when making a judgement on if a particular procedure should be recorded as an FDT. Examples include the guidance around Trans Urethral Resection of Bladder Tumour (TURBT) for bladder cancer (section 5.12.2) and polyp removal for colorectal cancer (section 5.7.1)
3.12.2 If a surgery is started and must be abandoned because the patient becomes too ill to continue, could it still be counted as an FDT?
If the surgery had commenced but it had to stop then yes it would still be the FDT. Although, if the patient is admitted for surgery and becomes ill prior to the surgical procedure beginning then the clock would continue until the patient is able to be treated.
3.12.3 How should stenting and clearing a stent be recorded?
Stenting should be recorded as a form of surgery but should only be included where it meets the criteria set out in the Enabling Treatments chapter 3.8.
3.12.4 Does a second excision/wide local excision count as a subsequent treatment even if no further tumour is found/margins are clear?
Yes, provided that the first excision has completely removed the cancer.
3.13 Other treatments
3.13.1 How should treatments using new technologies be recorded?
If there is not an appropriate category for a new technology, it should be recorded under CANCER TREATMENT MODALITY as Code 97 ‘Other Treatment’. If you are not sure about how a treatment using a new technology should be coded or if you are aware of new treatments coming on line that would need a new code in the future, please contact england.cancerwaitsdata@nhs.net.
3.13.2 How are transplants handled for cancer waits?
When the agreed treatment for a cancer is a transplant the DTT would be when the patient agrees to the care plan that includes the transplant.
The TREATMENT START DATE (CANCER) would be the date the patient is added to the transplant list.
For the purposes of monitoring the 62-day standards a transplant should only be considered first treatment if no other active anti-cancer treatment is planned in the interim.
- for CWT both bone marrow stem cell transplants and peripheral blood stem cells are treated the same.
- it is a clinical decision whether to record bone marrow/stem cell transplants as ‘surgery’ or ‘other.’
- CWT does not distinguish between whether the donor is allogeneic or autologous.
3.13.3 CAR-T therapy treatments.
Where a patient is receiving CAR-T therapy, the point at which cells are extracted can be classed as the start of FDT. This would still apply if the treatment was unsuccessful, and cells could not then be given back to the patient.
3.14 Combined treatments and treatment packages
For the purposes of the cancer waits dataset combined treatments are treatments of different modalities combined in a way that they must be scheduled to take place together. These should be regarded as single treatment packages.
Examples of combined treatments include:
- chemoradiotherapy – where radiotherapy and chemotherapy are delivered within a strict schedule so that they interact to make both treatments more effective (e.g. weekly 5FU during radiotherapy for rectal cancer, radiotherapy given synchronously with cycle 4 of CMF for breast cancer)
- pre-operative or intra-operative radiotherapy – where radiotherapy is given just before or during surgery to maximise the effect of both treatments.
- The definition of combined treatments excludes adjuvant therapies where each treatment can be scheduled separately (e.g. breast surgery followed by post-operative radiotherapy, chemotherapy for small cell lung cancer followed by consolidation radiotherapy).
3.14.1 How are individual supportive care packages recorded?
For the purposes of monitoring the 31-day subsequent treatment standards, supportive care packages (palliation of symptoms, symptomatic support etc) are to be considered as the whole. This means that whilst a patient may be receiving a range of care (transfusions, pain relief etc), if it is a single agreed package, the start of the package of care should be taken as:
- the date of the delivery of the first episode
- the consultation that results in the referral to a non-NHS specialist palliative care service (that are not contractually obliged to return these data independently); or
- the consultation at which the patient receives a prescription.
The recording of NHS supportive care provision will remain the responsibility of the organisation commissioned to provide that care unless there is a local agreement in place for this activity to be recorded by another organisation.
However, it is possible that additional palliative care/support might be agreed after or in addition to this package which would count as a new 31-day period
3.15 Treating Metastatic Disease
Where a patient receives treatment for a metastatic site tumour rather than the primary tumour first, this can be recorded as a first definitive treatment.
In this scenario the following should be recorded as the CANCER TREATMENT EVENT TYPE:
- Where primary is known – 12: First treatment for metastatic disease following a known Primary Cancer.
- Where primary is unknown – 07: First treatment for metastatic disease following an unknown primary
3.16 Subsequent Treatments and Earliest Clinically Appropriate Date (ECAD)
All subsequent treatments for primary and recurrent cancer need to have a 31-day period recorded.
A subsequent treatment could be:
- anti-cancer treatment (curative or palliative) aimed at shrinking (or delaying the growth/spread) of the tumour/cancer
- the provision of palliation for the symptoms resulting from the tumour/cancer
- symptomatic support by non-specialist palliative care teams where no active cancer treatment is planned
- active monitoring (where no active or palliative treatment is appropriate).
An individual patient may receive one or a combination of these interventions.
The 31-day subsequent treatment standards do not cover follow on treatments that are not directly related to shrinking or delaying growth/spread of the cancer (e.g. closure of stomas, reconstructive surgery following initial surgery, rehabilitative and psychological services etc).
Subsequent treatments act in a similar way to the first treatment recorded under the 31-day standard. However, subsequent treatments can either start with a Decision To Treat (DTT) date or the ECAD (both are recorded as CANCER TREATMENT PERIOD START DATE).
The ECAD is the earliest date that it is clinically appropriate for the next activity that actively progresses a patient along the pathway for that treatment to take place. This should be a previously agreed and clinically appropriate period of delay before the next treatment can commence. The activity may not always be the start of the treatment itself but could be the next appointment which deals with the planning of that treatment. When determining an ECAD only patient issues should be considered, not local capacity constraints.
The patient must be fully informed and agree to the ECAD.
The member of the consultant team liaising with the patient about the treatment in question would set the ECAD.
The ECAD can be with or without the presence of the patient and set at a number of points:
- at the clinical review with the patient following the preceding treatment. If it is not possible to decide at the review a further review could be arranged
- at the start of the preceding treatment if the patient will not be reviewed between treatments
- at the Multidisciplinary Team (MDT) meeting if it is possible to identify the likely ECADs between treatments in an agreed package
- following receipt of test results and prior to discussing with the patient if this is an appropriate date.
The patient does not have to be physically present on the date the ECAD is set as it can be set based on an earlier consultation.
The ECAD can be reviewed and changed any time up to the ECAD.
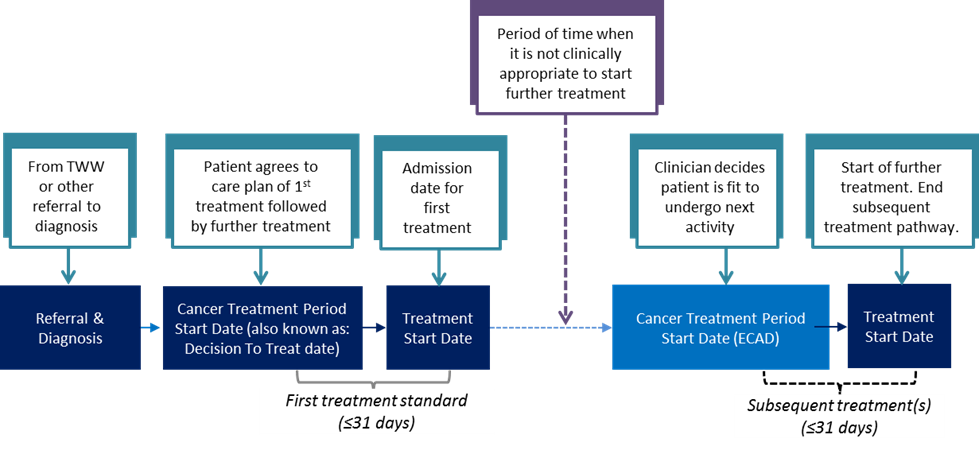
3.16.1 What counts as an activity in the context of an ECAD?
In the NHS Data Dictionary activity is defined as “A provision of services to a patient by one or more care professionals.” The ECAD relates to the next activity that actively progresses a patient pathway but not to an activity that relates to determining a patient’s fitness to continue their care plan.
3.16.2 Do subsequent treatments have to be uploaded sequentially?
Subsequent treatments are individual 31-day periods. It is therefore possible to upload details for a 31-day subsequent treatment period even if the details for the first treatment or previous subsequent treatment periods have not been entered onto the CWT system.
3.16.3 Do subsequent treatments have to be linked back to the initial referral (which could be years back, especially for a recurrence)?
No, each subsequent treatment will be a new 31-day period starting at the DTT or ECAD. The cases could be linked by the NHS number locally, if desired, for audit or trend analysis but this is not an automatic link and is not required nationally.
3.16.4 How long should patient records be kept open to consider possible recurrences and therefore the need for subsequent treatments in the future?
Indefinitely, records are not closed. There can be multiple 31-day periods over a number of years, each starting with a new DTT date or an ECAD.
A process needs to be in place locally to ensure that patients needing a subsequent treatment are identified and tracking restarts. Different models may be needed to capture information on patients with different cancer types and/or requiring different modalities of treatment.
3.16.5 How do you manage patients who receive an initial treatment from a private provider but then seek subsequent treatments through English NHS providers?
You need to have processes in place to identify and track any patient having subsequent treatments commissioned by English NHS providers, irrespective of whether earlier treatments were carried out by private providers. If a patient had a FDT in the private sector and then returns to the NHS for further treatment these further treatments would be classified as subsequent treatments (even if it is the first one they had on returning to the NHS).
3.17 Managing recurrences
When a patient, who has previously had cancer has a recurrent cancer diagnosis confirmed, the patient would proceed onto a 31 day subsequent treatment pathway.
The dataset includes a data item CANCER OR SYMPTOMATIC BREAST REFERRAL PATIENT STATUS. One option to complete this field is ‘Diagnosis of a recurrent cancer.’ Once this field is completed such patients would automatically be excluded from 62-day standard by the CWT system.
If the cancer diagnosed following a referral was in the same location or was the same type of cancer as a previously diagnosed cancer but is classed by the clinician as a new primary, then this would be a new 62-day pathway, not a recurrence. For example, if a patient has had a left breast cancer and then is referred via an urgent suspected cancer referral at a later date with a right breast cancer, then which pathway the patient is recorded under is dependent on the clinical decision as to whether this is a new primary or not.
3.17.1 Is there a time period after which a recurrence would be classed as a new primary?
There is no time limit. A recurrence is a recurrence, not a new primary, if that is the clinical diagnosis.
3.17.2 What is the difference between recurrences (potentially cured but recurs in the future) and progression (not cured and will progress at some point) in terms of the CWT standards?
A recurrence is where a patient has previously been informed that they are free of the disease.
A relapse or progression of a disease is where this has not happened.
Relapse and progression are terms more commonly associated with non-solid tumours (e.g. haematological malignancies) where it is more difficult to clearly identify if a neoplasm has been eradicated.
It is a clinical decision which category is most appropriate and in terms of cancer waits, it is used for the CANCER TREATMENT EVENT TYPE data items.
3.17.3 How will the patient pathway identifier work for recurrences?
The identifier for the primary cancer would be used for any recurrence of the same primary cancer in the future. This is because a recurrence is a continuation of the same patient pathway and a patient pathway lasts for the entire period of a single disease/condition, i.e. from the original referral date until a patient is cured or passes away. Even if a patient is discharged and then returns with a recurrence of the original condition, the identifier would be the same.
If a patient is diagnosed with a second primary cancer (i.e. not a recurrence of a cancer) this would have a new PATIENT PATHWAY IDENTIFIER (PPI).
3.17.4 How is transformation of benign and/or cancerous cells dealt with in CWT?
Transformations should be recorded as follows:
- if the initial condition had been within the scope of CWT and transforms then it would be classed as a recurrence
- if the initial condition was not within the remit of CWT and then transforms the new condition would be classed as a new primary
- if the initial condition had not been given a diagnosis but was being kept under surveillance and later transforms into a cancer within the remit of cancer waits this would be classed as being on the original 62-day pathway. (unless interval scanning as per section 4.2 has been recorded)
For example:
- follicular lymphoma transforming into a diffuse large B cell lymphoma or AML transforming to CML or CLL transforming to Hodgkin’s – would be classed as a recurrence as the initial conditions in each case had been covered by the cancer waits standards
- myeloid dysplastic syndrome transforming into AML – the AML would be classed as a new primary as MDS is not within the scope of cancer waits.
3.18 Clinical trials
If a patient has agreed to enter a clinical trial approved by an appropriate Ethics Committee (with a REC reference number) then the trial protocol will determine which treatments are classed as first or subsequent treatments respectively and they will be assigned as such under cancer waits standards.
For example, if the trial protocol sets out that the first treatment could potentially be surgery, hormonal drug treatment or a placebo depending on the arm of the trial the patient was on it would not matter which of these treatments the patient received it would be classed as the FDT.
Patients should be made aware if there is a possibility, they may receive a placebo as part of their treatment within a clinical trial.
The CANCER TREATMENT MODALITY for a placebo would be classed as Code 14 ‘anti-cancer drug regimen (other)’ for cancer waits reporting purposes assuming it is known which patient had received the placebo instead of another type of anti-cancer drug regimen. If it is a blind trial, and it is not possible to identify which patient received which type of drug, then the ‘anti-cancer drug regimen (other)’ category would be used for each drug arm.
For clinical trials where the protocol sets the timescales for delivery of treatment, the clock stop should be recorded as the date that consent is given by the patient to be entered into the trial.
For cases where a patient is entered into a clinical trial, and this has no impact on when a treatment cancer be delivered, for example a trial which relates to post-surgical care, the clock stop should be recorded in the same way as if a patient was not on a clinical trial.
Where combinations of treatments make up a single trial the enrolment date should be recorded as the treatment date, and the CANCER TREATMENT MODALITY should be recorded as the one which the patient is due to receive first.
3.19 Patient choice treatment adjustment
An adjustment for treatment can be applied in two scenarios:
- If the patient asks for a treatment after a specific date, then an adjustment can be taken up to this point, starting from the Decision To Treat.
- Where a patient is offered a date for treatment but declines this an adjustment can be taken as long as the offer is within a reasonable timeframe (i.e. within the 31-day standard). An additional adjustment could then be taken for any period after this offer which the patient is not available.
3.19.1 If a patient DNAs or cancels an agreed TCI date can an adjustment be made?
Yes, an adjustment would be applied from Decision To Treat/Earliest Clinically Appropriate date, to the date which the patient DNAs or cancels as long as this is within the 31-day Standard time-frame
3.19.2 What is the position on adjustments if a patient wishes to wait for a specific treatment option?
If the patient has been offered a choice of treatments and they opt for one where there is insufficient capacity to provide the treatment within the standard deadline then it would not be possible to use an adjustment. This would include examples where alternative modes of the same treatment are offered. (e.g. surgical approach).
If a patient is offered the same treatment with an alternative clinician in the same hospital and declines this, then an adjustment can be taken. An adjustment cannot be taken where the offer is made at a different provider, and the patient declines this.
3.19.3 What is the position on adjustments if a patient wishes to wait for a treatment under a specific consultant or provider?
If a patient is given a reasonable offer of treatment with provider/consultant/ location X but requests provider/consultant/location Y who cannot offer them an appointment within the standard time, then an adjustment can be made.
3.19.4 If a patient has been admitted for surgery but then changes their mind and does not proceed with the treatment can an adjustment apply?
If the patient decided that they did not want any treatment, then you would end the period on the date that this was agreed and record the CANCER TREATMENT MODALITY as code 98 ‘All treatment declined’.
If they agree to a different form of treatment, then you would use the DTT date for the newly agreed treatment as the starting point for the relevant 31-day period. You cannot make any adjustments for the 62-day period for a patient considering treatment X then declining and agreeing to treatment Y instead. With surgery, the clock would normally stop when the patient has been admitted (i.e. Prior to the surgery itself if it was planned for the next day etc), however, the admission date would not have stopped the 62-day clock in this example as the episode of care did not end with the treatment.
3.20 Clinically urgent treatment of another condition treatment adjustment
An adjustment can be applied if it is deemed clinically essential to treat another medical condition before treatment for cancer can be given, after a Decision To Treat the cancer has been made.
In such cases the adjustment would apply from the point at which it is confirmed that a patient needs treatment for the other medical condition, to the point at which after receiving treatment for this condition the patient is deemed clinically fit to commence their cancer treatment.
This would be recorded with the WAITING TIME ADJUSTMENT REASON (TREATMENT) being recorded as ‘Clinically urgent treatment of another condition.’
Where a patient is ordered diagnostics or referred to another specialist to exclude another medical condition, an adjustment cannot be applied for the period in which the patient is waiting to be seen for this assessment or diagnostics. An adjustment can only be taken from the point at which another condition is diagnosed, and the patient deemed clinically unfit for their cancer treatment, prior to the other condition being treated.
This adjustment cannot be applied for where a patient is advised to make lifestyle changes for example stop smoking, lose weight, or commence a period of prehabilitation prior to their cancer treatment. In these cases, the patient clock would continue. The 62-day and 31-day threshold is set to allow for patients in this scenario who will exceed the timescales.
3.20.1 Urgent treatment of another condition applied to patients with COVID-19 or influenza.
This adjustment should be applied as follows in relation to COVID-19 or influenza: Where a clinical decision is taken to offer a patient treatment now (i.e. the clinical view is that the risk of delay outweighs the COVID-19 or influenza risk), but the patient declines and requests a later date, an adjustment can be taken from the offered date to the date the patient is willing to come for treatment. In such situations, a process should be put in place to review the patient at fixed intervals to check whether their view has changed.
Where the clinical recommendation, agreed with the patient, is to delay treatment until the COVID-19/influenza risk decreases, a treatment adjustment cannot be taken.
If a patient chooses not to access treatment due to concerns about COVID-19/influenza they should remain on the appropriate active waiting list and remain visible. In line with current cancer waiting times standards, waiting times will not be ‘paused’ and clocks will continue through the period that the patient chooses not to attend.
If a patient waiting for treatment – with a Decision To Treat (DTT) – tests positive for COVID-19/influenza, then the clinically urgent treatment adjustment guidance can be applied. In such cases the adjustment would apply from the point at which it is confirmed that a patient has tested positive, to the point at which it is deemed that it is clinically appropriate to proceed with treatment.
3.21 Egg harvesting treatment adjustment
Where a patient opts for egg harvesting prior to their cancer treatment, an adjustment can be applied from the point at which the decision is made until eggs are harvested.
An adjustment cannot be applied for the period of time taken for the patient to wait to be seen by the egg harvesting service, only from the point at which the patient is seen by the service and agrees to egg harvesting to the point where harvesting takes place.
This would be recorded under the WAITING TIME ADJUSTMENT (TREATMENT) with the WAITING TIME ADJUSTMENT REASON (TREATMENT) being recorded as ‘Egg Harvesting.
4. Referral/upgrade to first treatment standard specifics.
4.1 Treatment standard overview
The CWT service standards are:
Maximum 62-day from receipt of an urgent GP (or other referrer) referral for urgent suspected cancer, breast symptomatic referral, urgent screening referral or consultant upgrade to First Definitive Treatment of cancer [Operational Standard – 85%]
4.2 Coverage of 62-day standards
The 62-day inclusion and clock start dates are included/referenced below:
- Urgent referral for suspected cancer
- Inclusion – Section 2.2
- Clock start – Section 2.2.3
- Breast Symptomatic
- Inclusion – Section 2.2.6
- Clock start – Section 2.2.3
- Urgent screening referrals
- Inclusion – Section 2.2.12
- Clock starts – Section 2.3.12
4.3 Starting the 62-day pathway (Clock start – Consultant upgrade)
If a consultant upgrades a patient for a first primary cancer the 62-day period starts at the CONSULTANT UPGRADE DATE. Only those upgrades that are diagnosed with a newly diagnosed cancer and go on to treatment need to be reported.
4.3.1 Who can upgrade a patient?
A consultant or an authorised member of the consultant team (as defined by local policy) should upgrade a patient if cancer is suspected. The ultimate responsibility for upgrades rests with the consultant responsible for the care of the patient who may have delegated their authority by local agreement. The upgrades could come from any part of the health service, not just from consultants and teams that most commonly see cancer patients. It is therefore important that local policies are agreed, and processes are in place to publicise and operate the upgrade system locally.
4.3.2 Can there be an upgrade from any source of referral?
Yes, except for the following as they are already on a cancer pathway:
- Urgent referrals for suspected cancer
- Referrals for breast symptoms (not suspicious of cancer)
- Urgent screening referrals.
4.3.3 Why not start the 62-day pathway from the receipt of the original referral which the consultant then went on to upgrade?
At the point when the original referral is received (recorded as the referral to treatment period start date or a RTT pathway) cancer is not suspected and it might be a few weeks before a consultant (or authorised member of a consultant team) decides to upgrade the patient to the 62-day pathway. It is not appropriate to calculate a timed 62-day period from this point (i.e. retrospectively starting the clock from the original referral) as the patients was not on the suspected cancer pathway at that point.
4.3.4 Circumstances where a Consultant upgrade would apply automatically.
Where a patient is referred to a Cancer Multidisciplinary Team meeting on suspicion or with a confirmed cancer, the date of this request must be counted as a consultant upgrade unless:
- The patient has already been upgraded on the pathway, e.g. where processes are in place to flag pathology reports of incidental findings
- A Decision To Treat has already been reached for the patient
- The patient has already received their first treatment for cancer, unless a new primary is suspected
- The patient is on an alternative 62-day pathway – Urgent Suspected Cancer, Breast symptomatic, or Urgent Screening.
Where a patient is discussed at more than one Cancer Multidisciplinary Team meeting, the date of request for the first meeting should be recorded.
Where a patient is referred for a Cancer Multidisciplinary Team meeting at another provider, even if for discussion only, the organisation making the referral is responsible for the consultant upgrade being made on or before the date of the inter-provider transfer.
In addition, a consultant upgrade would automatically apply in the following circumstances:
- Where a Trust radiology or pathology safety netting system flags a patient with suspicion or with confirmed cancer. The date of the radiology or pathology report would be used for the purposes of the Consultant Upgrade. This would only apply to patients under a consultant’s care within secondary care, not to those where the patient is still under the GPs care.
- From the point at which a patient on interval scan has an abnormal result suggesting that cancer is suspected.
- For Bowel Screening, where a patient initially declines all diagnostics, but then subsequently requests this, from the point at which the patient contacts the service and requests the diagnostic.
4.3.5 Recording of multiple primary cancers diagnosed on the same 62-day pathway.
Where more than one primary cancer is diagnosed as part of the same 62-day pathway, the pathway would run till treatment is commenced for any cancer. (i.e. the first cancer treated).
Where a Decision To Treat of other primaries has been made before the treatment of the first cancer, then the 31-day Decision To Treat/Earliest Clinically Appropriate Date (ECAD), to Treatment standard will apply. This could include applying an ECAD where it not possible to proceed with this treatment until the patient has completed their treatment for the first cancer treated.
Where a Decision To Treat for other primaries has not been reached at the point of first treatment of the first primary, then it may be appropriate to start a new 62 day Consultant Upgrade pathway, which would be mandated in the situations detailed in the section above (4.3.4).
An example of where this would apply would be a patient referred on an Urgent Suspected Skin Cancer pathway, who is found to have multiple independent Squamous Cell Carcinomas (SCC), and a decision is made to remove these on different dates. The original 62 day pathway would run, to the treatment of the first SCC, with the treatment of the other primary SCCs being recorded as separate 31 day Decision To Treat to Treatment pathways, but not on a separate 62 day pathway given the Decision To Treat was made before the first primary was treated.
4.4 Ending the 62-day pathway (Clock stops)
The clock stop for the 62-day standard at the point at which the patient receives their First Definitive Treatment for cancer as defined in chapter three of this guidance.
4.5 Adjustments
The same adjustments apply to the 62-day standards, as are applied to the first seen and treatment phases of the pathway, and these are taken off the total waiting time. Details of these adjustments can be found here:
- First seen adjustment – Section 2.3.6
- Treatment adjustment – Sections 3.19 to 21
The first seen adjustment would only be subtracted from the 62-day consultant upgrade standard if the CONSULTANT UPGRADE DATE was before the first seen date
4.6 Recording of inter-provider transfers and 62-day breach reallocation
4.6.1 When to record an inter-provider transfer.
Inter-provider transfers (IPTs) should be recorded when the responsibility for care is formally transferred.
The date that a referral request is received by the provider will mark the point at which the IPT is made.
Providers should review their pathways and agree when a patient transfers whether the responsibility of care remains with the referring provider or transfers to the receiving provider.
Where a request is made just for a diagnostic or MDT discussion only and the responsibility for care is not formally transferred this would not be recorded as an IPT in the CWT system.
Example scenarios where IPTs should be recorded are included below:
Scenario 1
Two IPTs would be recorded
- One from Trust A to Trust B as patient is discussed at MDT and followed up at Trust B (so transfer of care has taken place)
- One from Trust B to Trust C as patient has transferred to Trust C for treatment
Scenario 2
One IPT would be recorded
- One from Trust A to Trust C as patient has transferred to Trust C for treatment
- An IPT should not be recorded from Trust A to Trust B, as the patient just had a diagnostic at Trust B, and the patient was followed up with results at Trust A.
Scenario 3

No IPT would be recorded
- Transfer between Trust A and Trust B were just for diagnostics for which follow-up was at Trust A, so no IPT recorded as no transfer of patients care.
- Transfer between Trust A and Trust C was just for a Specialist Multidisciplinary Team (SMDT) discussion, the outcome of which was for treatment to commence at Trust A, so no IPT recorded as no transfer of patients care.
4.6.2 Principles around recording of Inter-provider transfers.
Requirements:
- Referral without a transfer of care
- Where a patient has only been referred for an MDT discussion, diagnostic test or second opinion.
- Referral with a transfer of care
- Patient has been informed they are being referred to another provider, meaning the receiving provider can act freely in arranging next steps of the pathway
- The purpose of the referral is clear
- All relevant clinical information is included. The details will vary by tumour type, and regionally and should be agreed locally.
- A complete IPT form is included with the referral or provided separately which includes the details of the pathway they are on (including if they already have a running 62-day pathway). All required diagnostics/imaging and associate results are available
Where a patient is able to be seen at the receiving provider for the next step of the pathway, then this would usually mean that that the inter-provider transfer has occurred and been accepted.
The exception would be where this provider was not notified that the patient was on an active 62-day pathway or further information is needed to progress the patient’s pathway. In these scenarios the Inter-provider transfer would only be recorded once this notification is received, or when a Decision To Treat is reached, whichever is the earliest.
4.6.3 Referral timelines.
The receipt of referral and IPT form should be recorded as the date of receipt of referral: – REFERRAL REQUEST RECEIVED DATE (INTER-PROVIDER TRANSFER)
- This includes when a referral is received on a non-working day and at any time of the day
- Where a completed referral information and/or IPT form is not received then the REFERRAL REQUEST RECEIVED DATE (INTER-PROVIDER TRANSFER) would be recorded as the date when the completed information is received
Receiving providers should acknowledge receipt of the referral/IPT, or feedback if additional information is needed within one working day.
4.6.4 Information required by receiving providers.
The CWT system requires submission of the REFERRAL REQUEST RECEIVED DATE (INTER-PROVIDER TRANSFER). This is the date when the receiving provider receives the referral request for the patient and the patient files/ records (minimum clinical data set) that will enable them to continue the patient’s diagnostic investigation pathway or commence treatment.
The following data items are mandated for an Inter-Provider Transfer:
| NHS NUMBER |
| NHS NUMBER STATUS INDICATOR |
| PATIENT PATHWAY IDENTIFIER* |
| CANCER REFERRAL TO TREATMENT PERIOD START DATE* |
*only one of these fields is required to be accepted by the system but both are preferable. Where a patient is on the 62-day consultant upgrade standard the PATIENT PATHWAY IDENTIFIER is mandated.
4.6.5 Details of provider breach reallocation.
The rules which assign 62-day performance where at least one transfer of care as occurred prior to first treatment are set out below.
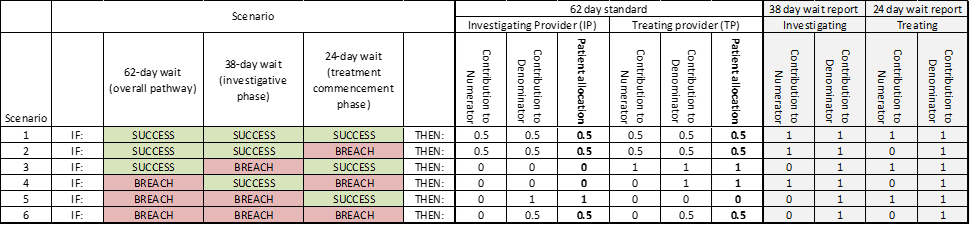
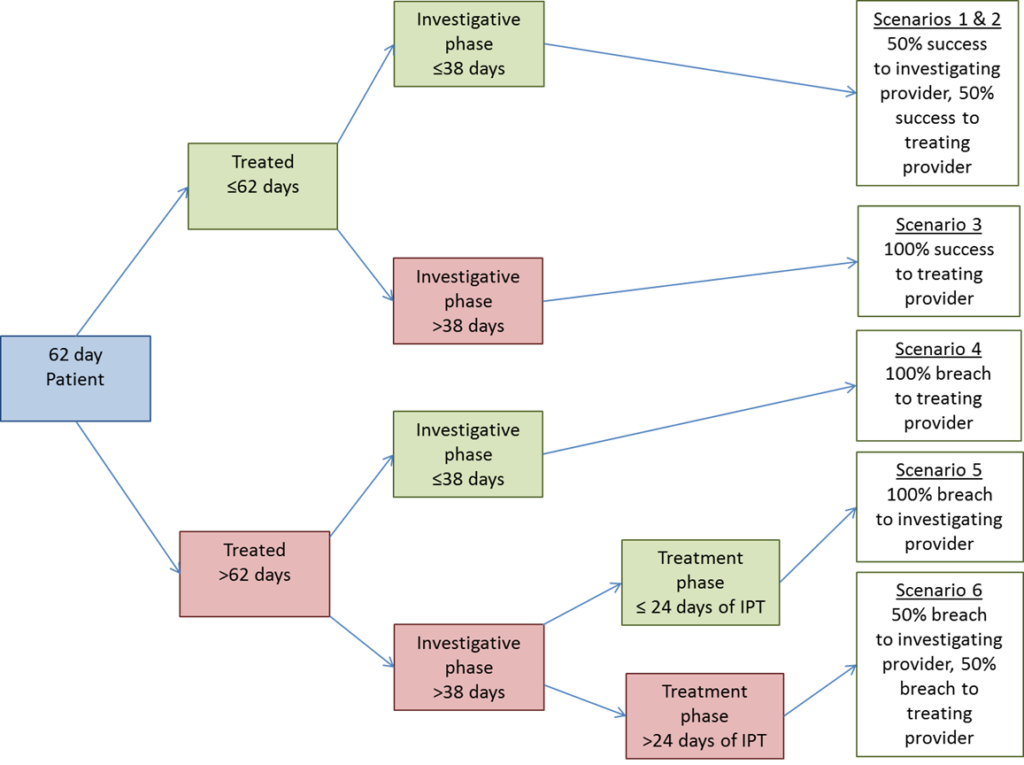
4.6.6 pathways with multiple Inter-Provider Transfers.
The pathway is split into two phases
- Investigating phase which is up to the final Inter-Provider Transfer
- Treating phase which is after the final Inter-Provider Transfer
Allocation of the investigating provider is made on the following basis
- If referral received to last IPT ≤38 days
- Allocation to provider with shortest pathway up to last ITT
- If one or more trusts have same length of pathway, pathway allocated to 1st provider on pathways
- If referral received to last IPT > 38 days
- Allocated to provider with longest pathway up to last ITT
- If one or more trusts have same length of pathway, pathway allocated to last trust on pathway
Once the allocation of investigation has been completed, then the rules to allocate cases and breaches apply in the same way as if there was only a single IPT in the pathway.
4.6.7 Pathways where treating provider is also involved in the investigative stage of the pathway.
If a provider is involved in the investigative stage and is also the treating provider (with another provider involved in between) the provider is considered separately in the calculations for responsibility for investigation and for treatment.
5. Tumour Specific guidance
The care pathways for patients with different tumour types can be very different and the operational standards are for overall performance, i.e. all tumours taken together. It is not expected that all tumour groups will meet that level of performance – this is not realistic and would not be in the best interest of all patients.
5.1 Cancers of the Brain and Central Nervous System (CNS)
In Scope:
- All malignant tumours
- ICD10 codes C47, C69-72.
Out of Scope:
- Von Hippel-Landau syndrome – a benign condition.
5.1.1 What cannot be classified as a First Definitive Treatment for Brain and CNS cancers?
- palliative care for any patient who is fit for active treatment, unless they decline active treatment options and wish to have only palliative treatment;
- surgical biopsy for diagnostic purposes, unless the tumour is effectively removed by the procedure; or
- Dexamethasone, unless described as palliative care with no other anti-cancer treatment being planned.
5.1.2 Can active monitoring be classed as a First Definitive Treatment low grade Brain and CNS cancers?
- active monitoring can be used where it is not appropriate to give any active anti-cancer treatment at that point in time, but an active treatment is still intended/may be required at a future date.
5.1.3 What cannot be classed as subsequent treatment for Brain and CNS cancers?
- any cosmetic procedures; or
- rehabilitation such as speech & language therapy and psychosocial support, e.g. cognitive behavioural therapy, physiotherapy etc.
5.1.4 Are kyphoplasty and vertebroplasty reported as subsequent cancer treatments?
Kyphoplasty and vertebroplasty would only apply as a treatment for patients where any vertebral compression fractures were caused by malignant disease. (both primary and secondary disease)
5.2 Breast Cancer
In Scope:
- ICD10 code C50;
- ICD10 code D05 (i.e. breast cancer in situ. Both ductal carcinoma in situ [DCIS] and lobular carcinoma in situ [LCIS] are covered by D05); and
- Paget’s disease of nipple/breast – clinical coders and cancer registries code this condition as ICD10 Code C50.
Out of Scope:
- Atypical Ductal Hypoplasia.
5.2.1 What cannot be classed as first treatment for breast cancers?
- surgical biopsy for diagnostic purposes, unless the tumour is effectively removed by the procedure;
- Sentinel Lymph Node Biopsy – this is a diagnostic staging procedure to determine whether the cancer has spread to the lymph nodes;
- Tamoxifen hormone treatment can only be classed as First Definitive Treatment if it is to be the sole treatment modality, or the treatment plan specifies that a second treatment modality should only be given after a planned interval. For example, unless the multi-disciplinary team has recommended that neoadjuvant therapy is necessary, Tamoxifen prior to surgery would not be a First Definitive Treatment. In this case, surgery should be reported as the First Definitive Treatment rather than the start of Tamoxifen, even if it is necessary to wait to assess a response to Tamoxifen; or
- palliative care for any patient who is fit for active treatment unless they decline active treatment options and wish to have only palliative treatment.
5.2.2 What cannot be classed as a subsequent treatment for breast cancer?
- reconstructive surgery (i.e. carried out at a different time to the mastectomy);
- cosmetic procedures (e.g. bilateral revision of mastectomy scars); or
- rehabilitation / psychosocial support (e.g. cognitive behavioural therapy, physiotherapy etc).
5.2.3 A patient is treated for breast cancer in the right breast and decides to have a left mastectomy, albeit there are no current issues with her left breast. Would removal of her left breast be exempt from cancer waits?
If this is a purely prophylactic treatment, then this would not be a subsequent treatment for cancer waits.
5.3 Children’s Cancer
A patient under 16 years of age at receipt of an urgent referral for suspected cancer (if relevant) or under 16 at Decision To Treat, is classed as a child.
To record a record for a child where cancer is suspected, use ‘02’ in the field TWO WEEK WAIT CANCER OR SYMPTOMATIC BREAST REFERRAL TYPE.
Children are in scope for all standards. For the purposes of the 28-day FDS, if cancer is ruled out, a letter to, or phone call with, a parent/guardian should be recorded as the stop clock date for the 28-day FDS pathway.
5.4 Gynaecological Cancers
In Scope:
- ICD10 codes C51-58
Out of Scope:
- colposcopy referrals from cervical screening programme, excluding those for moderate or severe dyskaryosis, invasive or glandular neoplasia.
5.4.1 What cannot be classed as first treatments for gynaecological cancers?
- cone or loop or LLETZ biopsy/hysteroscopy/colposcopy/vulvoscopy if diagnostic in intent only – however, if therapeutic in intent (i.e. if the intention of the procedure was to remove the tumour) then these would count as first treatment irrespective of whether the margins were clear. If the intention was diagnostic, but the tissue was found to be malignant, the procedure could count as first treatment if the tumour had effectively been removed by the excision;
- removal of polyps for diagnostic purposes – however, if the tissue was found to be malignant the procedure could count as first treatment if the tumour had effectively been removed by the excision;
- removal of para-aortic nodes before a patient starts radiotherapy or chemotherapy – however, if clinically involved nodes are having to be de-bulked prior to radiotherapy, this could be classed as first treatment;
- ileal conduit urinary diversion surgery to treat a bladder problem prior to active treatment (e.g. chemoradiation);
- removal/draining of ascites prior to chemotherapy, unless no other active treatment is planned;
- palliative care for any patient who is fit for active treatment unless they decline active treatment options and wish to have only palliative treatment).
5.4.2 What cannot be classed as a subsequent treatment for gynaecological cancers?
- reconstructive post-surgery; or
- rehabilitation / psychosocial support (e.g. cognitive behavioural therapy, physiotherapy etc).
5.4.3 Is the removal of pelvic lymph nodes considered a first treatment for cervical cancer?
The removal of pelvic lymph nodes as part of a two-part operation to treat cervical cancer can be classed as first treatment. The second stage treatment, determined by the status of the nodes, would be covered by the 31-day subsequent treatment standard.
5.4.4 Can pleural effusion/pleurodesis be a subsequent treatment for a gynaecological patient?
Pleural aspiration or drainage (for pleural effusion) or pleurodesis (surgical or medical) could be counted as a subsequent treatment.
However, if it is part of a palliative support package, the start of the package of care would be taken as the:
- date of the delivery of the first episode;
- the consultation that results in the referral to a non-NHS specialist palliative care service; or
- the consultation at which the patient receives a prescription.
So, unless any of these procedures was the first episode in the support package, they will not be counted.
- A patient was treated, and the full tumour was removed at that time. The patient was then given the choice of completion surgery (i.e. a hysterectomy) – is this classed as a subsequent treatment?
Yes. This would be classed as a 31-day subsequent treatment as it is part of the cancer care package.
5.5 Haematological Cancers
In Scope:
- ICD10 codes C81-C97 including:
- chronic lymphocytic leukaemia;
- chronic myelomonocytic leukaemia (CMML) – for the purposes of cancer this is classed as a form of leukaemia rather than a form of myelodysplastic syndrome, although it is noted that many are not clinically urgent;
- B-cell chronic lymphocytic leukaemia (CLL);
- Small Lymphocytic Lymphoma (SLL); and
- all cases of acute leukaemia.
Out of Scope:
- Myeloid dysplastic syndrome (D46.A or D46.B).
5.5.1 What cannot be classed as first treatment for haematological cancers?
- removal of lymph nodes – this will be a biopsy to establish a diagnosis of Lymphoma and there is likely to be additional disease throughout the body that will need active treatment;
- blood transfusions – unless a patient has no other active treatment planned, in this case the transfusions would be classed as palliative treatment; or
- palliative care for any patient who is fit for active treatment unless they decline active treatment options and wish to have only palliative treatment.
5.5.2 What cannot be classed as a subsequent treatment for haematological cancers?
Rehabilitation / psychosocial support (e.g. cognitive behavioural therapy, physiotherapy etc).
5.5.3 Are antibiotics a valid first treatment for low grade gastric lymphomas?
Antibiotics count as the start of treatment for low grade gastric lymphoma.
5.5.4 Are blood transfusions counted as subsequent treatments?
Palliative treatments can count as subsequent treatments so a blood transfusion could be covered by the 31-day treatment standard. However, an agreed package of palliative care would only count as one treatment, with the first treatment in the agreed palliative care package marking the end of the 31-day period.
5.5.5 If a patient is diagnosed with one haematological condition that transforms to a different type, how is this managed in cancer waits?
The relevant cancer waits data item is CANCER TREATMENT EVENT TYPE. This includes:
- code 09 (Treatment for relapse of primary cancer (second or subsequent)); and
- code 10 (Treatment for progression of primary cancer (second or subsequent)).
As haematological cancers do not spread/recur in the same way as solid tumours, haematologists consulted during the development of the updated cancer waits standards advised including these descriptions within the coding structure. It is for clinical teams locally to decide which is the most appropriate category to use for their haematological patients.
5.6 Head and Neck Cancers (including thyroid cancer)
In Scope:
- ICD10 Codes: C00 – C14, C30 – C32, C73, C77.0
Out of Scope:
- Barrett’s oesophagus
- What cannot be classed as first treatment for head, neck, or thyroid cancers?
Head and neck
- surgical biopsy for diagnostic purposes, unless the tumour is effectively removed by the procedure;
- palliative care for any patient who is fit for active treatment unless they decline active treatment options and wish to have only palliative treatment.
Thyroid
- surgical biopsy for diagnostic purposes, unless the tumour is effectively removed by the procedure;
- palliative care for any patient who is fit for active treatment unless they decline active treatment options and wish to have only palliative treatment.
5.6.1 What cannot be classed as a subsequent treatment for head, neck, or thyroid cancers?
Head and neck
- reconstructive surgery post treatment;
- rehabilitation, such as speech and language therapy and psychosocial support (e.g. cognitive behavioural therapy, physiotherapy etc);
- management of side effects;
- closure of tracheostomy;
- dealing with leaking/blocked voice prostheses, breathing/swallowing problems; or
- surgical voice restoration.
Thyroid
- rehabilitation, such as speech and language therapy and psychosocial support (e.g. cognitive behavioural therapy, physiotherapy etc).
- If a stent is changed every six weeks due to being outgrown or blocked, or laser treatment is used to unblock a stent – is each procedure classed as a subsequent treatment?
5.6.2 Each stent change or clearing does not need to be a subsequent treatment if it is not active treatment of the cancer.
5.7 Lower-Gastrointestinal Cancers – LGI (colon, rectal, anal)
In Scope:
- ICD10 Codes: C17 – C21, C26
Out of Scope:
- carcinoma in situ (CIS) found in polyps excised at colonoscopy – CIS includes cancer cells confined within the glandular basement membrane (intraepithelial) or lamina propria (intramucosal) with no extension through muscularis mucosae into submucosa; or
- carcinoids of the appendix (coded as ICD10 D37.3).
5.7.1 What cannot be classed as first treatment for LGI cancers?
- surgical biopsy, including polypectomy, for diagnostic purposes, unless the tumour is effectively removed by the procedure as confirmed by histopathology;
- palliative care for any patient who is fit for active treatment unless they decline active treatment options and wish to have only palliative treatment.
5.7.2 What cannot be classed as subsequent treatment for LGI cancer?
- closure of a temporary stoma; or
- rehabilitation, such as psychosocial support (e.g. cognitive behavioural therapy, physiotherapy etc).
5.7.3 Specific guidance around application of Cancer Waiting Times guidance to FIT implementation.
In line with existing CWT practice, Lower GI urgent suspected cancer referrals cannot be rejected by secondary care providers because a FIT result has not been included, or the FIT is negative on the referral form.
In these scenarios, providers can avoid patients inappropriately continuing on the Lower GI urgent suspected cancer pathway by contacting the referrer and asking them to agree to withdraw or downgrade the referral.
Providers can also remove patients from the LGI FDS pathway through the first seen appointment, this is explained in further detail below.
Local Lower GI urgent suspected cancer referral forms should be updated to include information on FIT, and education on use of FIT in the Lower GI pathway provided for primary care to support uptake.
The clock start remains when the urgent suspected cancer referral is received by secondary care. It is therefore recommended all GP practices follow the BSG/ACPGBI guidance[1] and provide FIT testing for all patients with colorectal symptoms (bar those with anal/rectal mass or anal ulceration) prior to the referral to support appropriate decision making and to make sure for those referred the result is available in time for clinical triage and therefore allows for prompt decision making.
A patient referred on an urgent cancer suspected pathway is required to have a first seen date. In line with the Best Practice Timed Pathway for colorectal cancer, this should be clinical triage appointment with the patient and can be performed virtually/by telephone. This process is normally led by a nurse with oversight from a consultant.
If at clinical triage, patients have a FIT fHb <10μg Hb/g, normal examination and full blood count and confirm they have no ongoing symptoms of concern, they should be discharged back to their GP or rerouted to an alternative pathway for definitive diagnosis.
As per section 2.4, the key principle allowing the ‘clock to stop’ for the Faster Diagnosis Standard is communication to the patient either that their cancer has been diagnosed, or that following reasonable diagnostic tests, they are no longer being investigated for suspected cancer. In this context, a FIT <10μg Hb/g a normal examination and a full blood count would be considered reasonable diagnostic tests for colorectal cancer, as the risk of colorectal cancer is <0.1% lower than the general population risk and therefore can ‘rule out’ or ‘exclude cancer’.
This decision to remove the patient from the urgent suspected cancer pathway can be taken by secondary care clinicians once they have been seen (this could be virtually) and the patient has been told they are no longer being investigated for suspected cancer. The GP should be informed of the decision.
Once the patient has been informed and discharged or moved to a non-cancer pathway this acts as the clock stop. Where colorectal cancer is no longer suspected but their symptoms could indicate another type of cancer, the clock does not stop, and the patient will remain on the pathway even if they are transferred between specialities within secondary care.
FIT is a reasonable diagnostic to ‘rule out’ or ‘exclude’ colorectal cancer as the risk in those with a FIT fHb <10μg Hb/g, a normal examination and a full blood count is <0.1%, lower than the general population risk.
5.8 Lung and Mesothelioma Cancers
In Scope:
- ICD10 Codes: C33 – C39, C45 (C78 for secondary after unknown primary)
5.8.1 What cannot be classed as first treatments?
Lung cancer
- drainage of a pleural effusion if further anti-cancer treatment is planned
- pleurodesis if further anti-cancer treatment is planned;
- mediastinoscopy, unless the excised tissue was found to be malignant, and the tumour had effectively been removed by the excision irrespective of whether the margins were clear – this is unlikely;
- stenting of the airway or superior vena cava if further anti-cancer treatment is planned;
- laser treatment of major airways obstruction if further anti-cancer treatment is planned;
- Video Assisted Thoracic Surgery (VATS) biopsy for diagnostic purposes unless procedure could be considered as de-bulking the tumour;
- palliative care for any patient who is fit for active treatment, unless they decline active treatment options and wish to have only palliative treatment; or
Mesothelioma
- drainage of a pleural effusion if further anti-cancer treatment is planned;
- pleurodesis if further anti-cancer treatment is planned;
- interventional analgesia (e.g. nerve block or cordotomy) if further anti-cancer treatment is planned; or
- palliative care for any patient who is fit for active treatment unless they decline active treatment options and wish to have only palliative treatment.
5.8.2 What cannot be classed as subsequent treatments?
Lung cancer
- antitussives such as codeine, morphine, dihydrocodeine or hydrocodone (for coughs)
- opioids (for breathlessness and pain management)
- corticosteroids for problems other than cerebral metastases
- bisphosphonates for bone pain
- rehabilitation such as psychosocial support, e.g. cognitive behavioural therapy, physiotherapy etc.
Mesothelioma
- non-invasive analgesia (e.g. opioids)
- corticosteroids for problems other than cerebral metastases
- rehabilitation such as psychosocial support, e.g. cognitive behavioural therapy, physiotherapy etc.
5.8.3 Does talc pleurodesis count as a subsequent treatment for lung cancer?
It can be classed as a subsequent treatment. However, if talc pleurodesis was one part of an agreed supportive care package then it is only the start of the package that counts. If it is in addition to an agreed package it could count in its own right.
5.8.4 How are pleural effusions recorded by cancer waits standards?
Managing pleural effusions could only be classed as first treatment if no further anti-cancer treatment is planned.
There is no perfect answer for coding pleural effusions. It is best to code them as either non-specialist palliative care (Code 09) or specialist palliative care (Code 07) as per advice of your local clinical team. Some pleurodesis procedures are carried out by surgeons under general anaesthetic (probably no more than 5% of the total) so some could legitimately be coded as surgical procedures.
5.8.5 How should management of ascites be covered by cancer waits standards?
Managing ascites would only be classed as first treatment if no further anti-cancer treatment is planned.
5.9 Sarcoma
In Scope:
- ICD10 C40-41,46, 48-49 &,79.5 (secondary with unknown primary)
- Kaposi’s sarcoma (malignant tumour arising from blood vessels in the skin) – rare in the western world except for patients with Aids
- Fibrosarcoma
Out of Scope
- Fibromatosis
5.9.1 How should the First Seen Date be recorded for sarcoma referrals made following direct access ultrasound?
A GP may make a suspected sarcoma referral based on a direct access ultrasound scan that does not exclude sarcoma. A specialist then reviews the image and determines that the patient does not need to be seen due to them being able to rule out cancer on the basis of the image alone. In this scenario, the specialist (or their team with delegated clinical responsibility) should undertake a consultation with the patient to communicate this, so that a short history can be taken at the same time to ensure that further investigations or clinical examination are not required.
The First Seen Date should be recorded as the date the patient is contacted.
5.9.2 What cannot be classed as first treatment for sarcomas?
- surgical biopsy for diagnostic purposes (unless the tumour is effectively removed by the procedure)
- palliative care for any patient who is fit for active treatment (unless they decline active treatment options and wish to have only palliative treatment).
5.9.3 What cannot be classed as a subsequent treatment for sarcomas?
- reconstructive surgery post definitive treatment
- rehabilitation, such as psychosocial support, e.g. cognitive behavioural therapy, physiotherapy etc.
5.10 Skin Cancers
In scope:
- ICD10 Codes: C43 – C44
including:
- Malignant Melanomas including: –
- Superficial Spreading Melanoma
- Nodular Melanoma
- Lentigo Maligna Melanoma
- Acral Lentiginous Melanoma
- Amelanotic Melanoma
- Merkel Cell Carcinoma
- Squamous Cell Carcinoma (SCC)
excluding the following conditions classified under C44:
- Basal Cell Carcinoma
- Multicentric Basal Cell Carcinoma
- Basal Cell Carcinoma, Morphoea
- Basal Cell Carcinoma, Fibroepithelial
- Basosquamous Carcinoma
- Metatypical Carcinoma
- Pilomatrix Carcinoma
Out of Scope:
- Lentigo Malignas (considered Carcinoma in Situ)
- Bowen’s Disease (considered Carcinoma in Situ)
- Intraepidermal Carcinomas (considered Carcinoma in Situ)
- Keratoacanthoma – benign condition not malignant.
5.10.1 How is the First Seen Date recorded for teledermatology pathways?
The First Seen Date in teledermatology would be the first of the following after referral:
- Appointment in secondary care where a dermoscopic image /photo is taken by a trained healthcare professional (this could include in a Community Diagnostic Centre)
- Date that a specialist reviews an image taken in primary care (before referral)
- When the patient is seen in the clinic
Where a specialist reviews a diagnostic image, the patient must be informed of the result, either that cancer is ruled out or that attendance at a dermatology clinic is required. The CANCER FASTER DIAGNOSIS PATHWAY END DATE should be recorded as the date of this communication if cancer is ruled out. This could be a phone call to the patient, or a letter explaining the result in which case the date the letter is sent would be recorded.
5.10.2 Do we only track the first skin squamous cell carcinoma (SCC) a patient has?
Each SCC is covered by the cancer waits standards not just the first.
Whether or not a SCC or Malignant Melanoma (MM) is classed as a new primary or a recurrence is a local clinical decision.
If a patient has multiple SCCs (or MMs) with the same ICD-10 coding and they can all be treated as part of the same appointment with the same treatment, pragmatically these could be classed as one 31-day pathway.
5.10.3 What cannot be classed as first treatment for skin cancers?
- surgical biopsy for diagnostic purposes (unless the tumour is effectively removed by the procedure)
- Sentinel Node Biopsy – this is a diagnostic staging procedure to determine whether the cancer has spread to the lymph nodes
- palliative care for any patient who is fit for active treatment (unless they decline active treatment options and wish to have only palliative treatment).
5.10.4 What cannot be classed as a subsequent treatment for skin cancers?
- reconstructive/cosmetic surgery post initial (definitive) treatment
- rehabilitation such as psychosocial support, e.g. cognitive behavioural therapy, physiotherapy etc.
5.10.5 A patient has a skin lesion excised at the GP surgery which is confirmed on histology as a malignant melanoma. The GP then makes an urgent suspected cancer referral. The patient is seen and listed for a wider excision. Histology shows no residual malignancy. How should this be recorded for cancer waits?
An urgent suspected cancer referral should be made when a GP suspects cancer. In this scenario a cancer has been diagnosed/treated prior to the referral.
It is advised that:
- if the margins were clear after the GP treatment (and they consider that to be the first treatment) then record the FDS end date as the date of 1st appointment. Any further treatment would be classed as a subsequent treatment, not linked to the original referral.
- if margins are not clear after the GP procedure (assuming it was diagnostic in intent) then you would class the 31-day period in the secondary care as the first treatment which would then link to the referral and create a 62-day period.
- How should the Faster Diagnosis Standard (FDS) End Date be recorded for skin cancers?
For suspected skin cancer, whether a Decision To Treat date should be recorded for a procedure would be based on the intention of the procedure and would apply to both those diagnosed with a reportable cancer and those who had cancer excluded. For those where the intention is diagnostic only (e.g. punch biopsy where a complete excision is not intended) you would not record a Decision To Treat, whilst if the intention is to fully excise or debulk a lesion with a procedure a Decision To Treat would be recorded.
The treatment date TREATMENT START DATE (CANCER) would only be recorded where a patient is diagnosed with a reportable cancer.
Example 1 – Full excision of lesion at first attendance, patient has reportable cancer (e.g. Melanoma)
- Urgent Suspected Cancer Referral received 20/09/2021
- Patient seen in clinic on 30/09/2021 – decision taken to fully excise a lesion
- Excision is undertaken on same day – 30/09/2021
- Pathology reported – Melanoma confirmed – 10/10/2021
- Patient seen in clinic – told they have melanoma – 20/10/2021
The following would be recorded for this patient:
- CANCER REFERRAL TO TREATMENT START DATE – 20/09/2021
- FIRST SEEN DATE – 30/09/2021
- FASTER DIAGNOSIS STANDARD END DATE – 20/10/2021
- CANCER TREATMENT PERIOD START DATE – 30/09/2021
- TREATMENT START DATE (CANCER) – 30/09/2021
The Faster Diagnosis Standard in this case would:
- Be reported in October as the FASTER DIAGNOIS STANDARD END DATE was reported on the 20/10/2021.
The waiting time against the Faster Diagnosis Standard would be recorded as 10 days, the difference between referral received (20/09/2021) and Decision To Treat (30/09/2021) as this is before the point at which the patient is told they have cancer.
Example 2 – Full excision of lesion at subsequent attendance, patient has reportable cancer (e.g. Melanoma)
- Urgent Suspected Cancer Referral received 20/09/2021
- Patient seen in clinic on 30/09/2021 – decision taken to fully excise a lesion; patient booked for future date
- Excision is undertaken on 10/10/2021
- Pathology reported – Melanoma confirmed – 20/10/2021
- Patient seen in clinic – told they have melanoma – 30/10/2021
The following would be recorded for this patient:
- CANCER REFERRAL TO TREATMENT START DATE – 20/09/2021
- FIRST SEEN DATE – 30/09/2021
- FASTER DIAGNOSIS STANDARD END DATE – 30/10/2021
- CANCER TREATMENT PERIOD START DATE – 30/09/2021
- TREATMENT START DATE (CANCER) – 10/10/2021
The Faster Diagnosis Standard in this case would:
- Be reported in October as the FASTER DIAGNOIS STANDARD END DATE was reported on the 30/10/2021.
- The waiting time against the Faster Diagnosis Standard would be recorded as 10 days, the difference between referral received (20/09/2021) and Decision To Treat (30/09/2021) as this is before the point at which the patient is told they have cancer.
Example 3 – Full excision of lesion at first attendance, patient does not have a reportable cancer (e.g. BCC)
- Urgent Suspected Cancer Referral received 20/09/2021
- Patient seen in clinic on 30/09/2021 – decision taken to fully excise a lesion
- Excision is undertaken on same day – 30/09/2021
- Pathology reported – BCC confirmed – 10/10/2021
- Patient seen in clinic – told they have BCC – 20/10/2021
The following would be recorded for this patient:
- CANCER REFERRAL TO TREATMENT START DATE – 20/09/2021
- FIRST SEEN DATE – 30/09/2021
- FASTER DIAGNOSIS STANDARD END DATE – 20/10/2021
- CANCER TREATMENT PERIOD START DATE – 30/09/2021
- TREATMENT START DATE (CANCER) – Not recorded
The Faster Diagnosis Standard in this case would:
- Be reported in October as the FASTER DIAGNOIS STANDARD END DATE was reported on the 20/10/2021.
- The waiting time against the Faster Diagnosis Standard would be recorded as 10 days, the difference between referral received (20/09/2021) and Decision To Treat (30/09/2021) as this is before the point at which the patient is told they do not have a reportable cancer.
Example 4 – Patient has punch biopsy intended just for diagnostic purposes at first attendance, patient does not have a reportable cancer (e.g. BCC)
- Urgent Suspected Cancer Referral received 20/09/2021
- Patient seen in clinic on 30/09/2021 – decision taken to do punch biopsy
- Punch biopsy is undertaken on same day – 30/09/2021
- Pathology reported – BCC confirmed – 10/10/2021
- Patient seen in clinic – told they have BCC – 20/10/2021
The following would be recorded for this patient:
- CANCER REFERRAL TO TREATMENT START DATE – 20/09/2021
- FIRST SEEN DATE – 30/09/2021
- FASTER DIAGNOSIS STANDARD END DATE – 20/10/2021
- CANCER TREATMENT PERIOD START DATE – Not recorded
- TREATMENT START DATE (CANCER) – Not recorded
The Faster Diagnosis Standard in this case would:
- Be reported in October as the FASTER DIAGNOIS STANDARD END DATE was reported on the 20/10/2021.
- The waiting time against the Faster Diagnosis Standard would be recorded as 30 days, the difference between referral received (20/09/2021) and Faster Diagnosis Standard End Date (20/10/2021) as this is before the point at which the patient is told they don’t have a reportable cancer.
10.7. CANCER CARE SPELL DELAY REASON (DECISION TO TREAT)
5.11 Upper Gastrointestinal Cancer (oesophageal, stomach, pancreatic, liver)
In Scope:
- ICD10 Codes: C15 – C16, C22 – C25
- gastrointestinal stromal tumours (GISTs) that are described as malignant, invasive, or as having metastases coded to the relevant ICD10 ‘C’ code for the part of the gastrointestinal tract involved.
Out of Scope:
- GISTs not specified as above, coded as borderline using the relevant ‘D’ code.
5.11.1 How should rare neuroendocrine tumours be coded – the diagnosis is not always specific to pancreatic origin?
The cancer waits database does not use morphology coding. It is therefore suggested that you code the primary site of origin of the tumour (i.e. record the ICD10 site as the primary site of origin) and not the fact that it is of a neuroendocrine type. Therefore, the tumours are called neuroendocrine even though they do not necessarily arise in an endocrine site. If the primary site is genuinely not known, then use a “Malignant neoplasm of other and ill-defined sites” code. This would be C76 but as the fourth digit is now required you should add more detail.
5.11.2 What cannot be classed as first treatment for upper GI cancers?
Pancreatic cancer
- surgical biopsy for diagnostic purposes (unless the tumour is effectively removed by the procedure)
- insertion of pancreatic/biliary stent – if the planned first treatment is resection for pancreatic or related cancers (ampullary, duodenal and distal bile duct) and the patient requires a stent due to having had to wait for the surgery
- insertion of pancreatic/biliary stent – for patients with mild obstructive jaundice (a serum bilirubin below 200 micromol/l) if local practice is that they do not require biliary stenting before resection if surgery and imaging are planned within 7-10 days
- palliative care for any patient who is fit for active treatment (unless they decline active treatment options and wish to have only palliative treatment).
Gastric/oesophago-gastric cancer
- surgical biopsy for diagnostic purposes (unless the tumour is effectively removed by the procedure)
- palliative care for any patient who is fit for active treatment (unless they decline active treatment options and wish to have only palliative treatment).
5.11.3 What cannot be classed as a subsequent treatment for UGI cancers?
- rehabilitation such as psychosocial support, e.g. cognitive behavioural therapy, physiotherapy etc.
5.11.4 When can a pancreatic stent be classed as first treatment?
It could be classed as a first treatment if planned to resolve jaundice before a patient has a resection or starts chemotherapy. However, many clinicians agree that patients with mild obstructive jaundice (a serum bilirubin below 200 micromol/l) do not require biliary stenting before resection if surgery and imaging are planned within 7-10 days. If this is the agreed clinical practice locally then stenting these patients will not count as first treatment.
5.11.5 Is a staging laparoscopy to determine whether a patient is suitable for major UGI surgery classed as first treatment?
The date of admission for the staging laparoscopy could be counted at the start date for first treatment if that treatment was surgery and the patient remained an in-patient between the admission for the laparoscopy and the surgery, i.e. if it is the same episode of care.
5.12 Urological Cancers (bladder, prostate, renal, testicular, upper tract transitional cell)
In Scope:
- ICD10 Codes: C66-C67 [Bladder]
- ICD10 Code: C61 [Prostate]
- ICD10 Codes: C64-C65 [Renal/Kidney]
- ICD10 Code: C60 [Penile]
- ICD10 Code: C62 [Testicular]
- ICD10 Codes: C65-66 [Upper tract transitional cell carcinoma (renal or ureter)].
Out of Scope:
- pTa – transitional cell carcinoma is regarded as non-invasive [Bladder]
5.12.1 What cannot be classed as first treatment for urological cancers?
- surgical biopsy for diagnostic purposes (unless the tumour is effectively removed by the procedure). This includes a TURBT procedure unless the tumour has been effectively removed, and the patient is now only on a surveillance follow up pathway. This should be documented in the MDT meeting, which can protocolise decision for straightforward cases.
- palliative care for any patient who is fit for active treatment (unless they decline active treatment options and wish to have only palliative treatment).
5.12.2 When would the FDS clock stop for patients with a bladder tumour seen on cystoscopy?
- A patient who is told they have a tumour at cystoscopy +/- TURBT would not normally be classed as the Faster Diagnosis Standard end date, as it would not be known if the patient has an invasive cancer or not (i.e. pTa or higher-grade tumour)
- A clock stop could be applied where the patient is told they have an invasive cancer, or conversely where the patient is reassured that they do not have an invasive cancer, although in most cases it is expected that pathology will need to be reported to reach this determination.
5.12.3 What cannot be classed as a subsequent treatment for urological cancers?
- reconstructive surgery
- rehabilitation such as speech & language therapy and psychosocial support, e.g. cognitive behavioural therapy, physiotherapy etc.
5.12.4 How do the rules around TURBT apply including how Mitomycin impacts different scenarios?
Mitomycin is often given at the time or shortly after a TURBT but is not an effective treatment for muscle invasive disease, so would not be classed as First Definitive Treatment where the tumour is muscle invasive.
Where the TURBT is shown to effectively remove the tumour, and the patient is put on a surveillance protocol, and Mitomycin is given at the time or shortly after a TURBT, this would be considered a combined treatment, so only the TURBT (i.e. surgery) would be recorded within the Cancer Waiting Times dataset.
Effectively this means that Mitomycin would never be recorded as a First Definitive Treatment.
6. CWT system – support and information
6.1 What other guidance documents are available?
All the available guidance is stored at: Cancer Waiting Times Data Collection (CWT) – NHS England Digital
This includes:
- National Cancer Waiting Times User Manual
- National Cancer Waiting Times Reports User Manual
- NHS Communications outlining the establishment of and changes to the national CWT data set
- This guidance (and previous versions of this guidance)
- The table of clinical codes acceptable to the CWT system
6.2 What support is available/how can I send questions?
Queries about the CWT rules or publications can be sent to england.cancerwaitsdata@nhs.net.
You can also contact the NHS Digital National Service Desk on 0300 303 5035 for queries about the data submission process or the automatically generated reports.
6.3 How is the data collected?
The CWT data is collected on a monthly basis. Submissions are sent to the CWT database hosted by NHS Digital.
Submission deadlines are published at on the NHS Digital website at: https://digital.nhs.uk/cancer-waiting-times
6.4 What do we do if an error is found after the monthly deadline has passed?
NHS England will normally publish revisions to the data on a six-monthly basis and revisions will relate to the six months prior to the revision’s publication date. The dates for submission of revisions are given on the NHS Digital website in the report generation section.
NHS England’s policy on revisions is detailed here: Revisions Policy for NHS data collected through SDCS
6.5 What do we do if an error is found after the 6-months deadline has passed?
When an error has occurred or records have been missed then you should contact england.cancerwaitsdata@nhs.net.
6.6 How do we upload data to the CWT system?
The data is uploaded to NHS Digital’s CWT system. Documents describing the process of uploading and downloading data can be found in the Document Library section of https://digital.nhs.uk/cancer-waiting-times
The database allows records to be automatically updated as a group. It is envisaged that most records will be uploaded in bulk once per month. Manual uploads are also possible although not recommended for many records or as a long term solution. An individual record can be manipulated manually (provided the correct NHS Number is submitted) after it has been uploaded up until the cut-off date at the end of the quarter.
6.7 Where can I see the official statistics publications produced from the CWT system data?
See here NHS statistics page for CWT.
6.8 How can we suggest changes to the CWT system?
If you have suggestions for how the CWT system could be enhanced and improved these should be logged with the NHS Digital National Service Desk on 0300 303 5035 or with the CWT team at england.cancerwaitsdata@nhs.net.
Annex: National Cancer Dataset: Waiting Times Subset (NCWTMDS)
7. Patient and Pathway Identification Information
7.1 NHS NUMBER
This is the 10-digit numeric number used to identify a patient uniquely within the NHS in England and Wales. It is a unique identifier for a patient and will not vary by any organisation of which a person is a patient.
7.1.1 What is the policy for a patient with no NHS number, e.g. service personnel , prisoners, and mobile populations such as non-UK citizens?
A patient with no NHS number should still be treated as clinically appropriate within the waits commitments but it will not be possible to upload related data on to the CWT system. NHS England expect there to be a certain proportion of such patients that data cannot be collected for.
7.2 NHS NUMBER STATUS INDICATOR CODE
A two-digit numerical code used as a validation for the NHS NUMBER.
Options:
- 01 Number present and verified
- 02 Number present but not traced
- 03 Trace required
- 04 Trace attempted – No match or multiple match found
- 05 Trace needs to be resolved – (NHS Number or Patient detail conflict)
- 06 Trace in progress
- 07 Number not present and trace not required
- 08 Trace postponed (baby under six weeks old)
7.3 PATIENT PATHWAY IDENTIFIER (PPI)
This is an identifier that is unique to a patient for a particular condition. It consists of an alphanumeric figure of up to 20 digits which, together with the ORGANISATION IDENTIFIER of the issuer, uniquely identifies a patient pathway for the length of a particular condition. The PPI is allocated by the organisation receiving the referral request which would result in the patient being seen for the first time for a particular condition/suspected condition.
The PPI should be the same as the one used for RTT and needs to be consistent across all provider systems.
The PPI needs to be unique within an organisation and must not contain the patient’s NHS number.
In the rare case where two organisations issue the same PPI the ORGANISATION IDENTIFIER (PATIENT PATHWAY IDENTIFIER ISSUER) will differentiate between the two records.
PPIs can only contain letters, numbers, and some special characters (=(equals), -(dash) or _(underscore)), as spaces can produce problems on some NHS systems.
7.3.1 Can a patient have multiple patient pathway identifiers (PPI)?
Yes, if a patient has multiple conditions (e.g. multiple primary cancers) then these would each get their own individual PPI.
However, once created, the same PPI should be used for every recording of an individual condition for the entire lifetime of the patient, and so a PPI could cover multiple episodes of care for the same cancer.
How is the PPI generated for patients coming through the NHS e-Referral Service route?
In this case the PPI is made up of two parts. The first part is 8 packing characters (X09UBRN=), the second part is the unique booking reference number (converted) which is a 12-digit alpha-numeric number.
7.3.2 How do we create a PPI for patients that come through A&E?
It is assumed that a patient coming though A&E and diagnosed with cancer, or a suspicion of cancer would be referred on to an elective pathway from A&E (e.g. following stabilisation) or admitted for assessment then referred internally within the provider to the relevant MDT. They should receive a PPI at the point of the referral to the cancer service. The same principle applies on RTT pathways for other acute services, for example, patients admitted with heart problems.
On the rare occasion where first treatment does take place following an emergency admission (e.g. at the time of emergency bowel surgery) a PPI would not be available related to this episode (i.e. where the patient is first treated) and therefore cannot be entered. As such the CWT system validation will allow you to enter a record without a PPI.
If a patient is admitted as an emergency prior to a planned first appointment for suspicion of cancer, then the PPI generated from the original referral on to the elective pathway should be used.
7.3.3 If a patient has been diagnosed and treated for a cancer and reported under a relevant PPI and then is referred in again with a suspected second primary to another site this patient will have a new PPI. If the new referral is subsequently diagnosed as a recurrence rather than a new primary cancer (e.g. colorectal patients who develop lung metastasis) what should we do?
You should try to link back to the original PPI if you find that a suspected second primary cancer is in fact a recurrence linked to the initial primary cancer, i.e. retrospectively change the record to ensure it links to the original PPI.
However, we need to be pragmatic. If it would take a disproportionate amount of work/time to retrospectively change the PPI on local systems, then it is acceptable to keep the new PPI albeit not strictly speaking correct.
7.3.4 If a patient is referred urgently for suspected cancer by their GP and no cancer is diagnosed, then is re-referred in six months’ time for suspected cancer at the same site (e.g. a breast patient initially diagnosed with cysts) should the same PPI be used or would a new one be appropriate?
If the patient was initially referred with a suspected breast cancer – they would have a PPI for that referral even if the referral ended with a non-cancer diagnosis (i.e. cysts). If they were then re-referred at a later date, and the referral was linked to the initial referral, i.e. cancer was once again suspected then this would come under the same PPI unless:
- the second referral was unrelated to the initial symptoms and/or diagnosis (this would then have a new identifier); or
- the patient had been formally given a benign diagnosis following the earlier pathway (this would also have a new identifier).
7.3.5 Will records uploaded without a PPI be rejected by the CWT system?
No, records uploaded without a PPI will not be rejected. However, the PPI is a mandatory field within the CWT dataset if applicable to the episode of care. Where applicable, all mandatory fields are expected to be complete on the database by the specified deadline date after the end of a month.
7.4 ORGANISATION IDENTIFIER (PATIENT PATHWAY IDENTIFIER ISSUER)
This is the code of the organisation issuing the PATIENT PATHWAY IDENTIFIER (PPI), i.e. the organisation that receives the referral request resulting in the patient being seen for the first time for a particular condition/suspected condition.
It is the use of this ORGANISATION IDENTIFIER, along with the 20 digit PPI that creates a unique reference number for a patient pathway and any timed periods it contains.
Where the NHS e-Referral Service has been used the Code X09 should be used.
8. Outpatient services
8.1 SOURCE OF REFERRAL FOR OUT-PATIENTS
This data item identifies the source of referral for the consultant-led out-patient episode which would generally be the DATE FIRST SEEN for the patient unless they went to a diagnostic clinic first. It can be initiated by a range of health professionals. An urgent suspected cancer or breast symptomatic referral can be initiated by any source of referral, provided there is local agreement, with the exception of ‘17 referral from a National Screening Programme’.
This data item is not applicable for subsequent treatments.
Options
Initiated by the CONSULTANT responsible for the Consultant Out-Patient Episode.
- 01 following an emergency admission
- 02 following a Domiciliary Consultation
- 10 following an Accident and Emergency Attendance (including Minor Injuries Units and Walk in Centres)
- 11 other – initiated by the CONSULTANT responsible for the Consultant Out-Patient Episode
- 03 referral from a GENERAL MEDICAL PRACTITIONER
- 92 referral from a GENERAL DENTAL PRACTITIONER
- 12 referral from a General Practitioner with Extended Role (GPwER) or Dentist with a Special Interest (DwSI)
- 04 referral from an Accident and Emergency Department (including Minor Injuries Units and Walk in Centres)
- 05 referral from a CONSULTANT, other than in an Accident and Emergency Department
- 06 self-referral
- 07 referral from a Prosthetist
- 13 referral from a Specialist NURSE (Secondary Care)
- 14 referral from an Allied Health Professional
- 15 referral from an OPTOMETRIST
- 16 referral from an Orthoptist
- 17 referral from a National Screening Programme
- 93 referral from a Community Dental Service
- 97 other – not initiated by the CONSULTANT responsible for the Consultant Outpatient Episode
8.1.1 Does this have to be completed for every referral for an out-patient appointment a patient receives for a particular cancer?
Within the CWT system upload of this data item is only required for the first referral by the NHS to a provider that initiates a cancer referral to treatment period and results in a DATE FIRST SEEN.
8.1.2 How do we code referrals made by specialist nurses in primary care?
Such referrals are made under the authority of the General Medical Practitioner leading their team and should therefore be classified as referrals from the GP (i.e. Code ‘03’). Referrals from Specialist Nurses in Secondary Care should be classified as code ‘13’.
8.2 PRIORITY TYPE CODE
This data item identifies the priority of a referral. This data item is not applicable for subsequent treatments.
Options
- 1 Routine
- 2 Urgent
- 3 Two Week Wait
8.2.1 Which of these priority type codes can be upgraded on to the 62-day pathway?
- Priority ‘3’ (Two Week Wait referrals) – this will cover urgent GP (or other referrer) referrals for suspected cancer and referrals for breast symptoms (cancer not suspected) – both sets of patients automatically go on to the 62-day pathway if cancer is diagnosed so patients referred under this PRIORITY TYPE CODE do not need to be upgraded. Although the Two Week Wait standard is no longer in force, urgent cancer referrals from a GP or other referrer should still be recorded using this priority type code.
- Priority ‘2’ (urgent referrals) – for cancer standards this would be used for patients coming on to 62-day pathway from screening programmes. An upgrade is not needed for these patients. Patients with this referral code not from cancer screening services could be upgraded on to the 62-day pathway if a clinician suspects cancer and a local protocol to upgrade is in place, or a patient meets the requirement for a mandatory upgrade.
- Priority ‘1’ (routine referrals) – for cancer standards any patient routinely referred could be upgraded to be covered by the 62-day standard if a clinician suspects cancer and a local protocol to upgrade is in place or a patient meets the requirement for a mandatory upgrade.
In terms of the upgrade, patients with a PRIORITY TYPE CODE of either ‘1’ (routine) or ‘2’ (urgent – but not from screening programmes) could be upgraded onto the 62-day pathway. Without the upgrade the patient would be on the RTT pathway.
8.2.2 If urgent referrals (not from screening programmes) or routine referrals are upgraded, should their PRIORITY TYPE CODE be changed to PRIORITY TYPE CODE ‘3’ (i.e. equivalent to Urgent Suspected Cancer referrals)?
No, the PRIORITY TYPE CODE is that which relates to the initial referral.
8.3. DECISION TO REFER DATE (CANCER OR BREAST SYMPTOMS)
This is the date on which:
- a general medical practitioner, general dental practitioner, optometrist, or other locally agreed referrer decides to refer a patient urgently to secondary care with suspected cancer.
- any health professional decides to make a referral to secondary care for breast symptoms where cancer is not suspected.
- a screening service decides to urgently refer a patient with suspected cancer.
- a consultant upgrade is made.
8.3.1 How do we get the DECISION TO REFER DATE?
This date may be, for example:
- the date on the letter, proforma or email from the general medical practitioner, general dental practitioner, or other health professional
- Date the request was made by the patient if the referral was a self-referral.
- the date on the letter for patients recalled for further assessment following a routine screening programme appointment.
The date may not be available to the health care provider if the initial service request to secondary care was made via the e-Referral Service system, and no supporting information was received.
8.4. CANCER REFERRAL TO TREATMENT PERIOD START DATE
This is the starting point for the 28-day FDS and 62-day standards. It will be one of the following:
- Receipt of referral – for urgent referrals for suspected cancer and referrals for patients with breast symptoms (cancer not suspected)
- Receipt of referral via Choose & Book UBRN conversion – the Unique Booking Reference Number conversion date for an appointment – for urgent referrals for suspected cancer and referrals for patients with breast symptoms (cancer not suspected)
- Receipt of referral for further assessment following a suspicious mammogram (recorded as original referral request received date) – this is for patients coming in via urgent referral from the breast screening programme with suspected cancer.
- Receipt of referral for an appointment with a specialist screening practitioner (SSP) to discuss suitability for colonoscopy (recorded as original referral request received date) – this is for patients coming in via urgent referral from the bowel screening programme with suspected cancer.
- Receipt of referral for a colposcopy appointment (recorded as original referral request received date or UBRN conversion) – this is for patients coming in via urgent referral from the cervical screening programme with moderate or worse cytology.
- Receipt of referral for further investigation following an initial screening test. For a TLHC referral this would be the receipt of referral to the multidisciplinary team to review the low dose CT scan results.
- Date of triage into secondary, where agreed locally for a patient to be directly triaged following an abnormal direct access diagnostic scan with a suspicion of cancer.
- Receipt of self-referral where this model is in place for referrals.
8.5. URGENT SUSPECTED CANCER OR SYMPTOMATIC BREAST REFERRAL TYPE
This data item is used to record the site where cancer is suspected when referring the patient as an urgent suspected cancer. It is also used to identify patients being referred because of exhibited (non-cancer) breast symptoms from any healthcare professional.
Options:
- 01 Suspected breast cancer
- 02 Suspected children’s cancer
- 03 Suspected lung cancer
- 04 Suspected haematological malignancies excluding acute leukaemia.
- 05 Suspected acute leukaemia
- 06 Suspected upper gastrointestinal cancers
- 07 Suspected lower gastrointestinal cancers
- 08 Suspected skin cancers
- 09 Suspected gynaecological cancers
- 10 Suspected brain or central nervous system tumours
- 11 Suspected urological cancers (excluding testicular)
- 12 Suspected testicular cancer
- 13 Suspected head and neck cancers
- 14 Suspected sarcomas
- 16 Exhibited (non-cancer) breast symptoms – cancer not initially suspected.
- 17 Suspected cancer- non-specific symptoms
- 18 Other suspected cancer (not listed)
8.5.1 When should Code 01 and Code 16 be used, i.e. how do we distinguish between suspected and non-suspected breast cancer?
Code 01 is used for GP referrals for suspected breast cancer. Code 16 is only to be used for referrals of patients with breast symptoms where cancer is not suspected.
The distinction between suspected breast cancer and exhibited (non-cancer) symptoms is necessary to support the monitoring of the NHS Operating Framework 2011-12. The differentiation might also help to monitor appropriateness of referrals and therefore identify any education needed about signs and symptoms of breast cancer amongst relevant healthcare professionals.
8.6. CONSULTANT UPGRADE DATE
This is the date that the consultant responsible for the care of the patient (or an authorised member of the consultant team – as defined by local policy) decided that the patient should be upgraded from an RTT period to a 62-day period as cancer is suspected.
This is also mandated where:
- Where a patient is referred to a Cancer Multidisciplinary Team meeting on suspicion or with a confirmed cancer.
- Where a Trust radiology or pathology safety netting system flags a patient with suspicion or with confirmed cancer. The date of the radiology report would be used for the purposes of the Consultant Upgrade. This would only apply to patients under a consultant’s care within secondary care, not to those where the patient is still under the GPs care.
- From the point at which a patient on interval scan has an abnormal result suggesting that cancer is suspected.
- For Bowel Screening, where a patient initially declines all diagnostics, but then subsequently requests this, from the point at which the patient contacts the service and requests the diagnostic.
These dates should be uploaded retrospectively by the provider delivering the treatment. It is for local policies to determine processes to facilitate this. If a patient is upgraded but cancer is not diagnosed, then there is no national requirement to collect this information, but local collection would aid local awareness and education about the magnitude and appropriateness of upgrades.
8.6.1 What referrals can and cannot be upgraded?
Referrals that can be upgraded are:
- any routine referrals (i.e. PRIORITY TYPE CODE ‘1’)
- urgent referrals that are not from the NHS cancer screening programmes (i.e. PRIORITY TYPE CODE ‘2’).
Referrals that cannot be upgraded are:
- urgent suspected cancer referrals recorded with PRIORITY TYPE CODE ‘3’ (Two Week Wait)
- referrals for breast symptoms (cancer not suspected) recorded with PRIORITY TYPE CODE ‘3’ (Two Week Wait)
- urgent screening referrals (PRIORITY TYPE CODE ‘2’) – these referrals can be identified by data item SOURCE OF REFERRAL FOR OUT-PATIENTS Code ‘17’ which is for a referral from an NHS cancer screening programme.
These are exceptions because the patient would automatically be covered by the 62-day standard if cancer was diagnosed.
8.6.2 Is there a time after which an upgrade is not allowed?
Yes. An upgrade must be on or before:
- a Decision To Treat with a patient has been agreed (i.e. before the Decision To Treat date recorded as the CANCER TREATMENT PERIOD START DATE)
8.7. ORGANISATION SITE IDENTIFIER (OF PROVIDER CONSULTANT UPGRADE)
This is the ORGANISATION SITE IDENTIFIER of the organisation acting as a commissioned health care provider when a decision is made to upgrade the patient from the RTT pathway to the 62-day standard, i.e. it is the ORGANISATION SITE IDENTIFIER of the organisation where a 62-day period for the consultant upgrade standard starts.
8.8. DATE FIRST SEEN
This is the date when the patient is seen for the first time by a consultant (or member of their team) or in a clinic following the referral receipt.
It will be one of the following (whichever is the earliest service relating to the referral request):
- First out-patient appointment – this is the attendance date with a consultant or member of the consultant team, either a face to face or virtual attendance
- First diagnostic procedure (if this precedes the first out-patient appointment), e.g. CT scan prior to appointment with chest physician, i.e. straight to test.
- Where manged through a tele-dermatology pathway, a specialist review of an image taken in primary care, appointment where a dermoscopic image/photo is taken, or when the patient is seen in clinic.
- First appointment following referral (or recall) from (or by) a screening provider i.e.
- Breast – appointment for further assessment following screening mammogram.
- Bowel – appointment with a specialist screening practitioner to discuss suitability for colonoscopy.
- Cervical – appointment for colposcopy.
- First seen as an emergency – this is the start date of the Hospital Provider Spell or the Arrival Date of the Accident and Emergency Attendance. If a patient is admitted as an emergency for the same condition, they have been referred under the urgent suspected cancer or breast symptomatic route, but have yet to have their first seen date, this admission would count as the DATE FIRST SEEN. The patient would no longer be counted as part of the Faster Diagnosis Standard cohort. This patient could be upgraded onto the 62-day period if a consultant (or authorised member of the team) suspected cancer.
8.9. ORGANISATION SITE IDENTIFIER (OF PROVIDER FIRST SEEN)
This is the ORGANISATION SITE IDENTIFIER of the organisation acting as a commissioned health care provider where the patient is first seen (where the DATE FIRST SEEN would be recorded).
8.10. WAITING TIME ADJUSTMENT (FIRST SEEN)
This records the number of days that should be removed from the calculated waiting time for the 28-day period and potentially the 62-day period (if cancer is confirmed) –, i.e. between receipt of the referral or decision of consultant upgrade (recorded as CANCER REFERRAL TO TREATMENT PERIOD START DATE or CONSULTANT UPGRADE DATE) and the DATE FIRST SEEN.
8.11. WAITING TIME ADJUSTMENT REASON (FIRST SEEN)
This data item is where you record the reason for using an adjustment prior to the first seen date.
Options:
- 9 No adjustment to waiting time.
- 3 Did Not Attend Out-Patient Appointment
Where the ATTENDED OR DID NOT ATTEND is National Code ‘Did Not Attend – no advance warning given’ or National Code ‘PATIENT arrived late and could not be seen’
8.11.1 Why do we need to select an option that no adjustment was used if no figure has been entered in the WAITING TIME ADJUSTMENT (FIRST SEEN) it is obvious no adjustment has been made?
If you enter a ‘0’ in the WAITING TIME ADJUSTMENT (FIRST SEEN) field, then you need to enter Code ‘9’ in this field. However, you can choose to leave both fields blank if you prefer. The option to include a ‘0’ and then Code ‘9’ has been included because some local systems find it easier to output zeros than blanks.
8.12 CANCER CARE SPELL DELAY REASON (FIRST SEEN)
This data item enables you to identify from a list of options an overarching reason why (after any adjustments have been removed) a delay/breach occurred to the first seen period. This reason will also be applicable if the patient went on to breach the related 62-day and 28-day standards.
Options:
- 01 Clinic cancellation
- 02 Out-patient capacity inadequate (i.e. no cancelled clinic, but not enough slots for this PATIENT)
- 03 Administrative delay
- 17 PATIENT choice delay relating to first Out-Patient Appointment
- 22 PATIENT care not commissioned by the NHS in England (waiting time standard does not apply) for treatment in an admitted care setting.
- 97 Other reason (not listed)
8.12.1 What option do we pick if there are multiple reasons for a breach?
You should pick the option that accounted for the longest wait for the patient.
8.13. CANCER CARE SPELL DELAY REASON COMMENT (FIRST SEEN)
This is the free text comment field to describe in more detail why the maximum two week wait period has been breached. It should not include the name of the patient, any other personal details or the clinician(s) involved in the case.
8.14. RAPID DIAGNOSTIC CENTRE PATHWAY COMPLIANCE INDICATOR
This data item is no longer in use and will be removed from the dataset at the next opportunity.
8.15. CANCER DIAGNOSIS REFERRAL ROUTE
This data item refers to if a patient was directly referred following a NG12 direct access diagnosis into secondary care or was referred via another route.
The options available are: –
- 01 Abnormal diagnostics result following a NICE guidance NG12 referral to a direct access diagnostics service.
- 02 Other (not listed)
9. Patient Status and Diagnosis
9.1. CANCER OR SYMPTOMATIC BREAST REFERRAL PATIENT STATUS
This data item enables local tracking of the status of patients referred with a suspected cancer (via GP/GDP/Optometrist or screening service) or referred from any health professional with breast symptoms where cancer was not suspected or upgraded onto the 62-day period.
Options (listed in logical sequence rather than numeric order)
- 14 Suspected primary cancer
- 09 Under investigation following symptomatic referral, cancer not suspected (breast referrals only) – this code can only be used for symptomatic breast referrals, i.e. those with a TWO WEEK WAIT CANCER OR SYMPTOMATIC BREAST REFERRAL TYPE of Code ‘16’ – Exhibited(non-cancer) breast symptoms – cancer not initially suspected)
- 03 No new cancer diagnosis identified by the Healthcare Provider
- 10 Diagnosis of new cancer confirmed – NHS funded first treatment not yet planned.
- 11 Diagnosis of new cancer confirmed – NHS funded first treatment planned.
- 07 Diagnosis of cancer confirmed – no NHS funded treatment planned.
- 08 First NHS funded treatment commenced.
- 12 Diagnosis of new cancer confirmed – subsequent NHS funded treatment not yet planned.
- 13 Diagnosis of new cancer confirmed – subsequent NHS funded treatment planned.
- 21 Subsequent NHS funded treatment commenced.
- 15 Suspected recurrent cancer
- 16 Diagnosis of recurrent cancer confirmed – first NHS funded treatment not yet planned.
- 17 Diagnosis of recurrent cancer confirmed – NHS funded first treatment planned.
- 18 Diagnosis of recurrent cancer confirmed – no NHS funded treatment planned.
- 19 Diagnosis of recurrent cancer confirmed – subsequent NHS funded treatment not yet planned.
- 20 Diagnosis of recurrent cancer confirmed – subsequent NHS funded treatment planned
- 22 Recurrent cancer NHS funded treatment commenced
- 23 Suspected cancer transformation
- 24 Diagnosis of cancer transformation confirmed – NHS funded first treatment not yet planned
- 25 Diagnosis of cancer transformation confirmed – NHS funded first treatment planned
- 26 Diagnosis of cancer transformation confirmed – no NHS funded treatment planned
- 27 Diagnosis of cancer transformation confirmed – subsequent NHS funded treatment not yet planned
- 28 Diagnosis of cancer transformation conformed – subsequent NHS funded treatment planned
- 29 Cancer transformation NHS funded treatment commenced
- 30 Suspected cancer progression
- 31 Diagnosis of cancer progression confirmed – NHS funded first treatment not yet planned
- 32 Diagnosis of cancer progression confirmed – NHS funded first treatment planned
- 33 Diagnosis of cancer progression confirmed – no NHS funded treatment planned
- 34 Diagnosis of cancer progression confirmed – subsequent NHS funded treatment not yet planned
- 35 Diagnosis of cancer progression confirmed – subsequent NHS funded treatment planned
- 36 Cancer progression NHS funded treatment commenced
9.1.1 Do these codes need to be updated throughout the patient journey?
It is mandatory to complete this field at the point of DATE FIRST SEEN and also at the TREATMENT START DATE (CANCER) and it is likely that the code will need to change at the second of these points, for example, changing from Code ‘14’ (suspected primary cancer) to Code ‘08’ (first treatment commenced). You might wish to update the codes more frequently locally to aid local tracking and record management – this might be particularly useful where Inter-Provider Transfers take place or to identify when records can be closed, e.g. when cancer is ruled out (Code ‘03’).
9.1.2 How are recurrent/metastatic cancers recorded?
Code ‘21’ can be used for recurrent/metastatic cancers as well as subsequent treatments for a new cancer.
If a suspected recurrent cancer is ruled out, then code ‘03’ can be used.
9.1.3 All treatments for recurrences are counted as a subsequent treatment but codes 16 and 17 allow you to record first treatments for these recurrences – why is that?
For local planning purposes you might wish to record that the first treatment for the recurrence is being planned. For the national standard, treatment of a recurrence, when given, is always assumed to be a subsequent treatment on the basis that the patient would have had some form of treatment for their initial cancer even if that was some years ago.
9.2. PRIMARY DIAGNOSIS (ICD)
This data item is used to record a four figure ICD-10 diagnosis of the primary tumour, i.e. the code which identifies the type of cancer. The codes which the CWT system will accept can be found at: Cancer Waiting Times Data Collection (CWT) – NHS England Digital
The PRIMARY DIAGNOSIS (ICD) field can only be completed when a full histologically confirmed diagnosis is available. This field is not the same as the PRIMARY CANCER SITE (CANCER FASTER DIAGNOSIS PATHWAY), which captures the type of cancer initially communicated to the patient at the end of the 28-day FDS, when a histologically confirmed diagnosis may not yet be available.
9.2.1 How do we code secondary, metastatic disease or an unknown primary?
For any recurrent or metastatic disease, the ICD-10 code of the primary site needs to be recorded as the PRIMARY DIAGNOSIS.
If you are treating metastatic disease of an unknown primary, you would use codes C78.0 – C79.8 as relevant to the metastatic site, you are treating.
9.2.2 Why aren’t the details of the metastatic site be included within the treatment record of the primary cancer?
We are aware that the first treatment record will not include the metastatic details. If metastatic details were included on the record for a known primary, it would not be clear if the treatment being reported on the CWT system was for the first treatment of the primary or for treatment of the metastases.
9.2.3 What happens if a primary cancer is diagnosed and confirmed AFTER a metastases has been treated?
If the primary cancer had not been diagnosed before a treatment was given you would have been treating metastases of an unknown primary. If the primary cancer is diagnosed at a later date the ICD-10 code can be included for any subsequent treatments.
9.3. TUMOUR LATERALITY
This identifies the position of a tumour within a patient.
Options
- L Left
- R Right
- M Midline
- B Bilateral
9.4. PROSTATE CANCER CLINICAL RISK CATEGORY
Prostate cancer diagnoses should be classified using the Cambridge Prognosis Group Classification as detailed in NICE guidance (NG131).
| Cambridge Prognosis Group Category | Cancer Waiting Times Classification | Criteria |
|---|---|---|
| 1 | Low | Gleason score 6 AND PSA <10 ng/ml AND Stages T1-T2 |
| 2 | Low – Intermediate | Gleason score 3+4 = 7 OR both PSA 10-20 AND stages T1-T2 |
| 3 | Intermediate – High | Gleason 3+4 = 7 AND PSA 10-20 ng/ml AND Stages T1-T2 OR Gleason 4+3 = 7 AND Stages T1-T2 |
| 4 | High | Gleason 8 OR PSA >20 ng/ml OR Stage T3 |
| 5 | High | Gleason 9-10 OR Stage T4 |
9.5. CANCER TREATMENT PERIOD START DATE
This is the date that the consultation between the patient and the clinician took place and a Planned Cancer Treatment was agreed. It marks the start of the 31-day period for both first and subsequent treatments. It will be either the DECISION TO TREAT DATE (DTT) or the EARLIEST CLINICALLY APPROPRIATE DATE (ECAD).
The DTT date (recorded as the CANCER TREATMENT PERIOD START DATE) is the date of the discussion in which the patient and clinician (or authorised member of the team), agree the treatment plan for first (and/or subsequent) treatments.
9.6. ORGANISATION SITE IDENTIFIER (OF PROVIDER CANCER DECISION TO TREAT)
This is the ORGANISATION SITE IDENTIFIER of the organisation acting as a commissioned health care provider where the Decision To Treat the patient (the starting point for the 31-day period for first treatments and for some subsequent treatments) was made – the CANCER TREATMENT PERIOD START DATE.
9.7. SERVICE REQUESTED DATE (INTER-PROVIDER TRANSFER)
This is the date that the transfer request is made by the referring organisation.
This item should only be recorded where a patient’s care is formally transferred.
This item can be used repeatedly to capture the sent date for all transfers in a patient pathway. It should be completed by the referring organisation.
9.8. REFERRAL REQUEST RECEIVED DATE (INTER-PROVIDER TRANSFER)
This is the date referral is received and accepted by the receiving provider.
This item should only be recorded where a patient’s care is formally transferred.
This item can be used repeatedly to capture the sent date for all transfers in a patient pathway. It should be completed by the referring organisation.
9.8.1 Who is responsible for completing this field?
The responsibility for completion of the REFERRAL REQUEST RECEIVED DATE field is with the provider receiving the patient, either to continue the diagnostic phase of a patient’s pathway, or to begin treatment. If the referring provider disagrees with the date the treating provider submits, then they should contact the treating provider.
9.9. ORGANISATION IDENTIFIER (REFERRING)
This is the ORGANISATION IDENTIFIER of the organisation that makes an Inter-Provider Transfer referral, i.e. it is the ORGANISATION IDENTIFIER of the sending organisation.
This item can be used repeatedly to capture the sending provider for all transfers in a patient pathway.
9.10. ORGANISATION IDENTIFIER (RECEIVING)
This is the ORGANISATION SITE IDENTIFIER of the organisation where an Inter-Provider Transfer referral is received, i.e.it is the ORGANISATION SITE IDENTIFIER of the receiving organisation.
This item can be used repeatedly to capture the receiving provider for all transfers in a patient pathway.
9.11. CANCER TRANSFER REFERRING REASON (INTER-PROVIDER TRANSFER)
This is the reason of why an inter-provider transfer was made.
Options:
- 01 Diagnosis
- 02 Staging
- 03 Second Opinion
- 04 Primary Treatment
- 05 Subsequent Treatment
This item can be used repeatedly to capture the reason for all transfers in a patient pathway as recorded by the referring organisation. The item needs to be completed by the referring organisation.
9.12. CANCER TRANSFER RECEIVING REASON (INTER-PROVIDER TRANSFER)
This is the reason of why an inter-provider transfer was received.
Options:
- 01 Diagnosis
- 02 Staging
- 03 Second Opinion
- 04 Primary Treatment
- 05 Subsequent Treatment
This item can be used repeatedly to capture the reason for all transfers in a patient pathway as recorded by the receiving organisation. This item needs to be completed by the receiving organisation.
9.13. CANCER FASTER DIAGNOSIS PATHWAY END REASON
The 28-day FDS requires a patient to be informed of a diagnosis of cancer, or ruling out of cancer, within 28 days. This data item records whether the patient was diagnosed, had cancer ruled out, or was excluded from the FDS pathway for any of the reasons in CANCER FASTER DIAGNOSIS PATHWAY EXCLUSION REASON.
Options
- 01 Diagnosis of cancer
- 02 Ruling out of cancer
- 03 Excluded from the Cancer Faster Diagnosis Pathway
- 04 Interval scanning
9.14. PRIMARY CANCER SITE (CANCER FASTER DIAGNOSIS PATHWAY)
The type of cancer communicated to the patient at the end of the 28-day FDS pathway.
This may be different to primary diagnosis (ICD) as it is the type of cancer first communicated to the patient; at which time, a full histological diagnosis may not yet have been made.
Options
- 01 Breast
- 02 Children’s
- 03 Lung
- 04 Haematological (Excluding Acute Leukaemia)
- 05 Acute leukaemia
- 06 Upper Gastrointestinal
- 07 Lower Gastrointestinal
- 08 Skin
- 09 Gynaecological
- 10 Brain/Central Nervous System
- 11 Urological (Excluding Testicular and Prostate)
- 12 Testicular
- 13 Head and Neck
- 14 Sarcoma
- 15 Metastatic disease of unknown primary
- 16 Prostate
- 17 Thyroid
- 18 Hepatobiliary
- 19 Pancreatic
- 20 Neuroendocrine tumour (NET)
- 98 Other (not listed)
9.15. CANCER FASTER DIAGNOSIS PATHWAY END DATE
The date on which the patient is told whether cancer is diagnosed or ruled out, or at which they are removed from the pathway for reasons recorded in CANCER FASTER DIAGNOSIS PATHWAY EXCLUSION REASON.
5.16. CANCER CARE SPELL DELAY REASON (OUTCOME COMMUNICATION CANCER FASTER DIAGNOSIS PATHWAY)
This data item enables you to identify from a list of options an overarching reason why (after any adjustments have been removed) a delay/breach occurred to the 28-day FDS, i.e. why the health care provider was unable to communicate the outcome to the patient within 28 days.
Options:
- 01 Clinic cancellation
- 02 Out-patient capacity inadequate (i.e. no cancelled clinic, but not enough slots for this PATIENT)
- 03 Administrative delay
- 04 Elective cancellation (for non-medical reason) for treatment in an admitted care setting
- 05 Elective capacity inadequate (PATIENT unable to be scheduled for treatment within standard time) for treatment in an admitted care setting
- 07 Complex diagnostic pathway (many, or complex, diagnostic tests required)
- 11 Diagnosis delayed for medical reasons (PATIENT unfit for diagnostic episode, excluding planned recovery period following diagnostic test)
- 13 Delay due to recovery after an invasive test (PATIENT DIAGNOSIS or treatment delayed due to planned recovery period following an invasive diagnostic test)
- 14 PATIENT Did Not Attend treatment APPOINTMENT
- 17 PATIENT choice delay relating to first Out-Patient Appointment
- 18 Health Care Provider initiated delay to diagnostic test or treatment planning
- 19 PATIENT initiated (choice) delay to diagnostic test or treatment planning, advance notice given
- 20 PATIENT Did Not Attend an APPOINTMENT for a diagnostic test or treatment planning event (no advance notice)
- 22 PATIENT care not commissioned by the NHS in England (waiting time standard does not apply) for treatment in an admitted care setting
- 23 Equipment breakdown
- 24 Inconclusive diagnostic result
- 25 Health Care Provider unable to make contact with PATIENT by telephone
- 26 PATIENT choice (PATIENT declined or cancelled an offered Appointment Date for follow up APPOINTMENT)
- 97 Other reason (not listed)
9.17. CANCER CARE SPELL DELAY REASON COMMENT (OUTCOME COMMUNICATION CANCER FASTER DIAGNOSIS PATHWAY)
This is the free text comment field to describe in more detail why the maximum 28-day FDS has been breached. It should not include the name of the patient, any other personal details or the clinician(s) involved in the case. This field is optional.
9.18. CANCER FASTER DIAGNOSIS PATHWAY EXCLUSION REASON
This data item enables you to identify from a list of options the reason why a patient has been removed from the 28-day FDS pathway.
Options
- 01 PATIENT died before diagnosis communicated
- 02 PATIENT declined all diagnostic investigations
- 03 PATIENT declined all APPOINTMENTS
- 04 PATIENT opted for private diagnostics (PATIENT may come back for NHS funded treatment)
- 05 Repeated Did Not Attend (DNA)/PATIENT-triggered multiple cancellations
- 06 PATIENT ineligible for NHS funded treatment
9.19. CARE PROFESSIONAL TYPE CODE (OUTCOME COMMUNICATION CANCER FASTER DIAGNOSIS PATHWAY)
This data item enables you to capture the Care Professional communicating the end reason 01 Diagnosis of cancer or 02 Ruling out of cancer in CANCER FASTER DIAGNOSIS PATHWAY END REASON. This field is optional.
Options
- 060 CONSULTANT
- 150 GENERAL DENTAL PRACTITIONER
- 160 GENERAL MEDICAL PRACTITIONER
- 180 NURSE
- 210 OPHTHALMIC MEDICAL PRACTITIONER
- 220 OPTOMETRIST
- 310 Radiographer
- XXX Other (not listed)
9.20. METHOD OF COMMUNICATION (END OF CANCER FASTER DIAGNOSIS PATHWAY)
This data item enables you to capture the method of communication of the end reason 01 Diagnosis of cancer or 02 Ruling out of cancer in CANCER FASTER DIAGNOSIS PATHWAY END REASON. This field is optional.
Options
- 01 Face to face communication
- 02 Telephone call
- 03 Email
- 04 Letter
- 98 Other (not listed)
9.21. ORGANISATION SITE IDENTIFIER (OF CANCER FASTER DIAGNOSIS END)
This is the ORGANISATION SITE IDENTIFIER of the organisation responsible for communicating the outcome (cancer diagnosis or cancer ruled out) to the patient on the 28-day FDS pathway, or where the patient was excluded from the pathway, i.e. the organisation where the CANCER FASTER DIAGNOSIS PATHWAY END DATE takes place.
10. Treatment Events
10.1. TREATMENT START DATE (CANCER)
This is the start date of the first or subsequent cancer treatments and marks the end of the 62-day period and the end of the 31-day period for first or subsequent treatments.
10.2. ORGANISATION SITE IDENTIFIER (OF PROVIDER CANCER TREATMENT START DATE)
This is the ORGANISATION SITE IDENTIFIER of the organisation acting as commissioned health care provider where a patient receives a treatment which ends a 62-day and/or 31-day period(s), i.e. the organisation where the TREATMENT START DATE (CANCER) takes place.
10.3. CANCER TREATMENT EVENT TYPE
This identifies the phase a treatment has reached during a cancer patient pathway for primary, recurrent, or metastatic cancer.
Options
- 01 First Definitive Treatment for a new primary cancer
- 02 Second or subsequent treatment for a new primary cancer
- 03 Treatment for a local recurrence of a primary cancer
- 04 Treatment for a regional recurrence of cancer
- 05 Treatment for a distant recurrence of cancer (metastatic disease)
- 06 Treatment for multiple recurrence of cancer (local and/or regional and/or distant)
- 07 First treatment for metastatic disease following an unknown primary
- 08 Second or subsequent treatment for metastatic disease following an unknown primary
- 09 Treatment for relapse of primary cancer (second or subsequent)
- 10 Treatment for progression of primary cancer (second or subsequent)
- 11 Treatment for cancer transformation of Primary Cancer (second or subsequent)
- 12 First treatment for metastatic disease following a known Primary Cancer.
10.3.1 Which of these codes are for first treatments that can end a 62-day period?
Only codes ‘01’, ‘07’ and ‘12’ will be considered to be first treatment events and therefore suitable for reporting as the end points to the 62-day period (from urgent GP (GMP, GDP or Optometrist) referral, referral with breast symptoms where cancer is not suspected, urgent referral from screening programmes or consultant upgrades) and against the original 31-day standard for first treatment.
10.3.2 What is code ‘06’ (Treatment for multiple recurrences of cancer (local and/or regional and/or distant)) used for?
This is for a patient who is treated for a cancer which has spread in more than one way.
- Local would be a recurrence very close to the site of the original primary cancer
- Regional would be, for example in the case of a breast patient, spread to the axilla, supraclavicular or inter-mammary nodes
- Distant would be metastatic spread to somewhere like the liver, brain, or lungs.
10.3.3 What are code ‘09’ (Treatment for relapse of primary cancer (second or subsequent)) and code ‘10’ (Treatment for progression of primary cancer (second or subsequent)) used for?
As haematological cancers do not spread/recur in the same way as solid tumours, haematologists consulted about cancer waits advised including this description within the coding structure.
10.3.4 This item seems like Cancer or Symptomatic Breast Referral Patient Status – what is the difference/why do we need both?
- This item (CANCER TREATMENT EVENT TYPE) is about reporting the final event that would mark the end of a 62-day and/or 31-day period;
- The CANCER OR SYMPTOMATIC BREAST REFERRAL PATIENT STATUS item is for local tracking and record management.
10.4. CANCER TREATMENT MODALITY
This data item identifies the type of treatment or care that a patient receives within the episode that ends a 31-day or 62-day period. The modality codes need to be used for both first and subsequent treatments.
Options
- 02 Anti-cancer drug regimen (Cytotoxic Chemotherapy)
- 03 Anti-cancer drug regimen (Hormone Therapy)
- 04 Chemoradiotherapy
- 05 Teletherapy (Beam Radiation excluding Proton Therapy)
- 06 Brachytherapy
- 07 Specialist Palliative Care
- 08 Active Monitoring (excluding non-specialist Palliative Care)
- 09 Non-specialist Palliative Care (excluding Active Monitoring)
- 10 Radio Frequency Ablation (RFA)
- 11 High Intensity Focussed Ultrasound (HIFU)
- 12 Cryotherapy
- 13 Proton Therapy
- 14 Anti-cancer drug regimen (other)
- 15 Anti-cancer drug regimen (Immunotherapy)
- 16 Light Therapy (including Photodynamic Therapy and Psoralen and Ultraviolet A Therapy (PUVA))
- 17 Hyperbaric Oxygen Therapy
- 19 Radioisotope Therapy (including Radioiodine)
- 20 Laser Treatment (including Argon Beam therapy)
- 21 Biological Therapies (excluding Immunotherapy)
- 22 Radiosurgery
- 23 Surgery (excluding enabling treatment)
- 24 Surgery (enabling treatment)
- 97 Other Treatment (not listed)
- 98 All treatment declined
10.5. CLINICAL TRIAL INDICATOR
This data item is used to record whether the treatment package recorded under CANCER TREATMENT MODALITY was part of a clinical trial of a new treatment (e.g. drug or procedure).
Options
- 01 Patient is taking part in a clinical trial
- 02 Patient is not taking part in a clinical trial
If the clinical trial relates to a part of the pathway other than the treatment episode, then code ‘02’ should be used.
10.6. CANCER CARE SETTING (TREATMENT)
This data item is used to record the type of care setting where the cancer treatment took place that marks the end of the 31-day and/or 62-day period (i.e. the TREATMENT START DATE (CANCER)).
Options
- 01 Cancer treatment delivered as part of a Hospital Provider Spell (where patient classification is National code 1 – Ordinary admission)
- 02 Cancer treatment delivered as part of a Hospital Provider Spell (where patient classification is National Code 2 – Day case admission)
- 03 Cancer treatment delivered in an Out-patient setting
- 04 Cancer treatment delivered in another care setting
10.7. CANCER CARE SPELL DELAY REASON (DECISION TO TREAT)
This data item enables you to identify from a list of options an overarching reason why (after any adjustments have been removed) a delay/breach occurred to the 31-day periods, i.e. why the health care provider was unable to provide the treatment in question within the service standard of 31-days.
Options
- 01 Clinic Cancellation
- 02 Out-patient capacity inadequate (i.e. no cancelled clinic, but not enough slots for this PATIENT)
- 03 Administrative delay
- 05 Elective capacity inadequate (PATIENT unable to be scheduled for treatment within standard time) for treatment in an admitted care setting
- 07 Complex diagnostic pathway (many, or complex, diagnostic tests required)
- 10 Treatment delayed for medical reasons (PATIENT unfit for treatment episode, excluding planned recovery period following diagnostic test) in an admitted care setting
- 11 Diagnosis delayed for medical reasons (PATIENT unfit for diagnostic episode, excluding planned recovery period following diagnostic test)
- 13 Delay due to recovery after an invasive test (PATIENT DIAGNOSIS or treatment delayed due to planned recovery period following an invasive diagnostic test)
- 14 PATIENT Did Not Attend treatment APPOINTMENT
- 16 PATIENT Choice (PATIENT declined or cancelled an offered Appointment Date for treatment)
- 17 PATIENT choice delay relating to first Out-Patient Appointment
- 18 Health Care Provider initiated delay to diagnostic test or treatment planning
- 19 PATIENT initiated (choice) delay to diagnostic test or treatment planning, advance notice given
- 20 PATIENT Did Not Attend an APPOINTMENT for a diagnostic test or treatment planning event (no advance notice)
- 21 PATIENT failed to present for elective treatment (choice) in an admitted care setting
- 22 PATIENT care not commissioned by the NHS in England (waiting time standard does not apply) for treatment in an admitted care setting
- 23 Equipment breakdown
- 24 Inconclusive diagnostic result
- 25 Health Care Provider unable to make contact with PATIENT by telephone
- 26 PATIENT choice (PATIENT declined or cancelled an offered Appointment Date for follow up APPOINTMENT)
- 97 Other reason (not listed)
10.8. CANCER CARE SPELL DELAY REASON COMMENT (DECISION TO TREAT)
This is the free text comment field to describe why the maximum 31-day period has been breached. It should not include the name of the patient, any other personal details or the clinician(s) involved in the case. This field is optional.
10.9. WAITING TIME ADJUSTMENT (TREATMENT)
This data item is used to record the number of days that should be removed from the calculated waiting time between the CANCER TREATMENT PERIOD START DATE and the TREATMENT START DATE (CANCER), i.e. the number of days that a clock can be paused for a 31 or 62-day period if a reasonable offer of treatment in admitted care has been declined.
10.10. WAITING TIME ADJUSTMENT REASON (TREATMENT)
This data item is where you record the reason for using an adjustment prior to admitted care.
Options:
- 6 Egg harvesting
- 7 Clinically urgent treatment of another condition
- 8 Patient pause
- 9 No adjustment to waiting time
10.10.1 Why do we need to select an option that no adjustment was used if no figure has been entered in the WAITING TIME ADJUSTMENT (TREATMENT) it is obvious no adjustment has been made?
If you enter a ‘0’ in the WAITING TIME ADJUSTMENT (TREATMENT) field, then you need to enter Code ‘9’ in this field. However, you can choose to leave both fields blank if you prefer. The option to include a ‘0’ and then Code ‘9’ has been included because some local systems find it easier to output zeros than blanks.
10.11. CANCER CARE SPELL DELAY REASON (REFERRAL TO TREATMENT)
This data item enables you to identify from a list of options an overarching reason why (after any adjustments have been removed) a delay/breach occurred to the 62-day period, i.e. why the health care provider was unable to provide treatment within the service standard of 62 days.
Options: – See options for CANCER CARE SPELL DELAY REASON (DECISION TO TREAT)
10.11.1 What option do we pick if there are multiple reasons for a breach?
You should pick the option that accounted for the largest proportion of the breach.
10.11.2 What do we do if patient choice is the reason for a delay?
Use code “patient choice” and use the related comment field (CANCER CARE SPELL DELAY REASON COMMENT (REFERRAL TO TREATMENT)) to describe the problem in more detail. If none of the patient choice options are relevant, then code ‘97’ “other reason” can be used, but this should be avoided if possible.
10.11.3 Are all 62-day standard breaches recorded using this field?
It is for any breach of a 62-day period starting from:
- receipt of urgent referral for suspected cancer
- receipt of urgent referral from any healthcare professional for breast symptoms (cancer not suspected)
- receipt of urgent referral from screening programmes (breast, bowel or cervical).
It is not to be used for breaches of the 62-day period following a consultant upgrade. These breaches are recorded under different data items (see CANCER CARE SPELL DELAY REASON (CONSULTANT UPGRADE) and CANCER CARE SPELL DELAY REASON COMMENT (CONSULTANT UPGRADE)) because the starting point of the upgrade period is different, i.e. date of decision to upgrade rather than receipt of referral.
10.12. CANCER CARE SPELL DELAY REASON COMMENT (REFERRAL TO TREATMENT)
This is the free text comment field to describe why the maximum 62-day period has been breached. It should not include the name of the patient, any other personal details or the clinician(s) or other staff involved in the case. This field is optional.
10.13. CANCER CARE SPELL DELAY REASON (CONSULTANT UPGRADE)
This data item enables you to identify from a list of options an overarching reason why (after any adjustments have been removed) a delay/breach occurred to the 62-day consultant upgrade period, i.e. why the health care provider was unable to provide treatment within the service standard of 62 days following a decision to upgrade.
Options – See options for CANCER CARE SPELL DELAY REASON (DECISION TO TREAT)
10.14. CANCER CARE SPELL DELAY REASON COMMENT (CONSULTANT UPGRADE)
This is the free text comment field to describe why the maximum 62-day consultant upgrade period has been breached. It should not include the name of the patient, any other personal details or the clinician(s) involved in the case. This field is optional.
Publications reference: PRN01855

