This guidance outlines roles, responsibilities, and accountabilities for delivering medicines optimisation in all parts of the NHS in England.
Introduction
Medicines optimisation is a person‑centred approach to the safe and effective use of medicines. It enables the best possible outcomes for patients and value for the NHS and taxpayers by tackling, for example, problematic polypharmacy, antimicrobial resistance, variation in access to effective treatments, medicines sustainability and the safety of high-risk medicines.
Medicines are used in almost all clinical pathways and spend on medicines consumes the second largest share of the NHS budget in England.
Integrated care boards (ICBs) have an important role in overseeing providers within their integrated care system and are accountable for the quality and safety of services, financial management and the performance of the local NHS.
The NHS Operating Framework sets out the ways of working and behaviours expected to deliver system-based approaches to health and social care and outlines the roles and responsibilities of NHS England, regions, ICBs and NHS providers.
The NHS is facing well documented financial challenges and the delivery of productivity and efficiency improvements, wherever possible, is being increasingly important. Medicines optimisation efficiency savings present a significant opportunity for integrated care systems (ICSs) to deliver balanced financial plans, and this guidance supports efficiency efforts in line with nationally identified medicines optimisation opportunities.
Purpose of this guidance
Before integrated care boards (ICBs) were formed, regional leadership for medicines optimisation was co-ordinated and delivered through regional medicines optimisation committees (RMOCs). With the changing NHS structures and statutory requirements, a review of the existing regional arrangements for medicines optimisation has been carried out.
NHS England has set up a framework of governance (see figure 1), policy, and clinical leadership, bringing together all aspects of medicines optimisation to support system delivery within and across the NHS.
Effective cross-organisational medicines optimisation delivery requires strategic leadership and collaborative working to ensure that all parts of the healthcare system identify, adopt, and support medicines optimisation opportunities. As part of this, NHS England has a role in bringing local, regional, and national expertise together to tackle long-standing medicines optimisation challenges, by learning from what has worked and how, and realising opportunities for national action at scale.
This guidance outlines roles, responsibilities, and accountabilities for delivering medicines optimisation in all parts of the NHS in England. It aligns to the NHS operating framework and offers flexibility in the delivery approach, allowing NHS regions and integrated care systems (ICSs) to establish arrangements that best serve their local populations and to collaborate across geographies, where it is beneficial and appropriate to do so.
Who the guidance is for
This guidance is for ICB leadership teams, regional medical directors, regional chief pharmacists, regional chief nursing officers, chief finance officers, directors of public health, other medicines accountable officers and all people involved in medicines optimisation. It has been developed in collaboration with national, regional and system leaders in medicines optimisation.
Roles, responsibilities, and accountabilities
National, regional, and system-level organisations have distinct responsibilities to deliver for medicines optimisation, but the NHS will need to work collaboratively for this to make a difference to patients. The flexibility in approach will enable regions and systems to establish appropriate local governance and oversight arrangements and agree priorities.
NHS England sets national policy and strategy and identifies opportunities to help ICBs realise their wider system objectives. Regional teams support ICBs to translate national strategy and policy into local system priorities and actions.
ICBs are responsible for bringing all system partners together and have a statutory role, and associated accountability, for:
- population health
- tackling inequalities in outcomes, experience, and access
- quality of care
- achieving financial balance.
NHS England national team
For each financial year we will develop a list of national medicines optimisation opportunities that ICBs, with the support of regions, will select from as their priorities to deliver locally. Most ICBs will have their own local processes for identifying medicines optimisation priorities, these should be based on data, knowledge and expertise of local population needs and aligned with local system priorities. The list (and supporting information) of national medicines optimisation opportunities will guide ICBs to those that have been identified nationally as a priority area.
To support systems in delivering their local medicines optimisation priorities, we will:
- Provide policy, guidance, recommendations, and resources to support systems in effecting change and sustained implementation.
- Engage and communicate with regions and systems on progress against medicines optimisation opportunities.
- Use the national medicines optimisation governance structure (see escalation section) as a mechanism for identifying, reviewing, and escalating complex issues for discussion and consideration.
- Oversee and monitor progress of chosen system opportunities using national, regional, and system-level data and metrics.
- Oversee services that are retained as NHS England directly commissioned services and involve medicines, such as health and justice, specialised commissioning and the NHS Genomic Medicine Service, and support regions providing these services.
- Signpost to existing, accessible data sources that have nationally defined metrics, to identify unwarranted variation and improvement. Data will be accessible at national, regional, ICS, sub-ICS, primary care network and practice levels to allow users to interpret, analyse and review as applicable.
- Set measures for improvement for each medicine optimisation opportunity; this information will be available in the supporting information to the national medicines optimisation opportunities list for each financial year.
- Use existing enablers such as CQUIN schemes to support implementation of the identified opportunities.
- Use key national guidance such as the NHS Long Term Plan, NHS England operational planning guidance, Major Conditions Strategy along with system intelligence and feedback from regional medical directors, chief nursing officers, regional chief pharmacists, directors of public health and others working on local medicines optimisation priorities, to:
- identify and suggest national medicines optimisation opportunities for subsequent years.
- identify processes where a ‘do once’ approach would benefit the system; for example, developing templates for local adaptation or providing training resources and packages to support implementation. These will be escalated through the NHS England Medicines Optimisation Delivery Group (MODG).
- Regularly communicate with national, regional, and system-level teams about medicines optimisation through existing forums and channels, providing updates on national policy, national guidance, and new treatments and associated outcomes. The national team will also share medicines optimisation news, including new tools and resources.
- Share best practice examples from systems, such as case studies, to demonstrate the ‘art of the possible’ so that other systems can learn, adapt, and adopt the approach or learning in their area based on populations, health inequalities and available resources.
NHS England regional teams
The NHS operating framework ensures that regional teams have a core leadership role within the geographies they oversee, supporting local health systems to deliver the best care for their populations and for commissioned services. Regions and their systems will need to develop ways of working that align to the overarching principles of this medicines optimisation guidance.
Engagement and support from regional accountable officers responsible for procurement, quality, safety, and financial balance will be necessary to ensure successful delivery of medicines optimisation priorities and their intended outcomes. These could include the regional medical director, regional chief pharmacist, regional nursing officer, chief financial officer, director of public health or a member of their respective teams, alongside regional leads for procurement, productivity, health inequalities, finance and commissioning (including specialised commissioning pharmacy leads).
Regional chief pharmacists will:
- Ensure appropriate regional governance structures are in place to support delivery of local medicines optimisation priorities where aligned to national medicines optimisation opportunities.
- Act as a formal link between national and local medicines policy decision-makers as needed, including, for example, health and justice, specialised commissioning, and community pharmacy commissioning teams.
- Facilitate collaboration and integrated working across and between integrated health systems to promote consistency, reduce duplication of work and make best use of available resources for medicines optimisation, for example, by providing a forum for local area prescribing committees (or equivalent) to identify and collaborate on common areas of work.
- Use data and available metrics to monitor and communicate progress against national medicines optimisation opportunities aligned to local priorities. Work with the national team to ensure intended benefits are realised.
- When progress is not going as intended:
- understand the barriers.
- work with other regional colleagues to determine if expected progress is limited in other parts of the country and understand why.
- raise issues with the NHS England MODG to explore reasons for variation; how to overcome common barriers; and whether a national ‘do once’ resource or approach would help.
- Work collaboratively with ICS medicines optimisation leads and wider regional programme leads with responsibility for medicines and prescribing, to:
- understand areas of variation, improvement opportunities and health inequalities within their individual systems using data and local intelligence
- identify local medicines optimisation priorities for delivery from the national opportunities list.
- Provide regional professional and strategic leadership:
- on medicines optimisation opportunities including medicines value, medicines safety, antimicrobial stewardship, medicines sustainability, genomic medicine informed prescribing, addressing overprescribing and inappropriate polypharmacy.
- for the safe use and management of controlled drugs, including statutory, regulatory, and advisory functions including for services directly commissioned by NHS England
- to build a regional network of ICS medicines optimisation leads to oversee delivery linked to this guidance.
- to ensure appropriate partnership working, public and patient involvement, and stakeholder engagement as necessary.
- Support appropriate and equitable uptake of new medicines and technologies in the NHS for example, those as recommended by the National Institute for Health and Care Excellence (NICE) technology appraisal process.
- Develop processes for identifying, collating, and using examples of best practice and lessons learnt from across the region and nationally, and encourage sharing of these between ICBs in the region and with regional and national colleagues as appropriate. Work with the national team as appropriate to share learning.
- Promote equality and reduce health inequalities in all work, linking in with the director of public health, or equivalent, where needed.
NHS England regions will operate as equal partners with ICBs, aligned with the principles in the operating framework, and underpinned by effective communication. They will provide expert advice and can help mobilise and support improvement efforts. If an ICB requires support to deliver its medicines optimisation opportunities, then this should be discussed and agreed between the relevant NHS England regional chief pharmacist and the ICB in terms of next steps. If issues need to be escalated, the regional chief pharmacist will help co-ordinate this and liaise with the national team.
Integrated care boards
- Use the list of national medicines optimisation opportunities to identify, prioritise and select at least 5 medicines optimisation opportunities that align to local priorities.
- Use available data and metrics to identify local medicines optimisation priorities to address variation in medicines access and use and seek improvement opportunities within key priority areas.
- Inform national strategy by providing feedback to the regional teams.
- Ensure appropriate stakeholder, partnership, and patient and public involvement.
- Ensure effective arrangements are in place across the ICS and region for collaborative working to effectively deliver against agreed medicines optimisation priorities and corresponding improvement goals.
- Maintain effective communication and collaboration with the regional team, ensuring that, where needed, issues are highlighted early.
- Discuss with and escalate concerns to the regional chief pharmacist early to ensure support can be provided in a timely manner. The regional chief pharmacist can engage with regional team colleagues to support as appropriate.
For success:
- The ICB Chief Pharmacist/ Director of Medicines and Pharmacy will provide clinical leadership for medicines optimisation across the system. Where this role is not in place, an alternative Senior Responsible Officer should be identified with accountability to, or membership on, the ICB Executive Team.
- ICBs should consider the development of an ICS integrated medicines optimisation committee (IMOC) where such an arrangement is not already in place. IMOCs (or equivalents) can ensure that all ICS partners are working together to develop and deliver a shared system-wide medicines optimisation strategy and operational plan, and that appropriate and effective governance arrangements are in place.
- Review medicines and prescribing budgets across the ICS, rather than in isolation by organisation, alongside consideration of potential clinical pathway and/or service redesign.
- Medicines and prescribing is a core component of clinical pathways; an ICS approach with multidisciplinary and service user input will ensure medicines are prescribed in the right setting, by the right person, at the right time. This also provides opportunities to improve safety and quality around medicines for improved health and social outcomes, as well as sustainability of resources.
Medicines optimisation governance structure
National medicines optimisation governance framework
NHS England develops national medicines optimisation policy and strategy, and related opportunities, underpinned by an agreed national medicines optimisation governance structure (see Figure 1).
Figure 1: National medicines optimisation governance structure
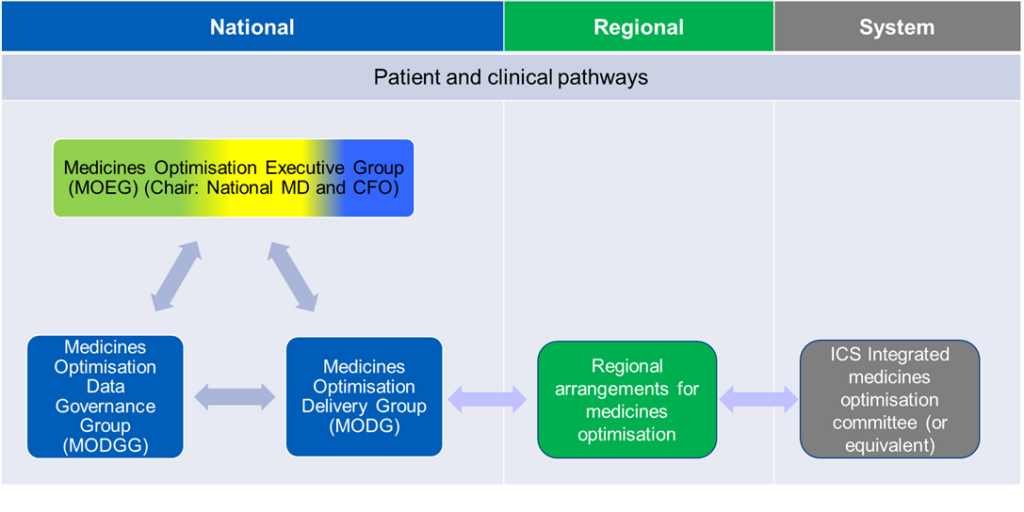
Figure description: this figure shows the two-way interaction at national level between the Medicines Optimisation Executive Group (MOEG), Medicines Optimisation Data Governance Group (MODGG) and Medicines Optimisation Delivery Group (MODG), and how the MODG communicates and receives feedback from regions, and how regional teams inform ICS integrated medicines optimisation committees (or equivalent) and receive feedback from them.
Medicines Optimisation Executive Group
The NHS England Medicines Optimisation Executive Group (MOEG) identifies and oversees the delivery of strategic medicines optimisation priorities across the NHS in England. The group is jointly chaired by the National Medical Director and Chief Financial Officer and Deputy Chief Executive Officer. The MOEG considers medicines issues in the context of organisational and system priorities aligned to the NHS Long Term Plan, operating framework, and operational planning guidance.
Medicines Optimisation Delivery Group (MODG)
The MODG translates the NHS England national medicines optimisation strategic priorities into a delivery programme to oversee the development of national recommendations and implementation guidance for adoption across the NHS. Its core membership consists of senior NHS England leaders, with attendance from other experts to ensure collaborative working with other national, regional and system representatives. This group is jointly chaired by the Deputy Chief Pharmaceutical Officer and Deputy Director of Medicines Policy (clinical).
Medicines Optimisation Data Governance Group (MODGG)
The MODGG brings all NHS England medicines optimisation metrics together to align them for quality and timeliness based on user needs. The group also oversees delivery of medicines optimisation opportunities across teams, directorates and programmes to support patients and the NHS secure the best outcomes and value from medicines. This work aligns to the national medicines optimisation opportunities, strategy, and priorities.
Where medicines optimisation initiatives are led through existing separate programmes, the MODGG provides the forum to ensure alignment and collaboration, so messaging across directorates and teams, to regions and systems is clear, concise, and coherent. This group is chaired by the National Clinical Director for Prescribing.
Alignment with other national NHS England medicines-related programmes
NHS England has several medicines-related work programmes that directly contribute to medicines optimisation. This medicines optimisation governance framework brings NHS England medicines optimisation work priorities together while acknowledging the wider medicines work across the organisation. These programmes and work areas will update MOEG as required and include:
- medicines value
- genomic medicines
- community pharmacy contracting
- medicines repurposing
- antimicrobial prescribing and medicines optimisation
- medicines sustainability
- infusions and specials medicines
- pharmacy integration
- specialised commissioning
- NHS Accelerated Access Collaborative.
For both ICBs and their partner NHS providers, the primary relationship with NHS England is through the relevant regional team. The regional chief pharmacist is responsible for providing a strong link between national, regional and system teams for medicines optimisation. The arrangements between regional teams, ICBs and providers are agreed locally.
Stakeholder engagement
Regional arrangements for medicines optimisation need to ensure timely and effective collaboration within and between regions. Regional teams engage with their leadership teams, including finance, commissioning, procurement, public health, quality and any other relevant clinical and non-clinical expertise for successful delivery.
Where needed, NHS England will engage with national bodies, such as government departments, arm’s length bodies, medical royal colleges, third sector, charities, patient groups and industry, to support development and delivery of the national medicines optimisation opportunities.
The national team will undertake internal engagement across NHS England to ensure other programmes or projects are aware of the medicines optimisation opportunities. This will ensure that programmes can collaborate on issues that may require cross-organisational working and alignment. This engagement will be overseen by MOEG and guided by the MODG and MODGG.
Assurance and accountabilities
Region-wide summaries of the ICB selected national medicines optimisation opportunities will provide an overall picture of the medicines optimisation areas local systems are prioritising. ICBs update their regional chief pharmacists on the chosen priority areas at agreed intervals, and regional chief pharmacists share updates with NHS England’s MODG every other month, unless any issues need to be flagged more frequently or escalation is required. ICBs provide assurance and accountability at system level, aligned to their established responsibilities, plans and associated assurance processes, as set out in the NHS England ICS guidance. Progress on the national medicines optimisation opportunities will be overseen by MOEG.
Escalation
Regional chief pharmacists will receive suggestions for future medicines optimisation opportunities from colleagues working in their geography in advance of annual business planning. These will be discussed and reviewed at regional level. If there is opportunity for a national ‘do once’ approach or an ‘adopt and spread’ model, this will be raised by the regional chief pharmacist to the NHS England MODG for discussion, potential inclusion and prioritisation in future national programmes. MODG has an established process within the governance framework to support this approach.
Stakeholder involvement in regional arrangements
Regional teams determine the most appropriate ways to provide assurance of delivery for selected national medicines optimisation opportunities aligned to implementation of their regional arrangements for medicines optimisation. The geography that the arrangements cover is determined locally, and the arrangements resourced locally, including any secretariat or project management support. Members of any groups should be able to speak with authority on behalf of the system or professional area they represent.
Principles for success
For successful delivery of medicines optimisation priorities nationally, regionally and across ICSs, the following principles should be considered:
- Take a clinically-led but operationally driven approach, with executive and senior responsible officer oversight across the whole system; this should align to the ICS guidance on clinical and care professional leadership.
- Ensure an ICB chief pharmacist/director of medicines and pharmacy has been identified to provide clinical leadership at system level for medicines optimisation. Where this role is not established, then an alternative SRO for medicines optimisation with accountability to the ICB should be identified.
- Prioritise and use medicines optimisation as an enabler for delivering the ICBs 4 key objectives.
- Use a data-driven approach to deliver outcomes for the population by focusing on quality improvement and efficiencies.
- Systems to identify and develop a plan to deliver at least 5 national medicines optimisation opportunities where a change in practice will improve local population health outcomes and that these align to, and compliment, local medicines optimisation priorities. The plan will be shared with the Regional Chief Pharmacist.
- Have regard for the wider impact of local medicines optimisation policy and decision-making, taking into account the impact on community contractors (such as pharmacy, dentistry and optometry services).
- When progress is not on track or as intended, escalate early within governance and relevant networks so that necessary support can be provided.
- Use co-production wherever possible, ensuring all opportunities are taken to capture the public and patients’ voice.
- Take a ‘do once’ approach to reduce unnecessary duplication of effort and resource, escalating through regional teams to the national medicines optimisation delivery group wherever possible.
- Collaborate and communicate regularly with system partners and wider networks to build and maintain successful relationships.
- Take a whole-system approach using quality improvement and change management methodologies, along with data and metrics, to identify and successfully drive change and demonstrate progress.
- Work together across medicines optimisation teams, pharmacy leaders, medical directors, commissioning and finance to understand current spend across commissioning budgets. Take into account that ICBs are funded for all locally commissioned services and this includes medicines (both primary care and hospital prescribed medicines).
The NHS England change model is a framework that can be used for any project or programme that is seeking to achieve transformational, sustainable change. Implementing change should be underpinned by four key principles: leadership, multiprofessional working, access to data and education.
Publication reference: PRN00586

