Publication date: August 2022
Version number: 1.0
Summary
Rituximab is recommended to be available as a routine commissioning treatment option acute thrombotic thrombocytopenic purpura (TTP) and elective therapy to prevent TTP relapse (adults and children aged 2 years and above) within the criteria set out in this document.
Committee discussion
Please see Clinical Panel reports for full details of Clinical Panel’s discussion.
What we have decided
NHS England has carefully reviewed the evidence to treat acute thrombotic thrombocytopenic purpura (TTP) and elective therapy to prevent TTP relapse (adults and children aged 2 years and above) Rituximab. We have concluded that there is enough evidence to make the treatment available at this time.
The evidence reviews which inform this commissioning position can be accessed here.
Plain language summary
TTP is a rare, potentially life-threatening condition that involves blood clots in the small blood vessels in the body. TTP happens when platelets (type of blood cell that forms blood clots) stick together too readily and form into clots. As these clots increase in number and size, they cause bleeding and reduction in blood supply to parts of the body, leading to organ damage, most commonly the brain, digestive tract, heart and kidneys. If the disease is untreated patient outcomes are poor, with a high mortality rate. Patients with TTP are treated with rituximab, an injection into the vein, in the acute episodes of the disease and also later in the patient pathway to prevent relapse.
About TTP
TTP is a critical medical condition requiring immediate transfer for treatment. Fifty percent of patients require intensive care admission and without treatment, the mortality in acute TTP is >90%.
TTP results from a deficiency of the enzyme ADAMTS 13, required for Von Willebrand factor (vWF) cleavage. The severely reduced enzyme levels are caused either by a genetic problem or through their destruction by antibodies.
Deficiency of the ADAMTS13 enzyme results in insufficient processing of the large vWF clotting factor which unravels during passage through the microcirculation leading to platelet aggregation and multi-organ microvascular thrombosis involving neurological, cardiac, gastro-intestinal and renal disease. The occlusion of small vessels also causes a fragmentation haemolysis (microangiopathic haemolytic anaemia) and platelet consumption resulting in thrombocytopenia.
Acute immune TTP is used throughout the document and includes both acute and relapsed episodes. Acute immune TTP definitions are confirmed by (Cuker A et al 2021).
About Rituximab
Rituximab provides a central role in the treatment pathway. It is a monoclonal anti-CD20 antibody meaning it binds to and acts on CD20, a molecule expressed on the surface of B lymphocytes (a type of white blood cell). These lymphocytes are responsible for producing antibodies (proteins involved in the immune system response in the body) so by binding to them and destroying them, fewer antibodies that destroy ADAMTS13 are produced. Thus, normalising ADAMTS13 activity levels and ultimately reducing clot formation in the blood.
Failure to remove ADAMTS13 antibodies results in a continuously low ADAMTS13 activity and ongoing disease, resulting in multiorgan damage.
The use of rituximab has resulted in significantly improved patient outcomes, including less plasma exchange and a smaller volume of plasma is required, quicker time to remission and reduced hospital stay. This is associated with reduced mortality (death) and morbidity (poor health) and markedly improved patient outcomes.
Current treatment
Rituximab as treatment in acute TTP and elective therapy to prevent TTP relapse has been the standard of care since 2005. The treatment was commissioned by clinical commissioning groups until 1 April 2020 when the service transferred to NHS England’s commissioning responsibility. Rituximab is currently not licensed for the treatment of acute disease or elective therapy to prevent TTP relapse.
Rituximab as treatment in acute immune TTP
Treatment of acute immune TTP is with both:
- Urgent plasma exchange (PEX) – to replace blood plasma with new plasma fluid in order to replenish stocks of
- Immunosuppression – to switch off the immune system response destroying the ADAMTS13 in the Immunosuppression is with high dose steroids initially and Rituximab.
Early administration of rituximab during acute episodes reduces time to remission and rituximab should be started within 72 hours of diagnosis. On average, inpatient stay is 14 days and treatment continues as an outpatient, aiming to normalise ADAMTS13 activity.
Rituximab as elective therapy to prevent TTP relapse
Rituximab is used to prevent relapse in patients with a fall in ADAMTS13 activity and symptoms based on the patient’s relapse history. The target is normalisation of ADAMTS13 activity as above.
As an elective therapy to prevent TTP relapse, rituximab is also given to the rare group of immune TTP patients who go into clinical remission (no active disease activity) after an acute episode but have persistent ADAMTS13 deficiency (lack) <10% despite having received rituximab and other immunosuppression for the acute episode.
Epidemiology and needs assessment
Acute immune mediated TTP can affect all ages, although it is exceedingly rare in children. It is more common in women than men and the median age at presentation is 30-40 years (University College London Hospitals NHS Foundation Trust, 2021). The national prevalence of TTP is 330 patients in England and annually there are 150 patients diagnosed acutely.
Relapse rate is approximately 30-50% though this is reducing with rituximab use in acute presentation of immune TTP. It is estimated that on average there are 100 patients treated with rituximab as an elective therapy every year.
Implementation
Inclusion criteria
Patients who present in the acute phase of the disease and meet all of the following inclusion criteria can be considered for treatment with rituximab
- aged 2 years and above
- have a baseline ADAMTS13 level ≤ 10%
Starting criteria
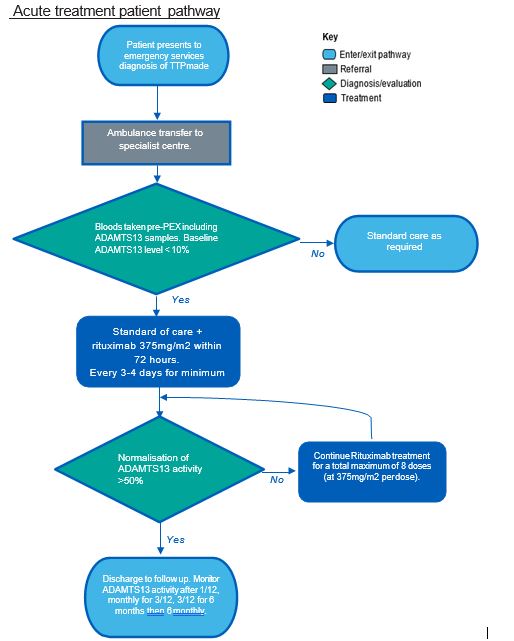
Treatment follows initial blood tests (including ADAMTS13 samples), PEX and high dose steroids.
Dose
Early administration during acute episodes reduces time to remission and rituximab should be started within 72 hours of diagnosis. For acute TTP rituximab is recommended at a dose of 375mg/m2 BSA (body surface area) repeated 3 times every 3-4 days (i.e. total 4 doses per cycle)1. A cycle may be repeated once (i.e. maximum of 8 doses). Inpatient stay is a median of 14 days and treatment continues as an outpatient, aiming to normalise ADAMTS 13 activity. The majority of patients normalise their ADAMTS13 activity levels following 4 rituximab infusions (i.e. one complete cycle).
Ideally, PEX should be withheld for at least four hours after a rituximab infusion, as there is evidence that the drug is removed by PEX. Rituximab is given more frequently, e.g. every 3-4 days, during the acute period whilst a patient is receiving PEX to overcome this, then administered weekly once PEX has stopped.
Stopping criteria
Treatment with Rituximab will be stopped if the following applies: EITHER:
- Normalisation of ADAMTS13 activity at ≥ 50% OR
- Maximum 8 doses of rituximab at 375mg/m2 BSA OR
- Acute or delayed serum sickness considered to be caused by rituximab
Elective (chronic or recurrent) treatment
1 Lower doses may be satisfactory with the aim of treatment to normalise ADAMTS 13 activity levels.
Inclusion criteria
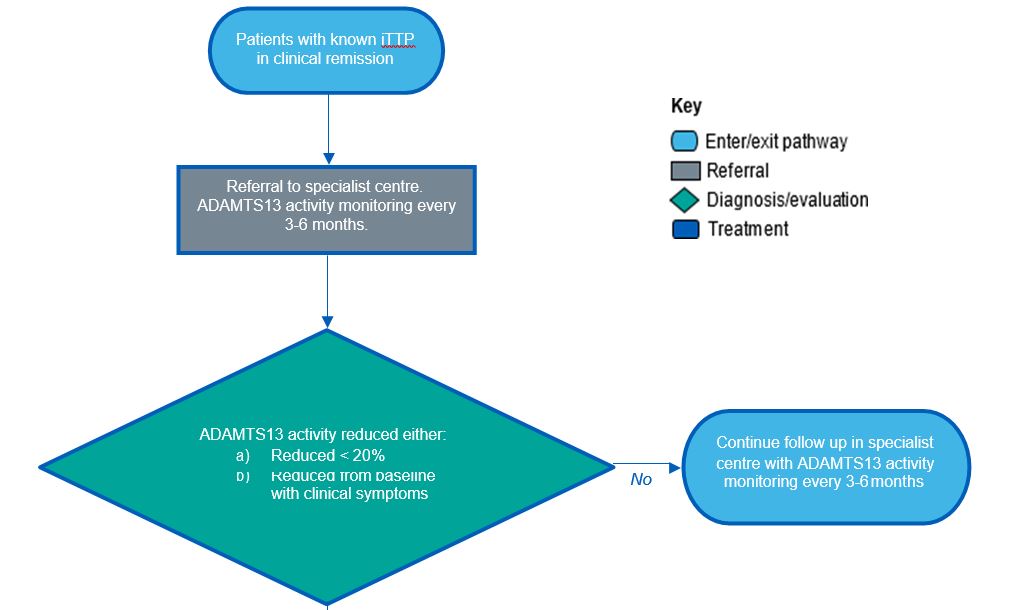
Patients who present for rituximab as an elective therapy to prevent TTP relapse who, meet the following inclusion criteria can be considered for treatment with rituximab
- Be aged 2 years and above and have the following:
- Clinical remission defined as absence of thrombotic and general symptoms AND
- Baseline ADAMTS13 level ≤ 20%
OR
- Baseline ADAMTS13 level reduced from baseline but still symptomatic
Starting criteria
Initiated in specialist centre following ADAMTS13 activity level monitoring.
Dose
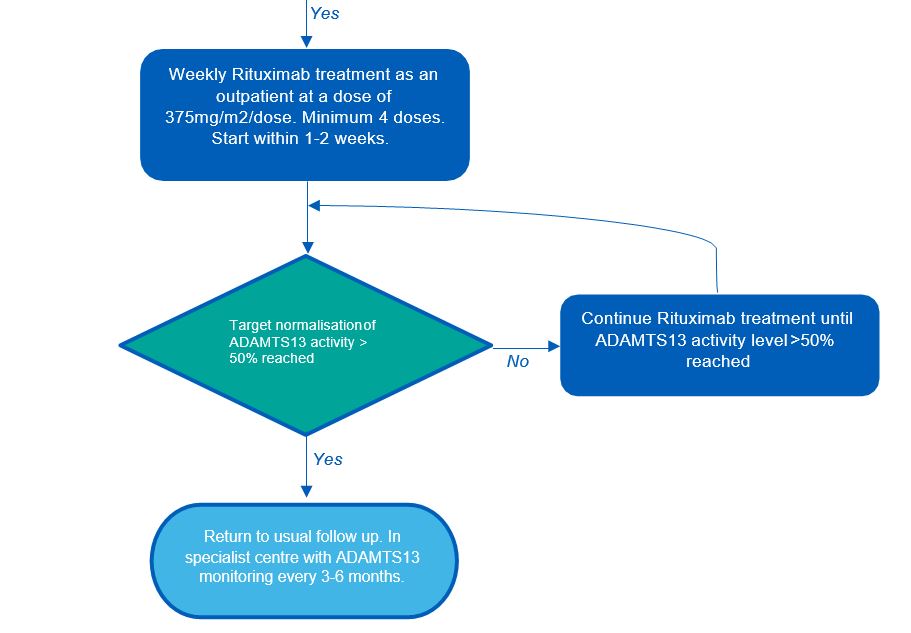
The standard regimen is to administer rituximab weekly as an outpatient at a dose of 375mg/m2 BSA for a minimum of four doses (up to 8 doses may be required). Some clinics may use alternative regimens. The target is normalisation of ADAMTS13 activity (see stopping criteria).
The interval for repeated courses of rituximab in chronic TTP is highly variable between patients and guided primarily by symptoms, in turn supported by serum ADAMTS13 levels. Repeat courses should not be any more frequent than 3 months from the end of the most recent course, and in practice are seldom required more often than 6 months from the most recent course. Many patients have prolonged intervals of 12 months or more.
Stopping criteria
Treatment with Rituximab will be stopped when ADAMTS13 activity ≥ 50%. Patients will usually complete one course (or cycle) of 4 doses of rituximab regardless, as this is sufficient for most patients.
Exclusion criteria
Patients who meet the following criteria are not eligible for rituximab:
- Previous failure to respond to rituximab therapy
- Known acute hypersensitivity reaction to rituximab
- Treatment within previous 2 months
- Active malignancy
- Organ or stem cell transplant
- Congenital TTP
- Acute or delayed serum sickness to previous rituximab
Patient pathway
Acute treatment patient pathway
Elective treatment patient pathway
Governance arrangements
This policy should be used in conjunction with the service specification for TTP, NHS England. (2021). Thrombotic Thrombocytopenic Purpura (TTP) all ages. Available at https://bidstats.uk/tenders/2021/W13/747962872.
Any provider organisation treating patients with this intervention will be required to assure itself that the internal governance arrangements have been completed before the medicine is prescribed. These arrangements may be through the Trust’s Drugs and Therapeutics committee (or similar) and NHS England may ask for assurance of this process.
Provider organisations must register all patients using prior approval software and ensure monitoring arrangements are in place to demonstrate compliance against the criteria as outlined.
Mechanism for funding
The mechanism for funding is via established mechanisms for high-cost tariff-exempt drugs to NHS England specialised commissioning teams.
Audit requirements
In line with the service specification requirements, all providers will contribute consented patient activity to the United Kingdom TTP Registry.
Policy review date
This document will be reviewed when information is received which indicates that the policy requires revision. If a review is needed due to a new evidence base then a new Preliminary Policy Proposal needs to be submitted by contacting england.CET@nhs.net.
Our policies provide access on the basis that the prices of therapies will be at or below the prices and commercial terms submitted for consideration at the time evaluated. NHS England reserves the right to review policies where the supplier of an intervention is no longer willing to supply the treatment to the NHS at or below this price and to review policies where the supplier is unable or unwilling to match price reductions in alternative therapies.
Equality statement
Promoting equality and addressing health inequalities are at the heart of NHS England’s values. Throughout the development of the policies and processes cited in this document, we have:
- Given due regard to the need to eliminate discrimination, harassment and victimisation, to advance equality of opportunity, and to foster good relations between people who share a relevant protected characteristic (as cited under the Equality Act 2010) and those who do not share it; and
- Given regard to the need to reduce inequalities between patients in access to, and outcomes from healthcare services and to ensure services are provided in an integrated way where this might reduce health
Definitions
|
Monoclonal antibody |
is an antibody that has been designed to recognise and attach to a specific structure called an antigen that is found in the body. Rituximab has been designed to attach to the cell surface marker called CD-20. This is involved in causing inflammation. By preventing CD-20 attaching to its receptors, rituximab reduces inflammation. |
|
Relapsed disease |
describes when a condition has recurred following response to previous treatment, this may occur at any time following completion of treatment. |
|
Rituximab |
a monoclonal antibody that targets CD-20, which is a cell surface marker that is widely expressed on B- cells, leading to B cell depletion. |
|
Plasma Exchange (PEX) |
is where blood is taken out of a vein and goes into a machine that separates the plasma from the blood cells and platelets. The blood cells and platelets are combined with donated plasma and returned to the body. For TTP the process removes the antibodies in the blood that block the ADAMTS13 enzyme. |
References
NHS England. (2021). Thrombotic Thrombocytopenic Purpura (TTP) all ages. Available: https://bidstats.uk/tenders/2021/W13/747962872. Last accessed 3rd Sept 2021.
University College London Hospitals NHS Foundation Trust. (2021). What is Thrombotic Thrombocytopenia Purpura (TTP)? Available at: https://www.uclh.nhs.uk/our-services/find-service/cancer-services/blood-diseases-clinical-haematology/blood-diseases-types-and-services/red-cell-diseases/TTP/what-thrombotic-thrombocytopenia-purpura-t.

