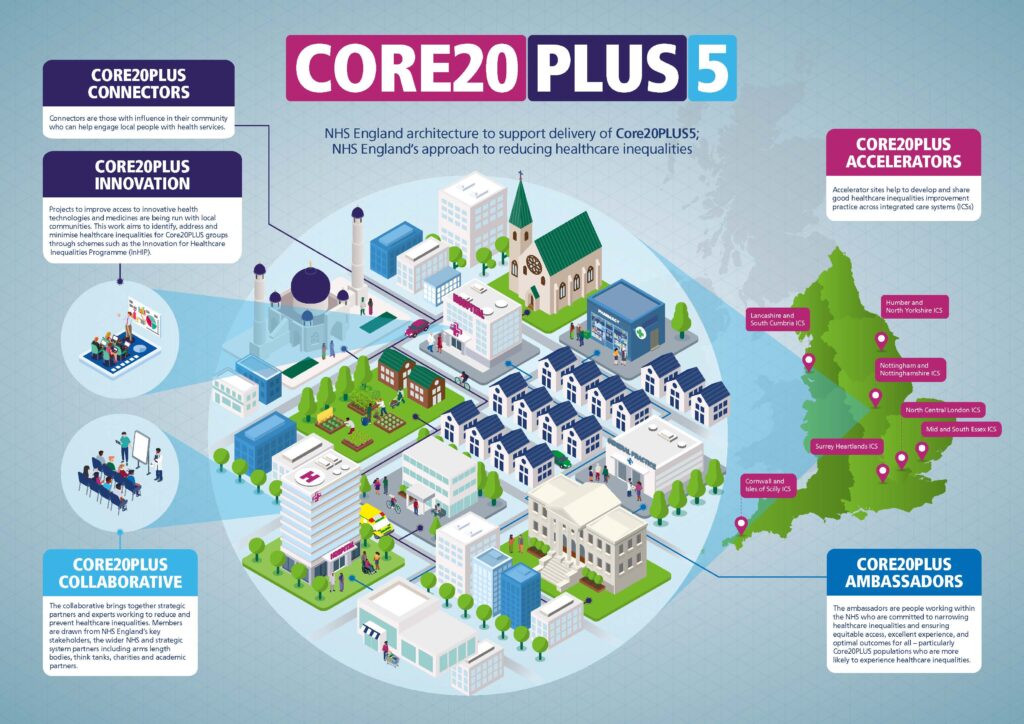
Innovation for Healthcare Inequalities Programme
NHS England’s Innovation for Healthcare Inequalities Programme (InHIP) aims to address local healthcare inequalities experienced by deprived and other under-served populations.
Project teams (comprising of clinical and non-clinical expertise) from across the country are working together with their local communities to identify, address and minimise healthcare inequalities through projects to improve access to the latest health technologies and medicines.
These technologies and medicines are focused on five clinical areas of priority that closely align with the national Core20PLUS5 approach to reducing healthcare inequalities, which includes maternity, mental health, respiratory, cancer diagnosis and cardiovascular disease.
Nationally a total of £4.2 million was made available to support the programme and to fund eligible projects across England. These projects have been designed and developed by local teams to make sure that those most in need within their geography will benefit from this investment. Key to the projects are voices from local communities who are guiding this work.
Through this unique approach to addressing local healthcare inequalities, InHIP is helping build new relationships with system leaders and laying the foundations for other programmes of work to adopt similar approaches.
Read the report which sets out how the first wave of projects generated knowledge and insights on the interventions and approaches to improve access for underserved populations to proven innovations.
NHS England’s InHIP programme is a collaboration between the Accelerated Access Collaborative (AAC), NHS England’s National Healthcare Inequalities Improvement Programme and the Health Innovation Network (previously Academic Health Science Network) and delivered in partnership with integrated care systems (ICSs).
The programme is supporting ICSs to generate evidence in piloting new approaches to the spread and adoption of innovation to underserved and deprived communities. This foundation of learning will pave the way for continuing locally led initiatives and work in tackling healthcare inequalities at a system level working in partnership with the Health Innovation Network.
This programme builds on the AAC and the Health Innovation Network’s achievements and learning to date in improving access to innovations in healthcare for the general population.
Local projects
This is a map showing the spread of projects and clinical area of focus. For the full list of projects (including the population focus and specific clinical pathway) please see below.
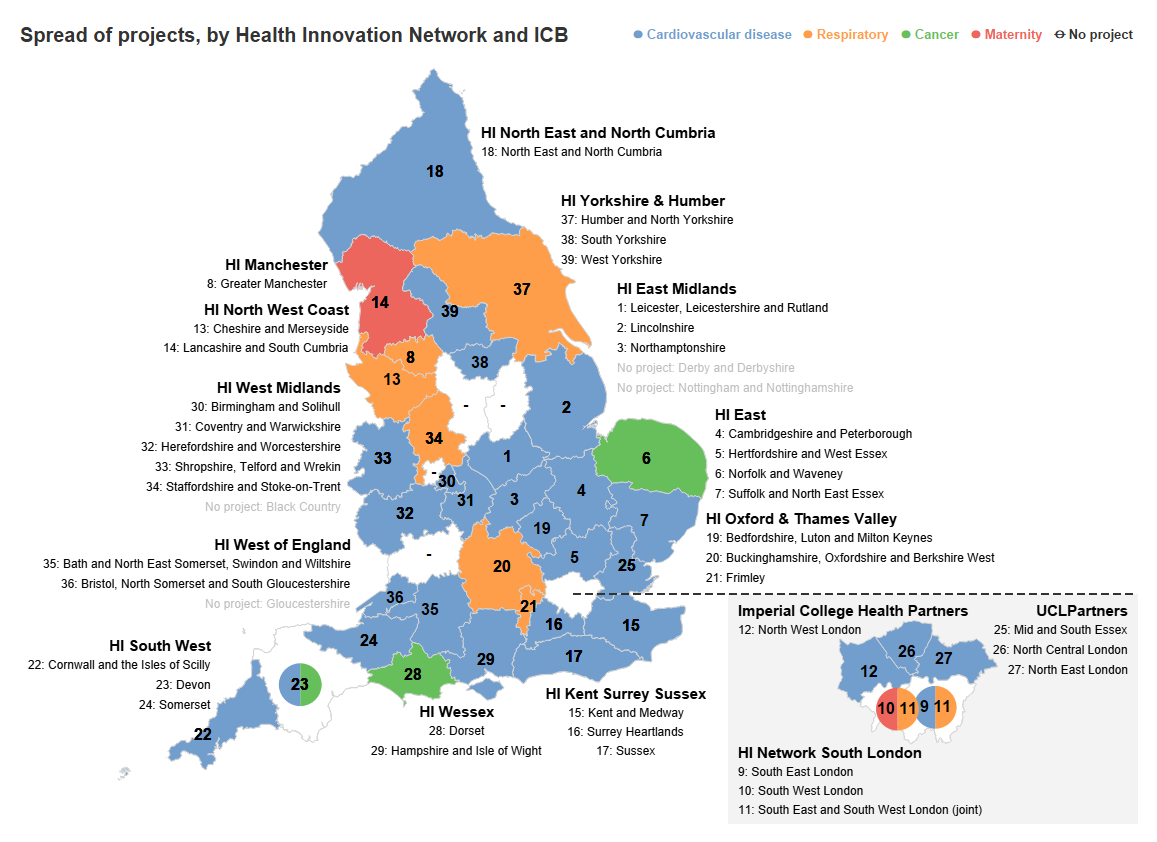
*Areas without projects did not/or were not able to submit an InHIP project proposal in Oct 2022.
Detailed summary of projects
| Primary Network for InHIP | ICS | Clinical area(s) | Clinical Pathway | Target Core20Plus5 population |
| Health Innovation Oxford and Thames Valley |
Bedfordshire, Luton and Milton Keynes |
Cardiovascular Disease | Lipid Management and Heart Failure | Core20 (IMD 20% Most Deprived) |
| UCLPartners | Mid and South Essex | Cardiovascular Disease | Heart Failure | Core20 (IMD 20% Most Deprived); Ethnic minority communities |
| Health Innovation East | Cambridgeshire and Peterborough | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived), Ethnic Minorities, Traveller Communities |
| Health Innovation East | Hertfordshire and West Essex | Cardiovascular Disease | Atrial Fibrillation Pathway | Core20 (IMD 20% Most Deprived); Ethnic minority communities |
| Health Innovation East | Norfolk and Waveney | Cancer Diagnosis | Colorectal Cancer | Core20 (IMD 20% Most Deprived); People experiencing homelessness |
| Health Innovation East | Suffolk and North East Essex | Cardiovascular Disease | Atrial Fibrillation Pathway | Core20 (IMD 20% Most Deprived); rural deprivation |
Further information
For more information on InHIP activities in your area please contact your local AHSN.
You can also contact the national team with any queries via email at aac.innovation@nhs.net
| Primary Network for InHIP | ICS | Clinical area(s) | Clinical Pathway | Target Core20Plus5 population |
| UCLPartners | North Central London | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived); Ethnic Minority Communities |
| UCLPartners | North East London | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived); Ethnic Minority Communities |
| Health Innovation Network South London | South East London | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived) 4 PCNs in Bexley; Ethnic minority communities; age (18-39 year group – lower number on treatment compared to average in England) |
| Health Innovation Network South London | South West London | Maternity | Pre-eclampsia | Ethnic minority communities (women also need to be BMI 30 and over) |
| Health Innovation Network South London | South East London and South West London | Respiratory | Asthma Pathway | Core20 (IMD 20% Most Deprived) deprived communities Bromley (SE) and Croydon (SW) ; Ethnic minority communities; People experiencing homelessness; Gypsy, Roma and Traveller communities; People with multi-morbidities |
| Imperial College Health Partners | North West London | Cardiovascular Disease | Atrial Fibrillation Pathway | Core20 (IMD 20% Most Deprived); Ethnic minority communities |
Further information
For more information on InHIP activities in your area please contact your local AHSN.
You can also contact the national team with any queries via email at aac.innovation@nhs.net
| Primary Network for InHIP | ICS | Clinical area(s) | Clinical Pathway | Target Core20Plus5 population |
| Health Innovation West Midlands | Birmingham and Solihull | Cardiovascular Disease | Atrial Fibrillation Pathway | Core20 (IMD 20% Most Deprived) 3PCNs; Ethnic minority communities |
| Health Innovation West Midlands | Coventry and Warwickshire | Cardiovascular Disease | Atrial Fibrillation Pathway | Core20 (IMD 20% Most Deprived); rural communities |
| Health Innovation West Midlands | Herefordshire and Worcestershire | Cardiovascular Disease | Atrial Fibrillation Pathway | Core20 (IMD 20% Most Deprived); rural communities |
| Health Innovation West Midlands | Shropshire, Telford and Wrekin | Cardiovascular Disease | Atrial Fibrillation Pathway | Core20 (IMD 20% Most Deprived); Ethnic minority communities; Rural communities |
| Health Innovation West Midlands | Staffordshire and Stoke-on-Trent | Respiratory | Asthma Pathway | Core20 (IMD 20% Most Deprived) – 3 PCNs where >50% patients live in the most deprived 20% |
| Health Innovation East Midlands | Leicester, Leicestershire and Rutland | Cardiovascular Disease | Coronary and Peripheral Artery Disease | Ethnic minority communities (S. Asian within most deprived areas) |
| Health Innovation East Midlands | Lincolnshire | Cardiovascular Disease | Heart Failure | Core20 (IMD 20% Most Deprived); coastal |
| Health Innovation East Midlands | Northamptonshire | Cardiovascular Disease | Atrial Fibrillation Pathway and Hypertension Pathway | Ethnic minority communities; Core20 (IMD 20% Most Deprived)- (Black, Black African and Black Caribbean populations of Wellingborough) |
Further information
For more information on InHIP activities in your area please contact your local AHSN.
You can also contact the national team with any queries via email at aac.innovation@nhs.net
| Primary Network for InHIP | ICS | Clinical area(s) | Clinical Pathway | Target Core20Plus5 population |
| Health Innovation Yorkshire and Humber | Humber and North Yorkshire | Respiratory | Asthma Pathway | Regional variation in asthma related mortality |
| Health Innovation Yorkshire and Humber | West Yorkshire | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived); Ethnic minority communities |
| Health Innovation Yorkshire and Humber | South Yorkshire | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived); Ethnic minority communities; “white non-British” and “older” working aged men within the North / North East are a local priority |
| Health Innovation North East and North Cumbria | North East and North Cumbria | Cardiovascular Disease | Lipid Management, Hypertension Pathway and AF Pathway | Core20 (IMD 20% Most Deprived) and South Asian and African-Caribbean populations |
Further information
For more information on InHIP activities in your area please contact your local AHSN.
You can also contact the national team with any queries via email at aac.innovation@nhs.net
| Primary Network for InHIP | ICS | Clinical area(s) | Clinical Pathway | Target Core20Plus5 population |
| Health Innovation Manchester | Greater Manchester | Respiratory; Other (inc. smoking cessation) | Asthma Pathway and Smoking Cessation | Core20 (IMD 20% Most Deprived); Ethnic minority communities; CYP at Royal Oldham Hospital |
| Health Innovation North West Coast | Cheshire and Merseyside | Cardiovascular Disease; Respiratory | Asthma Pathway, Lipid Management, Heart Failure, Coronary and Peripheral Artery Disease, Neuronal Ceroid Lipofuscinosis Type 2 (CLN2), Hyperkalaemia and Chronic Obstructive Pulmonary Disease (COPD) | Core20 (IMD 20% Most Deprived); Other (Fuel deprivation, chronic illnesses, mental health mortality risk) |
| Health Innovation North West Coast | Lancashire and South Cumbria | Maternity | Pre-eclampsia | Core20 (IMD 20% Most Deprived); Ethnic minority communities |
Further information
For more information on InHIP activities in your area please contact your local AHSN.
You can also contact the national team with any queries via email at aac.innovation@nhs.net
| Primary Network for InHIP | ICS | Clinical area(s) | Clinical Pathway | Target Core20Plus5 population |
| Health Innovation Oxford and Thames Valley | Buckinghamshire, Oxfordshire and Berkshire West | Respiratory | Asthma Pathway | Core20 (IMD 20% Most Deprived) |
| Health Innovation Oxford and Thames Valley | Frimley | Respiratory | Chronic Obstructive Pulmonary Disease (COPD) and Asthma Pathway | Core20 (IMD 20% Most Deprived); Ethnic minority communities |
| Health Innovation Wessex | Hampshire and Isle of Wight | Cardiovascular Disease | Atrial Fibrillation Pathway and Hypertension Pathway | PCN’s with the IMD 20% most deprived populations, focussing on ethnic minority population most affected by COVID, people with SMI and undocumented migrants. |
| Health Innovation Kent Surrey Sussex | Kent and Medway | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived); Ethnic minority communities; Protected characteristic groups (women 40-59) |
| Health Innovation Kent Surrey Sussex | Surrey Heartlands | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived); Ethnic minority communities; People with a learning disability and people with autism who are ineligible for an NHS health check due to age. |
| Health Innovation Kent Surrey Sussex | Sussex | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived); Ethnic minority communities; Protected characteristic groups (Women 40-59); Gypsy, Roma and Traveller communities |
Further information
For more information on InHIP activities in your area please contact your local AHSN.
You can also contact the national team with any queries via email at aac.innovation@nhs.net
| Primary Network for InHIP | ICS | Clinical area(s) | Clinical Pathway | Target Core20Plus5 population |
| Health Innovation South West | Cornwall and Isles of Scilly | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived); Ethnic minority communities; Fishermen; People with a learning disability and people with autism; People experiencing homelessness; People in contact with the justice system |
| Health Innovation South West | Devon | Cardiovascular Disease | Heart Failure | Core20 (IMD 20% Most Deprived); Ethnic minority communities; People with a learning disability and people with autism; those with severe mental illness |
| Health Innovation South West | Somerset | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived); People experiencing homelessness |
| Health Innovation Wessex | Dorset | Cancer Diagnosis | Colorectal Cancer | Coastal communities with pockets of deprivation hidden amongst relative affluence |
| Health Innovation West of England | Bath and North East Somerset, Swindon and Wiltshire | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived); <60 and previous CVD event in IMD 1-3 deprivation area |
| Health Innovation West of England | Bristol, North Somerset and South Gloucestershire | Cardiovascular Disease | Lipid Management | Core20 (IMD 20% Most Deprived) and previous CVD event in IMD1 deprivation area |
Further information
For more information on InHIP activities in your area please contact your local AHSN.
You can also contact the national team with any queries via email at aac.innovation@nhs.net