NHS Infusions and Special Medicines Programme: Guidance to replace EL(97)52 in England
This video summarises the guidance and explains why it is important for acute trusts and integrated care boards
1. Purpose
This guidance sets out the governance and regulatory arrangements for aseptic preparation of medicines for NHS patients in England and replaces the 1997 NHS Executive letter EL(97)52, Aseptic dispensing in NHS hospitals.
It defines the roles and responsibilities for:
- NHS organisations in meeting quality standards, responding to audits and inspections, and reporting quality indicators when performing aseptic preparation activities. This includes the statutory responsibilities of chief pharmacists as established by The Pharmacy (Preparation and Dispensing Errors – Hospital and Other Pharmacy Services) Order 2022.
- The NHS Specialist Pharmacy Service Quality Assurance service (SPS QA) in providing regulatory oversight and inspection of aseptic preparation activity, auditing services against quality standards.
- NHS England as responsible for commissioning the overarching governance and assurance process, providing oversight and ensuring the delivery of enforcement where necessary.
It also describes the oversight provided by the Care Quality Commission (CQC) and the inter-relationships between the regulatory bodies, primarily the CQC, Medicines and Healthcare products Regulatory Agency (MHRA) and General Pharmaceutical Council (GPhC).
2. Introduction
Since 1997 the NHS has operated within an assurance process for the aseptic preparation of injectable medicines issued under a Department of Health Executive letter – EL(97)52. This requires audits every 12 to 18 months of NHS sites in England that perform any aseptic preparation not covered by an MHRA manufacturer’s ‘specials’ (MS) authorisation. SPS QA officers undertake the audits and reported. There have been concerns about the transparency of the results of these audits and the lack of understanding at NHS trust board level of the board’s responsibility and accountability, particularly the importance of implementing required remedial actions and the need to prioritise associated works.
NHS organisational structures, policies and governance frameworks have changed since 1997. The Department of Health and Social Care 2020 report Transforming NHS pharmacy aseptic services in England recommends “strengthen the accountability and responsibility around the unlicensed preparation of aseptic medicines under EL(97)52 guidance and the role of the Chief Pharmacist”.
This guidance addresses the above recommendation, is consistent with the current NHS infrastructure and replaces EL(97)52 in England with immediate effect. It applies to provision of all aseptically prepared medicinal products within NHS pharmacy aseptic facilities, other than those products manufactured under an MHRA MS authorisation (see Scope of guidance section below).
Most such services operate under Section 10 exemption from the Medicines Act 1968 (as referenced in The Human Medicines Regulations 2012), but as detailed below, the scope of this guidance also encompasses a range of aseptic activities that sit outside the Section 10 exemption and outside the scope of MHRA MS authorisation. These include, for example, aseptic preparation/reconstitution of investigational medicinal products (IMPs) and advanced therapy medicinal products (ATMPs) within NHS pharmacy aseptic facilities in England.
This guidance specifies the process for auditing, monitoring, assuring and reporting compliance of services and facilities with critical quality and patient safety parameters, and for responding to serious concerns about non-compliance. It also specifies the process for escalation of unresolved concerns and signposts to other key guidance and standards applicable to NHS aseptic preparation services and activities.
The guidance is predicated on universal implementation of a template for a digital, good manufacturing practice (GMP) based audit and compliance management system termed iQAAPS (interactive quality assurance of aseptic preparation services). This is supported by a web-based process that enables trust chief pharmacists to develop and improve their services (see Revised assessment process section and Appendix 1 for further details).
The priority is that preparation is carried out to a consistently high standard and the products issued have an assurance of quality to the level of safety that patients legitimately expect.
This guidance has been developed for NHS England by a short life working group (see Appendix 5). It will be formally reviewed no later than month/+ three years from the date of publication, or sooner in the event of any significant change.
3. Definitions
For the purposes of this guidance, ‘aseptic preparation of medicines’ focuses on activity performed within an aseptic facility with pharmacy oversight. This definition therefore encompasses reconstitution of injectable medicines when undertaken within NHS pharmacy aseptic facilities in England. For the avoidance of doubt, reconstitution of injectable medicines in clinical areas is out of scope of this guidance.
Manufacturing under a MHRA MS authorisation, a manufacturer/importer authorisation (MIA) or manufacturer/importer authorisation for IMPs (MIA IMP) are also out of scope of this guidance.
From a legal and regulatory perspective, aseptic preparation and dispensing should be seen as two separate but linked activities:
- Aseptic preparation is defined as: reconstitution of an injectable medicine or any other aseptic manipulation when undertaken within NHS aseptic facilities to produce a labelled ready-to-administer presentation of a medicine, in accordance with a prescription provided by a practitioner, for a specific patient.
- Dispensing is defined as: supply of a finished product to a specific patient, or to the person responsible for its administration, in accordance with a prescription.
This guidance focuses on aseptic preparation and covers all directly associated activities up to the point of issue from the pharmacy aseptic facility.
Compliance with GMP (MHRA 2022) is required in line with Royal Pharmaceutical Society standards, documented in the current edition of Quality assurance of aseptic preparation services.
For further detail regarding the definition of terms used in this guidance, see the Glossary section and explanatory notes in Appendix 2.
4. Scope of guidance
The principles and processes described in this guidance apply to all aseptic preparation of medicinal products in NHS pharmacy aseptic facilities in England, other than those products manufactured under an MHRA authorisation. The guidance therefore applies to all NHS pharmacy aseptic facilities in England undertaking preparation of sterile medicinal products under Section 10 exemption to the Medicines Act 1968 (as amended), even when provided from facilities that concurrently hold an MHRA MS authorisation.
The guidance applies to:
- Aseptic reconstitution of any medicinal products or IMPs, where this is performed in a pharmacy aseptic facility.
- Aseptic reconstitution of innovative therapies such as ATMPs where this is performed in any NHS aseptic facility with pharmacy oversight.
- All types of preparations made aseptically (ie it applies to formulations such as eye drop preparations under limited and defined circumstances, in addition to injections).
This guidance does not apply to:
- Clinical service elements such as organisational injectable medicines policies and National Patient Safety Alerts (NPSAs). Compliance with standards for such matters is regulated and monitored by the CQC.
- Reconstitution of medicinal products in a clinical area; such activity should be in accordance with the MA holder’s summary of medicinal product characteristics (SmPC) or, for IMPs, in line with the requirements of the clinical trial protocol and any clinical trial pharmacy manual.
- Products manufactured aseptically under an MHRA MS authorisation, a manufacturer/importer authorisation (MIA) or manufacturer/importer authorisation for investigational medicinal products (MIA IMP).
- NHS radiopharmacy services in England, whether within or outside pharmacy oversight. Arrangements for radiopharmacy services will be specified separately to this guidance.
- Aseptic preparation services provided by non-NHS facilities such as independent sector hospital pharmacies.
- The devolved administrations.
5. Restrictions for aseptic preparation
All NHS pharmacy aseptic preparation services operating under Section 10 exemption must meet the five criteria below. It should be noted that some of these criteria have been updated from those specified in Medicines Control Agency’s Guidance to the NHS on the licensing requirements of the Medicines Act, published in 1992, and which were documented in EL(97)52 and the related 1997 Department of Health publication Aseptic dispensing for NHS patients – a guidance document for pharmacists in the United Kingdom (the Farwell report). The original five criteria are therefore specified within other extant published pharmacy standards documents, but this guidance updates the original requirements.
- Preparation is carried out by or under the supervision of a pharmacist. (It is noted that the definition of supervision for the pharmacy profession is being reviewed.) It is critical that the person responsible for supervision has the necessary competence and technical expertise in GMP.
- Preparation uses only closed systems with the exception that emergency eye drop preparation is allowed under defined circumstances (see note 1 below).
- Preparation uses only licensed sterile medicinal products or, when appropriate, a sterile medicinal product with sufficient evidence to provide assurance of pharmaceutical quality.
- Finished products are allocated a shelf-life of no more than 8 days and the allocated shelf-life is supported by validated stability data and an objective risk assessment (see note 2 below).
- Preparation is carried out in accordance with the NHS professional standards referred to in this guidance.
Notes
- Note 1 – For exceptional use of open systems, see Emergency preparation of eye drops in unlicensed aseptic units (SPS 2019).
- Note 2 – The extension of the maximum shelf-life from seven to eight days enables supply of full 7-day treatment courses and limits wastage, but only where it is safe and appropriate to do so, being supported by an objective risk assessment and microbiological, chemical and physical stability data. This may enable rescheduling of patients where clinically appropriate or the use of 7-day drug reservoirs more readily.
6. Roles and responsibilities
Overarching organisational responsibilities for NHS organisations performing aseptic preparation, SPS QA, NHS England and the CQC are specified below. Requirements for key individuals and services, and the roles of primary stakeholders are documented in Appendix 3.
NHS organisations performing aseptic preparation
- To ensure all aseptic preparation is carried out in accordance with the current edition of the professional standards, Quality assurance of aseptic preparation services (QAAPS).
- To adhere to the requirements of this guidance, reporting quality indicators monthly to NHS England via the iQAAPS system.
- To facilitate and respond to audits by SPS QA.
- To implement and update action plans to agreed timescales.
- To report and appropriately escalate serious incidents relating to aseptically prepared medicines.
Specialist Pharmacy Service Quality Assurance (SPS QA)
- To audit NHS aseptic units.
- To monitor quality indicators and progress against audit action plans.
- To report audit outcomes and situation reports.
- To support enhanced compliance management of elevated risk units.
- To escalate concerns regarding aseptic services risks.
NHS England
- To commission the overarching governance and assurance process.
- To oversee and support delivery of the governance and assurance process.
- To ensure that relevant NHS bodies follow up escalated concerns.
- To impose restrictions and measures on NHS pharmacy aseptic facilities where deemed appropriate.
- To ensure that enforcement is applied where necessary.
Care Quality Commission
- To seek assurance that the governance and assurance arrangements for aseptic services are in place.
7. Revised assessment process
Audit process to assess risk
Inspection of aseptic preparation is undertaken to audit practice and processes against the standards outlined in the current edition of the Quality assurance of aseptic preparation services. However, other relevant standards for assessment include, but are not limited to, MHRA’s Good manufacturing and good distribution practice, Guidance for specials manufacturers and other guidance notes including good clinical practice, health and safety law and guidance, NHS guidance and NPSAs.
SPS QA officers with suitable knowledge and experience in aseptic preparation and quality assurance perform these audits. The specialists are the lead regional quality assurance pharmacists in their local area or trained and nominated auditors accountable to the lead regional QA pharmacist. Audit teams cover each of the NHS regions in England and report to the Head of SPS for England via the SPS QA hub lead. Each audit team maintains a local list of sites to be audited, including details of aseptic services provided, and sets the local audit programme.
Auditors document detailed audit findings in an audit report and summary report via the IQAAPS system (see Reporting section for further details).
Audit findings that include deficiencies against the defined standards are assessed and reported as one of four levels of significance:
- Critical – deficiencies that require immediate action (within 24 hours).
- Major – deficiencies that require corrective actions to start as soon as possible and progress to be demonstrated within three months.
- Minor (sometimes termed ‘other’) – deficiencies that require corrective actions and progress to be demonstrated within 12 months.
- Satisfactory – no adverse findings although suggestions for further improvement may be noted.
These deficiencies are grouped by the chapter headings in the Quality assurance of aseptic preparation services standards. A summary of the findings is documented and one of the four levels of significance above is assigned. Any standards or chapters that are not audited are identified as ‘not reviewed’.
An overall risk rating linked to patient safety is assigned to the aseptic unit. The categories of overall risk are:
- High
- Medium (this replaces the previous risk rating of ‘significant’)
- Low.
The audit frequency is risk-based but the maximum interval between audits is no more than two years.
Note: There may be situations or exceptional circumstances where short notice and/or targeted audits are necessary or where the audit period is reduced or extended from that originally proposed. Any escalation of risk results in more frequent service audit, and an aseptic unit that receives a critical deficiency or an overall risk assessment of ‘high’ is reviewed within one month.
Application of iQAAPS (interactive quality assurance of aseptic preparation services)
iQAAPS is a digital, GMP-based audit and compliance management system implemented to manage a number of activities linked to the audit process and provide greater oversight of aseptic preparation. Specifically it:
- facilitates the completion and submission of the pre-audit questionnaire and any other documentation aseptic facilities request
- facilitates the production and distribution of audit reports
- facilitates the creation, updating and close-out of action plans in response to audit findings by aseptic facilities
- enables auditors to have oversight of progress against agreed actions and timeframes in response to audit deficiencies
- provides a ‘live’ audit status for all aseptic facilities
- facilitates the submission of monthly quality indicators (QIs) for aseptic facilities
- enables auditors to have oversight of the QIs for aseptic facilities
- collates data to enable trending and reporting as detailed in the Reporting section of this guidance.
In addition, a self-assessment function enables aseptic facilities to self-declare their compliance with the QAAPS standard. Evidence statements can be created to support the self-assessment rating. Action plans can also be produced in response to internal audit findings to drive quality improvement.
For further detail about iQAAPS, see Appendix 1.
8. Assurance and performance management
Quality indicators (QIs)
Each facility is responsible for reporting a set of predefined quality indicators to NHS England via the iQAAPS system on a monthly basis. These are liable to evolve over time; the QIs in place at the time this guidance is published are detailed in Appendix 4.
The trust chief pharmacist is responsible for ensuring that:
- QI data is submitted monthly
- QI data is routinely monitored locally
- appropriate actions are taken when QIs begin to drift from the ‘norm’
- any significant results are reported to the auditor
- supporting information is provided to the auditor on request, including Pharmaceutical Quality System records and investigations.
Note: Failure to submit QIs or to take appropriate action in response to results may impact on the re-audit frequency of the site or escalation in accordance with the Compliance management and escalation section of this guidance.
Patient safety incidents
Any patient safety incident that is categorised as a ‘serious untoward incident’ arising from work undertaken in a pharmacy aseptic service is to be addressed and reported in accordance with NHS policy and following local organisational procedures. At the same time as undertaking a critical root cause analysis under the trust’s pharmaceutical quality system, the trust chief pharmacist is required to provide a summary of any such incident to the SPS auditor and regional chief pharmacist.
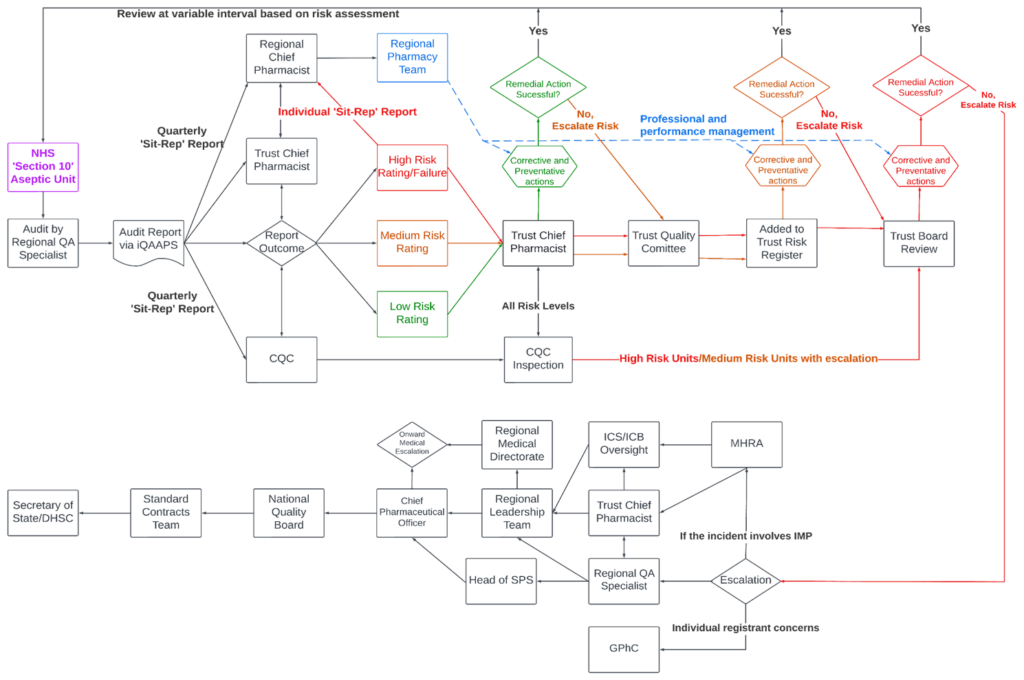
Process for communication of audit findings
Following a site inspection:
- Auditor documents audit findings on the iQAAPS system.
- Auditor publishes audit report and summary report on iQAAPS system.
- Trust chief pharmacist and accountable pharmacist are issued audit report and summary report via iQAAPS.
- Auditor sends the summary report to the trust chief executive.
- SPS QA sends quarterly regional situation report to the regional chief pharmacist (generated from iQAAPS).
- SPS QA sends quarterly national situation report to Head of SPS and NHS England Chief Pharmaceutical Officer (generated from iQAAPS).
- SPS QA provides site-specific audit reports or access to iQAAPS to other regulators on request.
Process for ongoing oversight
- Auditor approves via iQAAPS action plans created in response to audit deficiencies, including timeframes for completion.
- Trust chief pharmacist and auditor monitor progress against action plans through to closure of deficiencies.
- Trust chief pharmacist and auditor monitor QIs.
- Escalation of facilities that fail to make adequate progress with corrective and preventative actions, fail to submit QIs or for which the QI data demonstrates a loss of control, in accordance with the Compliance management and escalation section of this guidance.
- Review of re-audit frequency by auditor based on received intelligence.
9. Compliance management and escalation
Compliance management is automatically triggered for every service that is identified as having a high risk rating, and also for any site identified as having a medium risk rating that is failing to make adequate progress with corrective and preventive actions, submit QIs or for which the QI data demonstrates a loss of control.
The trust chief pharmacist is responsible for informing the trust board when sites are placed under compliance management and for ensuring that ongoing updates of progress and risk are provided.
Compliance management comprises two stages:
Stage 1 compliance management: Enhanced oversight
- Service placed under stage 1 compliance management by SPS QA.
- SPS QA imposes service restrictions if deemed necessary.
- Site-specific situation report prepared and distributed in accordance with the Reporting section of this guidance.
- Auditor closely monitors progress with action plans and QIs until service is removed from compliance management.
- Auditor reviews re-audit frequency in line with service response.
Stage 2 compliance management: Escalation
- Service placed under stage 2 compliance management by SPS QA for failing to meet stage 1 compliance management requirements.
- Site-specific situation report prepared and distributed in accordance with the Reporting section of this guidance.
- Additional restrictions and measures to be imposed by NHS England regional chief pharmacist and integrated care system (ICS) chief pharmacist, as deemed appropriate.
(Note: in extreme cases this may include enforced unit closure.) - Further escalation by NHS England in accordance with the Improvement process section of this guidance, if deemed appropriate.
Appeal process
Services placed under stage 1 enhanced oversight or stage 2 escalation may appeal the decision. The trust chief pharmacist must make any appeal in writing to the head of SPS; this must detail any concerns being raised, provide evidence to support the appeal and must be countersigned by the trust chief executive.
An appeal generates a formal peer review of the audit by a senior auditor who is independent of the previous audit. Their decision is final.
10. Improvement process
Provider collaboratives, integrated care system (ICS) and NHS England regional teams should appropriately resource pharmacy quality assurance services in their respective areas. This resourcing should be used to develop teams of individuals with the relevant expertise to assist underperforming units (as identified through auditing with the iQAAPS system), applying corrective and preventive actions as appropriate. These teams should also develop and deliver training materials for aseptic services across the area to ensure workforces are kept suitably up to date on current practices.
11. Reporting
Audit reports
The auditor documents all audit findings in an audit report and summary report. Reports are prepared and published electronically via the iQAAPS system.
- The trust chief pharmacist and accountable pharmacist are alerted by email when a report is published and available to be viewed via the iQAAPS system. They should confirm that they have read the report via the iQAAPS system.
- A copy of the summary report is sent to the trust chief executive.
Situation reports
SPS QA produces a quarterly national situation report to provide an overview of the status of all NHS pharmacy aseptic facilities in England. Reports are distributed to the NHS England Chief Pharmaceutical Officer, Head of SPS and Head of the NHS Infusion and Specials Medicine Programme.
SPS QA produces a quarterly regional situation report to provide an overview of the status of all NHS pharmacy aseptic facilities in the appropriate NHS region. Reports are distributed to all NHS England regional chief pharmacists.
SPS QA produces site-specific compliance management reports for all sites placed in stage 1 enhanced oversight and stage 2 escalation. Updates to the report are produced monthly. The reports are distributed to the NHS England Chief Pharmaceutical Officer, Head of SPS, Head of the NHS Infusion and Specials Medicine Programme, regional chief pharmacist, trust chief executive and trust chief pharmacist. Reports are shared with other regulators on request.
Audit summary of findings report
SPS QA periodically reviews audit data collated via the iQAAPS system to identify common areas of weakness and areas of good practice. An anonymised summary of findings report is published annually.
12. Glossary
Trust chief pharmacist
A chief pharmacist, in relation to a pharmacy service, is a pharmacist who:
- plays a significant role (irrespective of whether other individuals also do so) in:
- the making of decisions about how the whole or a substantial part of the activities of the pharmacy service are to be managed or organised, or
- the actual managing or organising of the whole or a substantial part of those activities
- has the authority to make decisions that affect the running of the pharmacy service so far as concerns the sale or supply of medicinal products, and
- is responsible for securing that the pharmacy service is carried on safely and effectively.
Manufacturing
Activity that produces medicinal products under an MHRA manufacturer’s ‘specials’ (MS) authorisation, manufacturer/importer authorisation (MIA) or manufacturer/importer authorisation for investigational medicinal products (MIA IMP).
Manufacture includes any process carried out in the course of making a medicinal product but specifically excludes dissolving or dispersing the product in, or diluting or mixing it with, some other substance used as a vehicle for the purposes of administering it.
Reconstitution
Manipulation (dissolving or dispersing the product in, or diluting it or mixing with, some other substance used as a vehicle) to enable the use or administration of a medicinal product in accordance with the marketing authorisation (MA) holder’s summary of medicinal product characteristics (SmPC) (or, for investigational medicinal products (IMPs), in line with the sponsor pharmacy approved documentation).
Reconstitution may be performed within either a clinical area or an NHS pharmacy aseptic facility.
Preparation
Aseptic preparation is the reconstitution of an injectable medicine or any other aseptic manipulation when undertaken within NHS aseptic facilities to produce a labelled ready-to-administer presentation of a medicine, in accordance with a prescription provided by a practitioner, for a specific patient.
Assembly
Relates to packaging and labelling only and not to the preparation of medicines from their ingredients/starting materials.
Dispensing
Supply of a finished product to a specific patient, or to the person responsible for its administration, in accordance with a prescription.
Abbreviations
- EL(97)52: NHS Executive letter 97(52) – Aseptic dispensing in NHS hospitals, 1997
- SPS: NHS specialist pharmacy service
- QAAPS: Quality assurance of aseptic preparation services, this being the title of the Royal Pharmaceutical Society standards publication
- iQAAPS: Interactive quality assurance of aseptic preparation services, a digital, good manufacturing practice (GMP) based audit and compliance management system implemented to support the audit process for aseptic preparation
- QI: Quality indicator
- IMP: Investigational medicinal product
- ATMP: Advanced therapy medicinal product
- MHRA: The Medicines and Healthcare products Regulatory Agency, an executive agency of the Department of Health and Social Care in the UK, which is responsible for ensuring that medicines and medical devices work and are acceptably safe
- MS: Manufacturer’s ‘specials’ authorisation granted by the MHRA to manufacture, assemble or import medicines in accordance with The supply of unlicensed medicinal products (“Specials”) MHRA Guidance Note 14
- MIA: Manufacturer/importer authorisation granted by the MHRA to manufacture, assemble or import medicinal products
- MIA IMP: Manufacturer/importer authorisation for investigational medicinal products granted by the MHRA to manufacture, assemble or import investigational medicinal products.
Appendix 1: iQAAPS
- iQAAPS is a browser-based, electronic audit tool developed by SPS QA to support external audits of unlicensed NHS pharmacy aseptic units.
- iQAAPS incorporates the quality assurance of aseptic preparation services, 5th edition standards by kind permission of the Royal Pharmaceutical Society and NHS Pharmaceutical QA Committee.
- iQAAPS is accessible via all modern internet browsers at vision.quiqcloud.com/login, but access is restricted. An account to access the system will be created for all trust chief pharmacists and accountable pharmacists. The accountable pharmacist may request access for additional users by emailing admin@quiqsolutions.com.
- Training resources including a system user guide, ‘how to’ videos and a recording of a training session are available on the iQAAPS system. For further information please contact iQAAPSadmin@liverpoolft.nhs.uk.

Appendix 2: Explanatory notes regarding definitions
As detailed in the Glossary, for the purposes of this guidance, ‘preparation’ is undertaken in an NHS aseptic facility with pharmacy oversight to produce a labelled ready-to-administer presentation of a medicine. This may or may not be performed in line with the summary of product characteristics (SmPC) for marketed medicines.
‘Reconstitution’ is the activity of dissolving or dispersing the medicine in, or diluting it or mixing it with, some other substance used as a vehicle for the purposes of administration. Where reconstitution is performed in a clinical area, this should be in line with the SmPC for marketed medicines or as specified in sponsor pharmacy approved documentation (often a specific pharmacy manual) in a clinical trial.
Although reconstitution may be performed in a clinical area or within a pharmacy aseptic unit, it is classed as a preparation activity within the scope of this guidance when it is performed in the latter.
The regulatory explanation for this is as follows:
The definition of ‘manufacture’ specifically excludes reconstitution activities. Manufacture of medicinal products must occur under a MHRA authorisation in England.
The definition of ‘assembly‘ relates to packaging and labelling. It is therefore clear that while the reconstitution of an aseptic product is not an assembly activity, the act of labelling the syringe is considered to be assembly. Assembly activity is a type of ‘manufacturing’ activity and is routinely performed under a manufacturer’s authorisation.
Section 10 of the Medicines Act gives a specific exemption for the need for a manufacturer’s authorisation where activities, including preparation and labelling, are performed under the defined conditions laid out in this guidance. Section 10 exemption applies to products that hold a marketing authorisation (licensed medicines) and to unlicensed medicines.
In the context of investigational medicinal products, Regulation 37 of the Medicines for Human Use (Clinical Trials) Regulations 2004 provides an exemption for hospitals and health centres to allow labelling activity to be undertaken by a person operating under the supervision of a pharmacist.
Hence, although reconstitution is neither manufacture nor assembly and is therefore technically outside the scope of both Section 10 and MHRA licensing, the act of labelling the reconstituted products requires the use of the S10 exemption or the Regulation 37 exemption for licensed and unlicensed medicines, and IMPs, respectively. Hence this guidance is applicable where reconstitution and the subsequent necessary labelling is undertaken within pharmacy facilities, so we encompass it within the term ‘preparation’.
This guidance is therefore also applicable to aseptic services reconstituting ATMPs according to an SmPC or sponsor approved clinical trials documentation within pharmacy aseptic facilities.
Appendix 3: Roles and responsibilities
See Roles and responsibilities section for overarching organisational responsibilities for NHS organisations performing aseptic preparation, Specialist Pharmacy Service Quality Assurance (SPS QA) and NHS England.
Requirements for key individuals and services, and the roles of primary stakeholders are documented below:
| Organisation/role | Medicines reconstituted in clinical area (in line with SmPC) immediately prior to administration | Medicines prepared in a pharmacy aseptic facility |
|---|---|---|
| SPS supports the use of medicines to help people live longer, fuller lives by joining together experts to create a rich source of impartial advice for pharmacists and other professionals using medicines. | – Provides a tool to allow self-assessment for clinical area activities. | – Sets standards and specifies user requirements for iQAAPS. – Undertakes audits and feeds back via iQAAPS. – Escalates unresolved concerns to regulators where required. – Reviews and trends iQAAPS QI data and shares findings with key stakeholders. – Produces and distributes reports in accordance with this guidance. – Provides advice to promote improved compliance by units exhibiting increased risks. – Imposes restrictions on activity as required to manage risk and protect patient safety. – Undertakes interim compliance monitoring between scheduled audits. |
| CQC is the independent regulator of health and adult social care in England. It makes sure health and social care services provide people with safe, effective, compassionate, high quality care and encourages care services to improve. It monitors, inspects and regulates services. Then it publishes what it finds, including performance ratings, to help people choose care. Where it finds poor care, it will use its powers to take action. | – Ensures that reconstitution occurs in line with SmPC by trained staff in line with hospital policies. – Reviews completed self-assessments as part of CQC inspections. – Ensures governance with respect to clinical area preparation is appropriate. – Identifies inspection topics in association with SPS. | CQC will seek assurance that the governance and assurance arrangements for aseptic services are in place as part of regulatory framework and routine engagement conversations with NHS trust chief pharmacists. Specifically: – Reviews iQAAPs audit ratings and trends as part of CQC inspection. – Identifies inspection topics in association with SPS. |
| GPhC regulates pharmacists, pharmacy technicians and pharmacies in Great Britain, working to assure and improve standards of care for people using pharmacy service. | The GPhC will respond to concerns escalated regarding registrants. This may include: – Checking iQAPPs audit ratings and trends. – Checking SPS audit reports. | |
| The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the UK which is responsible for ensuring that medicines and medical devices work and are acceptably safe. | – Enforcement – exceptionally, MHRA will investigate in the event of serious incidents. Where this is the case, civil or criminal prosecutions may result. | |
| Trust chief pharmacist undertakes the responsibilities encompassed by their statutory role in accordance with The Pharmacy (Preparation and Dispensing Errors – Hospital and Other Pharmacy Services) Order 2022. | Provides governance to ensure the safe and effective use of medicines within the healthcare organisation. | – Appoints the GMP technical expert to provide leadership to the aseptic unit. – Ensures that individuals supervising aseptic preparation are competent to do so. – Provides oversight for all aseptic processing of medicines in the healthcare organisation. – Ensures all relevant information and changes regarding pharmacy aseptic services are provided to SPS QA. – Ensures QIs are reported in a timely manner. – Implements action plan in response to SPS audit deficiencies. – Acts on adverse trends noted in QI situational reports. |
| Trust chief executive | Receives CQC report. | – Receives SPS summary report. – Supports chief pharmacist to enable robust action plan implementation. – Escalates to ICS lead where required. |
| ICS/ICB chief pharmacist and medical director | – Receives report via NHS England regional medical director and regional chief pharmacist, and trust chief executive. – Reviews (with regional chief pharmacist and regional medical director) service commissioning of sites under compliance management. | |
| Regional chief pharmacist | – Receives report via SPS. – Escalates to NHS England through Regional Medical Director, and ICS/ICB, where required. – Reviews (with ICS/ICB chief pharmacist and medical director, and regional medical director) service commissioning of sites under compliance management. |
Appendix 4: iQAAPS quality indicators
Each site needs to continually monitor a range of quality indicators (QIs), and from these a number have been specified for review in iQAAPS to provide auditors, trust chief pharmacists and accountable pharmacists with oversight of the site’s performance. These are liable to evolve over time and the QIs in place at the time of publishing this guidance are as detailed below:
- number of operator validations, process validations, EOSMF and sterility tests performed
- number of operator validations, process validations, EOSMF and sterility tests failed
- number of grade A samples performed, eg settle plates, contact plates, swabs and finger dabs
- number of grade A samples out of specification, eg settle plates, contact plates, swabs and finger dabs
- number of errors detected internally, eg prior to release
- number of errors detected externally, eg post release
- number of items produced
- internal error rate (number of errors detected prior to release, expressed as % of number of items produced)
- external error rate (number of errors detected post release, expressed as % of number of items produced)
- number of days over locally defined safe working capacity this month
- number of EL audit actions past agreed target date.
Appendix 5: EL(97)52 Review Working Group
- Chair: Stephen Brown
- NHS England: Elizabeth Dimond, Matthew Greening
- Specialist Pharmacy Service: Tim Root
- Specialist Pharmacy Service QA: Mark Jackson, Anne Black, Rob Lowe
- Department of Health and Social Care: Susan Grieve, Nancy Brobbey
- Pharmacy Aseptic Services Group: John Landers
- Royal Pharmaceutical Society of UK: Oweikumo Eradiri
- Association of Pharmacy Technicians UK: Philip Jones
- Care Quality Commission: Morag Ross
- General Pharmaceutical Council: Timothy Snewin, Lisa McCreesh, Aileen O’Hare
- Medicines and Healthcare products Regulatory Agency: Ian Jackson, Alan Moon, Shirley Stagg
- Secretariat: Phillip Rudd

