1. Introduction
1.1 Since April 2021 the provision of all NHS-funded routine vaccination and immunisation (V&I) services for children and adults has been an essential service, except for the COVID-19, childhood and seasonal influenza (adults and at risk) vaccination programmes which continue to be commissioned as enhanced services.
1.2 Practices are required to offer eligible registered patients routine vaccinations in line with the UK Vaccination and immunisation schedule and Statement of financial entitlements (SFE). A set of vaccination and immunisation standards (V&I standards) were introduced, together with 5 global sum funded core contractual requirements. These V&I standards apply to the vaccination programmes outlined in the SFE but practices are encouraged to apply them, as appropriate, to the seasonal vaccination programmes.
1.3 This document outlines the V&I standards and core contractual requirements as set out in the Regulations and the previously issued 10 March 2021 NHS England letter.
1.4 The Regulations have been updated to clarify the V&I standards, and from April 2024 include the addition of the data quality requirements following the 2024/25 contract consultation. These V&I standards, together with the core contractual requirements, underpin delivery and organisation of these essential V&I services. It is recognised that most practices are already working to the V&I standards and core contractual requirements, but where practices may not be achieving against them, this document will support practices in understanding what is required to meet them.
2. Vaccination and immunisation standards
2.1 The National Health Service (General Medical Services Contracts) Regulations and the National Health Service (Personal Medical Services Agreements) Regulations outline the V&I standards that underpin delivery of general practice V&I services. The V&I standards cover:
- invitations for immunisation appointments when patients first become eligible for relevant vaccines or immunisations;
- the steps to be taken if no response is received to an invitation;
- the provision of immunisation appointments;
- the steps to be taken if an eligible patient does not attend an immunisation appointment;
- requests for relevant vaccinations or immunisations made by patients who are eligible but have not previously received them for any reason;
- the identification of gaps in the vaccination records of registered patients, and the offer, and provision of, immunisation appointments to those patients; and
- the processing of records relating to patient vaccinations and immunisations, including records relating to patient vaccination status and administration.
2.2 Practices must also ensure they adhere to the V&I standards on processing of data relating to relevant vaccinations and immunisations, where “processing” has the same meaning as in section 3(4) of the Data Protection Act 2018 and covers the sharing, recording and storage of data as follows:
“Processing”, in relation to information, means an operation or set of operations which is performed on information, or on sets of information, such as —
(a) collection, recording, organisation, structuring or storage,
(b) adaptation or alteration,
(c) retrieval, consultation or use,
(d) disclosure by transmission, dissemination or otherwise making available,
(e) alignment or combination, or
(f) restriction, erasure or destruction,
(subject to subsection (14)(c) and sections 5(7), 29(2) and 82(3), which make provision about references to processing in the different Parts of this Act).
2.3 Under the relevant parts of data processing practices are required to:
- share vaccination status data (both vaccinated and unvaccinated) with the local Child Health Information Service (CHIS) and any other system nationally required by NHS England;
- record the vaccination status for all patients, including those patients who have arrived from overseas;
- use relevant SNOMED codes to record vaccination events; and
- maintain accurate and up-to-date patient vaccination records, including correcting vaccination records when practices are made aware of any errors.
3. Core contractual requirements
3.1 Underpinning and supporting delivery of the V&I standards are 5 core contractual requirements, namely:
- a named lead for vaccination services
- standards for call/recall programmes and opportunistic vaccination offers
- provision of sufficient convenient appointments
- standards for record keeping and reporting
- participation in national agreed catch-up campaigns
A named lead for vaccination services
3.2 Each practice is required to clearly identify a named lead for vaccination and immunisation services. The named lead does not necessarily have to be a clinician although for many practices this is likely to be the preferred option. Where the named lead is not a clinician, then they must work alongside and be supported by a clinician to ensure that the core standards are met.
3.3 The role of the named lead is to:
- Take responsibility for the oversight of all V&I services. This will include that the V&I standards and core contractual requirements are being met and that opportunities for vaccination are maximised within the practice; and
- to work closely with others within and outside of the practice, including the primary care network (PCN), NHS England public health commissioning, CHIS, school-aged vaccination services and local authority public health colleagues (who work with health visitors and school nursing teams), to understand current performance and where this can be improved, if required.
Standards for call/recall programmes and opportunistic vaccination offers
3.4 As a minimum, all patients should be proactively offered all routine immunisations as they become eligible, unless otherwise specified. The detail of each programme’s requirements are set out in Annex A.
3.5 Practices should ensure that their call/recall and opportunistic offers of vaccination are made in line with the agreed national standards as set out below.
Initial call requirements and the role of CHIS
3.6 The patient should be sent the initial call or invitation just before or as they become eligible for the relevant immunisation programmes. The invitation can be made using various channels of communication including a personalised letter, telephone call and/or text message. Ideally the practice should use the patient’s preferred method of communication where this is known. Practices are expected to move towards text-based reminders as the required infrastructure becomes available.
3.7 As a minimum, for both adults and children, this invitation should include information on how to book an appointment. Where invitations include a pre-booked appointment slot, it should include information on how to change the appointment if it is unsuitable.
3.8 For children, the initial contact should normally provide a pre-booked appointment slot. In most areas, parents will be informed of their child’s eligibility for the routine childhood immunisations by their local CHIS, with some local CHIS arrangements including management of both call and recall and offering appointments. Where the local CHIS does not operate the call/recall requirements described below, it will be the practice’s responsibility to have safe and effective systems in place to ensure that all children are offered a minimum of 3 invitations for each vaccination. Practices can find details for their local CHIS service, including whether they manage call/recall, by contacting their local commissioner.
Recall requirements
3.9 A call/recall programme is one that supplements the initial invitation with follow-up activity in the case of non-response. Patients and parents/carers who do not respond to the initial invitation or attend a booked or pre-booked appointment, should be recalled on a minimum of 2 separate additional occasions as needed to ensure vaccination. In most cases, best practice recall activity should continue beyond 3 contacts until vaccination has been completed – especially for routine childhood immunisations – to ensure maximum individual and population protection. Practices should have protocols in place to ensure timely follow-up of these patients and patients should be contacted to confirm receipt of this 2nd invitation.
3.10 Where the patient does not respond to the 2nd invitation, a healthcare professional should make a 3rd contact, either a face-to-face or a telephone conversation. The UK Health Security Agency (UKHSA) has published resources to aid these discussions and to support informed patient choice and improved uptake.
3.11 Patients who remain unvaccinated following this 3rd contact should be flagged on the GP record as unimmunised, to maximise opportunities for opportunistic vaccination.
3.12 In the case of children, practices are required to ensure that the local CHIS is notified of children who are vaccinated and those who remain unvaccinated. Practices should also ensure the local school-aged immunisation teams are notified of those who remain unvaccinated, to enable follow-up where this is practicable within current systems.
Opportunistic delivery
3.13 Opportunistic vaccine delivery will remain an important delivery mechanism, irrespective of whether a programme is designated as call/recall, especially for those programmes where primary responsibility for delivery sits outside the practice, such as HPV vaccination. Opportunistic delivery can be triggered by:
- a patient requesting vaccination for which they are eligible and that they have not received; or
- the practice’s identification of gaps in a patient’s vaccination record when they present for an unrelated issue, or at other key points such as new patient registration.
3.14 In these circumstances, the practice should offer to vaccinate the patient during this appointment unless there are clinical reasons not to do so. If vaccination is not possible during this appointment, then a specific appointment for vaccination should be offered before the patient leaves the practice.
Provision of appointments
3.15 Practices should ensure that they have availability of sufficiently trained staff and convenient, timely appointment to cover 100% of their eligible registered population. Practices can collaborate across their PCN to achieve vaccination coverage during both core and enhanced hours from 1 April 2024, as outlined in the Network Contract Directed Enhanced Service and using the relevant schedule in the PCNs Network Agreement. However, practices must ensure that appointments are acceptable and convenient to their practice’s registered population.
3.16 Appointments should be available at a range of times across the week including during Network Standard Hours offering enhanced hours appointments in evenings and weekends, providing maximum flexibility for working adults and parents/carers. Any appointment time lost to non-attendance should be repurposed to proactive follow-up.
3.17 Practices should ensure that patients can book appointments for vaccinations and immunisations online as these services develop. Practices should work towards integrating online bookings with other digital developments such as the eRed Book and the NHSApp.
Participation in national annual catch-up campaign
3.18 Catch-up campaigns are time-limited programmes aimed at an unvaccinated cohort of eligible patients.
3.19 Participation in an annual catch-up campaign is a core requirement for practices with funding provided through global sum. Where a catch-up campaign focuses on a vaccine that accrues an item of service payment, then this will be payable against each vaccine delivered as part of the catch-up campaign. Details of the annual catch-up campaign will be provided by NHS England following consultation with the General Practitioners Committee England.
3.20 Practices are reasonably required to participate in these campaigns. The ask of practices should be reasonable and mainly linked to continuation and reinforcement of existing activities, with practices required to demonstrate what they have done to identify and call-in eligible patients, follow the core standards for call/recall, offering vaccination where required and updating patient records accordingly. The expectation is that the practice would be able to demonstrate proactive action to support the campaign. Practices are required to inform their local commissioner of the outcomes of the campaign, which may include verifying what the practice did to help deliver the relevant campaign (such as providing information on the activities undertaken and whether this led to vaccination).
Standards for record keeping
3.21 Practices must adhere to the standards for recording vaccination events for contract monitoring and payment purposes. Practices are required to use the nationally specified SNOMED codes to record this activity and to return performance data to UKHSA and any successor organisation. SNOMED codes can be found on the NHS Digital website.
3.22 Practices must ensure that the following records are kept for each vaccination event:
- any refusal of immunisation
- where an offer of immunisation is accepted
- details of the informed consent to the immunisation
- the batch number, expiry date and name of the vaccine
- the date of administration
- when 2 or more vaccines are administered in close succession, the route of the administration and injection site of each vaccine
- any contraindication to the vaccine or immunisation
- any adverse reaction to the vaccination or immunisation.
4. Ordering and administration of vaccine
4.1 Practices should ensure all vaccine ordering is conducted in line with the national guidance and adheres to any limits on stock to be held at any one time.
4.2 All vaccinations provided by practices must be with the appropriate vaccine using the correct dosage as clinically appropriate.
4.3 All healthcare professionals involved in the administration of vaccines must have the necessary skills and training, including the treatment of anaphylaxis and must have referred to current clinical guidance.
4.4 Where a patient has indicated they wish to receive the vaccination but are physically unable to attend the practice, the practice must make all reasonable efforts to ensure the patient is vaccinated.
5. Recoding vaccination events
Recording retrospective vaccination events
5.1 To code retrospective vaccination events, the practice should:
- Backdate the event date of the vaccination SNOMED code to accurately reflect when the vaccination was delivered.
- Set the GMS flag to ‘No’ (for EMIS and Cegedim practices) or the ‘Event done’ flag to ‘No’ (for TPP practices)*.
- Add free text associated with the vaccination SNOMED code to note the date the vaccine was given and where.
* The purpose of the GMS flag is to denote when an activity was delivered in fulfilment of the practice’s GMS (inclusive of PMS and APMS) contract (GMS=True), or either delivered by the practice outside the GMS contract or delivered by another healthcare provider (GMS=False). TPP has not implemented a GMS flag but offers analogous functionality in the form of an ‘Event done’ flag which, if set to false, denotes that the practice did not deliver the activity.
Recording vaccination events for individuals from overseas or from another setting
5.2 When a patient or their representative reports that a vaccination has been delivered overseas or in another setting, individual clinicians should exercise their judgement to determine that a vaccination has been delivered and to record it in the patient record. The Green Book sets out that, where children and adults come to the UK and do not have a documented or reliable verbal history of immunisation, practices should assume the person is unimmunised and a full course of required immunisations should be planned. Where patients arrive from overseas with a documented or reliable verbal history of immunisation, then the practice can assume the person is immunised and record the details as such in the GP patient record – noting that in the case of reliable verbal histories, it may not be possible to record the batch number or exact vaccination date.
Current and valid SNOMED codes
5.3 SNOMED codes in use to support practice payments are available at Primary Care Domain (PCD) Reference Set Portal. To find the relevant code cluster content, select ‘PCD Refsets’, then choose the relevant programme from the ‘Ruleset’ dropdown menu. See example below of an image of the portal showing an example of the Primary Care Domain code clusters for measles:
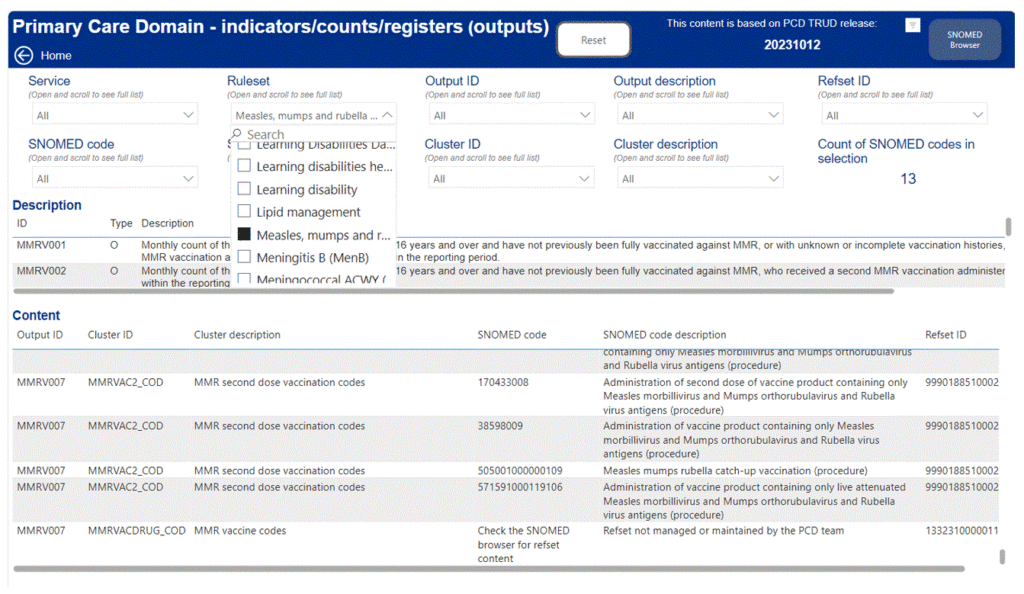
5.4 This data provides the content of the PCD clusters/refsets.
5.5 Additionally, information on drug codes can be found by searching the SNOMED CT Browser.
6. Calculating Quality Reporting Service (CQRS) participation
6.1 Commissioners should continue to ensure that all practices accept V&I services on CQRS, to ensure accurate and timely payment. NHS England’s Digital team will publish a full list of the extraction criteria and eligible codes for payment purposes.
7. Payments
7.1 Practices will be eligible to claim an item of service payment for all doses of vaccine as set out in the Statement of Financial Entitlements (SFE), even when multiple vaccines are administered in a single appointment. Annex A provides details of the vaccinations outlined in the SFE. For vaccinations commissioned under an enhanced service, practices will be eligible to claim an item of service payment in line with the specification requirements.
7.2 Practices will only be eligible for the item of service payment where all requirements, as set out in the SFE or relevant enhanced service, have been met.
7.3 To ensure payment is received, practices must ensure they follow the payment and validation terms and use the correct SNOMED codes to record the vaccinations administered. Under the SFE, practices are required to submit claims for item of service payments within 6 months of administering the vaccination.
7.4 Where a vaccine is centrally supplied, no claim for reimbursement of the vaccine costs or personal administration fee applies.
8. Helpful resources
8.1 The latest information and guidance on vaccines and vaccination procedures for all the vaccines, including completing the schedule of vaccines, can be found in The Green Book: immunisation against infectious disease (GOV.UK)
8.2 Further information to support practices can be found at the links below:
- Statement of Financial Entitlements (SFE)
- Vaccine update – a regular newsletter on the latest developments in vaccines and vaccination policies and procedures
- UK Health Security Agency
- GO.UK’s immunisation page – information for immunisation practitioners and other health professionals
Annex A: vaccination and immunisation programmes which attract an item of service payment under the GMS contract and details of eligibility criteria
The content outlined in this annex was correct at the time of publication, but providers should ensure they refer to the Green Book for the latest information on the vaccination programmes.
These vaccinations and immunisation programmes are eligible for an appropriate item of service payment under the GMS contract.
- Childhood vaccination and immunisation schedule – for all children starting the programme at 8 weeks will follow the immunisation schedule and be offered vaccinations routinely. Practices should strive to vaccinate any children with interrupted, incomplete or ‘unknown’ immunisation status where possible and in line with the Green Book.
- Adult routine vaccination and immunisation schedule – all adult routine immunisation programmes should be offered routinely to those cohorts of patients.
- Other programmes – the immunisation programmes outlined below should be offered routinely to those cohorts of patients.
Childhood immunisations
Practices are required to provide the following vaccination and immunisation programmes in line with the routine childhood immunisation schedules.
Diphtheria containing vaccines
| Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| 6 in 1 vaccine: diphtheria, tetanus, pertussis, polio, Haemophilus influenzae type b (Hib) and hepatitis B (DtaP/IPV/Hib/HepB). | 8 weeks 12 weeks 16 weeks | Call/recall |
| 4 in 1 vaccine: diphtheria, tetanus, pertussis and polio. | 3 years 4 months old or soon after | Call/recall |
| 3 in 1 vaccine: diphtheria, tetanus and polio (this is predominantly given via the school’s programme, but where administered via a practice then an item of service fee will only be applicable). | Boys and girls aged 14 years (only attracts an IoS payment if not given in school) | Opportunistic or if requested |
Meningococcal group b (MenB)
| Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| Practices are required to provide to each child registered with the practice a Men B vaccination in line with the childhood immunisation schedule. | 8 weeks 16 weeks 1 year old (on or after the child’s 1st birthday) | Call/recall |
Rotavirus gastroenteritis
Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| Practices are required to provide to each child registered with the practice the rotavirus vaccine in line with the childhood immunisation schedule. Where the vaccine status of the child is unknown and unable to receive the first dose before the age of 15 weeks no vaccine should be given. | 8 weeks 12 weeks | Call/recall |
Pneumococcal (13 serotypes)
Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| Practices are required as part of the childhood immunisation schedule and in non-routine cases to provide PCV vaccinations to eligible registered patients. This is a 2-dose schedule. | 12 weeks 1 year old (on or after child’s 1st birthday | Call/recall |
| Children who are severely immunocompromised or have complement deficiency, asplenia or splenic dysfunction must receive the PVS and Hib/MenC as outlined in column 2. | Infants under 1 year old PCV 1st set of doses (2 injections 8 weeks apart) 1 year old PCV 2nd set of doses (2 injections 8 weeks apart) | Call/recall |
Haemophilus B and Meningitis C booster
Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| Practices are required to provide to each child registered with the practice a Haemophilus B and Meningitis C booster vaccination in line with the childhood immunisation schedule. | 1 year old (on or after the child’s 1st birthday) | Call/recall |
Measles, Mumps and Rubella (MMR)
Practices are required to vaccinate all registered patients who are eligible and who have not previously received a completed course of the MMR vaccination.
Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| MMR vaccinations for those under the age of 6 as set out in the Routine Immunisation Schedule with additional doses to be given where clinically indicated. Further guidance can be found in the Green Book. | 1 year old (on or after the child’s 1st birthday) 3 years 4 months old or soon after (check 1st dose has been given) | Call/recall Call/recall
|
| MMR vaccinations for those aged 6 and over for those who have not received a completed course of the vaccination where clinically indicated, or with an unknown or incomplete vaccination history where clinically indicated. | Aged 6 years and over | Opportunistic or if requested* |
*If requested, the decision on when to vaccinate older adults needs to take into consideration the past vaccination history, the likelihood of an individual remaining susceptible and the future risk of exposure and disease. Individuals born before 1970 are likely to have had all 3 natural infections and are less likely to be susceptible. MMR vaccine should only be offered to such individuals on request or if they are risk assessed to be at high risk of exposure.
Human papillomavirus (HPV)
| Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| Practices are required to provide HPV vaccinations to those (eligibility for boys include males born on or after 1 September 2006) who have attained the age of 14 years but who have not attained the age of 25 years who have missed vaccination under the school’s programme. An item of service fee will only be applicable for those vaccinations administered by the practice. | 1 dose – Aged 14 until attain the age of 25 years | Opportunistic or if requested |
| Individuals who are immunocompromised must receive HPV as outlined in column 2. | A 3-dose schedule of HPV is required | Opportunistic or if requested |
Meningococcal A.C.W.Y (MenACWY)
Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| Practices are required to provide MenACWY vaccinations to those who have attained the age of 14 years but who have not attained the age of 25 years. This includes those who may have missed the school’s programme (14 not yet 25 years) and those (19 not yet 25 yeas) who are attending University for the 1st time. An item of service fee will only be applicable for those vaccinations administered by the practice. | Aged 14 until attain the age of 25 years | Opportunistic or if requested |
Details of the wider UKHSA published routine childhood immunisation schedule is available here: Complete routine immunisation schedule – GOV.UK (www.gov.uk)
All adult routine vaccinations and immunisations should be offered routinely to all eligible cohorts of patients outline in Table 2 below:
Adult routine immunisations
Pneumococcal Polysaccharide Vaccine (PPV)
Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| Practices are required to offer pneumococcal polysaccharide vaccination to all eligible patients; unless contra-indicated and is usually a single dose of vaccine. | 65 years old | Proactive call at 65, opportunistic or if requested thereafter. |
| Booster doses may be required at 5 yearly intervals for individuals with no spleen, splenic dysfunction or chronic renal disease (as per Green Book). | 2-64 years in defined clinical risk groups (see Green Book) | Call/recall if in a defined clinical risk group. |
Shingles vaccinations programme
Practices are required to offer Shingles vaccination to all eligible patients as outlined below. Further information on the shingles programme is available General Practice Shingles Vaccination Programme Technical Guidance.
| Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| Immunocompromised cohort – 2 doses of Shingrix vaccine, 2nd dose 8 weeks to 6 months after the 1st dose. | Aged 50 years and over | Call/recall; opportunistic or if requested |
| Immunocompetent routine cohort – 2 doses of Shingrix vaccine, 2nd dose 6 to 12 months after the 1st dose* *Includes those aged 70-79 years on or before the 31 August 2023 who remain eligible until they attain the age of 80 years for whom Zostavax stocks were used until depleted and then eligible for Shingrix. | Aged 70 years and at point of vaccination until attain the age of 80 years | Call/recall; opportunistic or if requested |
| Immunocompetent catch-up cohort – 2 doses of Shingrix vaccine, 2nd dose 6 to 12 months after the 1st dose. | Aged 65 years on or after 1 September 2023 and at the point of vaccination until 69 years. | Call/recall; opportunistic or if requested |
Selective immunisations
Babies born to hepatitis B infected mothers
Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| Practices are required to vaccinate those babies born to mothers who have Hepatitis B. Vaccination should commence as soon as possible after birth. If the baby has not already been vaccinated immediately after the birth by the hospital the baby’s registered practice should administer the vaccine. Practices need to ensure that the results of the baby’s blood test to ascertain the existence of Hepatitis B infection is recorded in the baby’s patient record as part of the delivery of this programme. | At birth (normally administered by hospital) At 4 weeks old At 12 months old | Call/recall |
Pertussis in pregnancy
Vaccination and Immunisation Programme | Age eligibility | Type of offer |
|---|---|---|
| The optimal time for pertussis vaccination is from 16 weeks of pregnancy, or soon after, to maximise transplacental transfer of antibodies to the unborn child. Practices are required to offer vaccination to pregnant women who reach or are already at the 16th week of their pregnancy at the time of vaccination. The offer of vaccination can also be between 16 to 32 weeks of pregnancy, ideally between 20 and 32 weeks. However, women who miss vaccination and who are beyond week 32 of pregnancy should still be offered immunisation. It is important to all women to be offered the pertussis vaccine during each pregnancy. | Pregnant women from 16 weeks | Opportunistic or if requested |
Publication reference: PRN01285

