Best practice timed diagnostic pathways
Best practice timed pathways support the ongoing improvement effort to shorten diagnosis pathways, reduce variation, improve patient experience of care, and meet the Faster Diagnosis Standard (FDS). The guidance will support cancer alliances and constituent organisations to adopt consistent, system-wide approaches to managing this diagnosis pathway.
This guidance sets out how diagnosis within 28 days can be achieved for the suspected lung cancer pathway. Alongside the pathway itself, resources are highlighted to support implementation of the pathways.
This lung pathway is part of a series, published since April 2018. From previous pathways implemented by cancer alliances, implementation guidance was shared in June 2021, identifying areas that are key to success, such as setting up with clinical and operational engagement, auditing pathways, allocating project management resources, ensuring leadership, analysing data, and sharing successes.
This guidance complements existing resources such as NICE guidelines (including NG12) and should therefore be read alongside such guidance.
The best practice timed pathway in this document draws on the National Optimal Lung Cancer Pathway (NOLCP), which was published by the NHS England Clinical Expert Group for Lung Cancer in August 2017, and updated in 2020, and sets out how to deliver the pathway from referral to treatment. Pathway redesign at University Hospitals of South Manchester achieved minimum waiting times through optimal processes.
For any questions about this document please email england.cancerpolicy@nhs.net.
Professor Peter Johnson, National Clinical Director for Cancer, NHS England
Professor David Baldwin, Clinical Lead for Clinical Advisory Group, NHS Cancer Programme
The Faster Diagnosis Standard
We committed in the NHS Long Term Plan to provide a faster diagnosis for people through the introduction of the Faster Diagnosis Standard (FDS). This standard will ensure people are told they have cancer, or that cancer is excluded, within a maximum of 28 days from referral. The new standard is intended to:
- reduce the time between referral and diagnosis of cancer
- reduce anxiety for the cohort of people who will be diagnosed with cancer or receive an ‘all clear’
- reduce unwarranted variation in England by understanding how long it is taking people to receive a diagnosis or ‘all clear’ for cancer
- represent a significant improvement on the current two-week wait to first appointment target, and a more person-centred performance standard.
FDS performance data, including a breakdown by suspected cancer pathway, has been published since June 2021, and faster, more streamlined pathways will be a priority.
As the key system-wide organisations for cancer services, cancer alliances will need to work across the local system to ensure that implementation is prioritised by senior stakeholders, clinical leaders, and operational colleagues, and that capacity is prioritised to enable the standard to be delivered.
The FDS has been formally performance managed since October 2021 activity, in line with cancer services recovery, with an initial threshold of 75% rising to 80% in 2025/26. Cancer alliances will need to ensure that they have plans to meet the threshold, which will need to be increased in subsequent years if we are to contribute to achieving the early diagnosis ambitions in the NHS Long Term Plan.
The case for change
Lung cancer is the third most common diagnosed cancer in England, and accounts for the most deaths.
The UK has low lung cancer survival when compared with Europe and the USA. Estimated five-year survival (2016-20) is among the lowest in Europe at 21%. This varied by cancer alliance with a range of 15% to 29%.
For lung cancer patients in England diagnosed from 2016 to 2020, one-year age-standardised net survival was 45%. This varied by cancer alliance with a range of 39% to 52%.
In 2020, only 29% of all staged lung cancers were diagnosed at an early stage (stages 1 and 2). While this has improved since 2016, this varied by sub-integrated care board (ICB) with a range of 16% to 45%.
Figure 1: Percentage of staged lung cancers diagnosed at early stage (stages 1 and 2) in 2020, by sub-ICB, with a median average of 28%
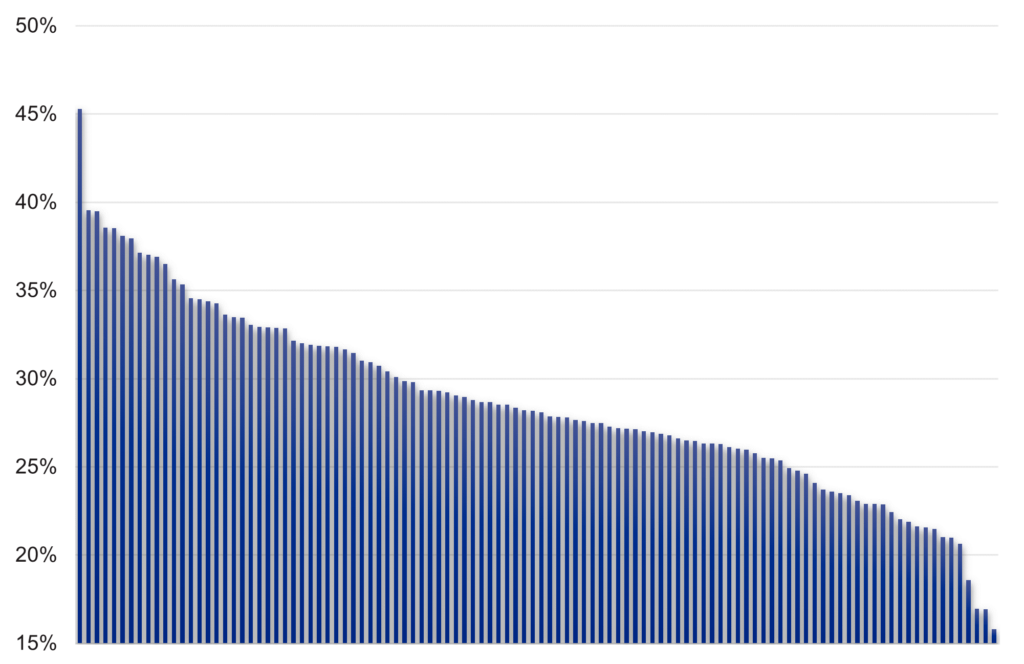
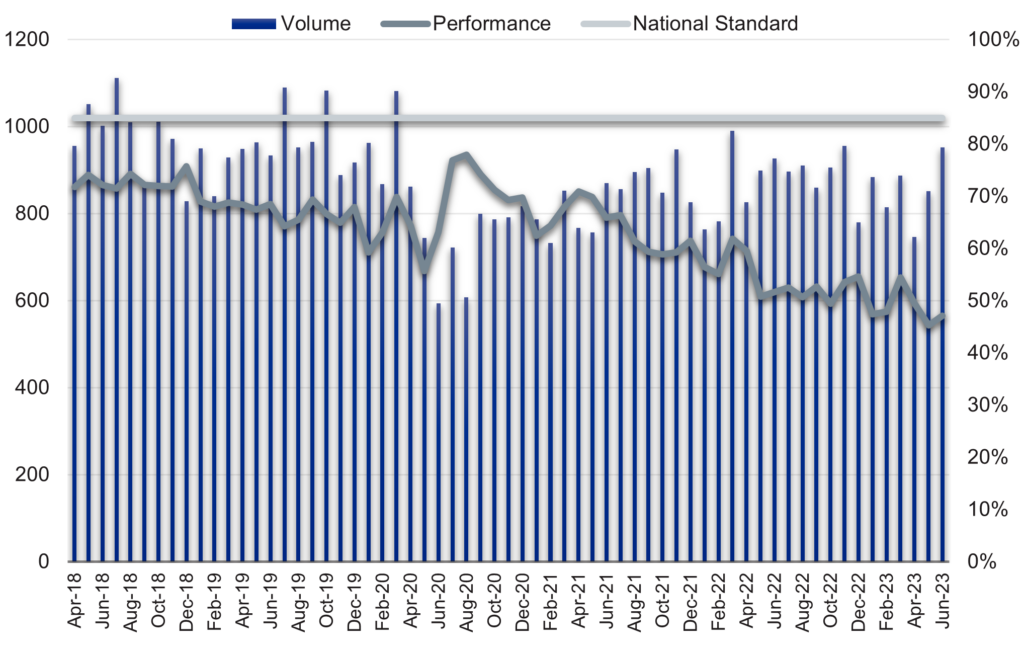
Figure 2: Lung cancers referred for urgent suspected cancer and commencing treatment (62-day standard) by volume and performance, 2016/17 to June 2023
Actions for cancer alliances
In 2023/24, all systems were asked to develop plans to:
- Implement and maintain priority pathway changes.
- Increase and prioritise diagnostic and treatment capacity, including ensuring that new diagnostic capacity, particularly via community diagnostic centres (CDCs), is prioritised for urgent suspected cancer.
- Maximise the pace of roll-out of additional diagnostic capacity, delivering the second year of the three-year investment plan for establishing Community Diagnostic Centres (CDCs) and ensuring timely implementation of new CDC locations and upgrades to existing CDCs.
- Deliver a minimum 10% improvement in pathology and imaging networks productivity by 2024/25 through digital diagnostic investments and meeting optimal rates for test throughput.
- Increase GP direct access in line with the national rollout ambition and develop plans for further expansion in 2023/24
NHS England provides support, funding and guidance to help cancer alliances improve outcomes and reduce variation. The following support is available:
- funding and programme management to support delivery to achieve the FDS and best practice timed pathway milestones
- implementation guidance for achieving pathways
- collaboration and networking events to share best practice.
“The National Optimal Lung Cancer Pathway is potentially the most important initiative to improve times to treatment, increase the proportion of patients treated through better performance status, and in reducing variation in clinical practice. Performance status worsens rapidly in some patients and is one of the strongest independent predictors of both receipt of treatment and outcome.
“Many clinicians have embraced the pathway as a method to improve speed of diagnosis, adherence to guideline-driven management and improve patient experience. Outcomes are anticipated to improve to those seen in other countries.”
Professor David Baldwin, Clinical Lead for Clinical Advisory Group, NHS Cancer Programme.
Benefits of pathway change
For patients and unpaid carers
- Reduced anxiety and uncertainty of a possible cancer diagnosis, with less time between referral and receiving the outcome of diagnostic tests.
- Improved patient experience from fewer visits to the hospital, particularly to specialist centres if possible, and avoiding emergency admission.
- Potential for earlier recognition and initiation of pre-optimisation for treatment that could reduce complications and adverse outcomes.
For systems
- Reduced demand in outpatient clinics with increased straight to test provision and use of pathway navigators.
- Allow resources to be targeted at patients with cancer by removing non-cancer patients earlier in the pathway.
- Improved quality, safety, and effectiveness of care with reduced variation and improvement in outcomes.
Experience of care
- Patients and carers know they are urgently referred for investigation of suspected cancer and should expect diagnosis within 28 days.
- Ensure that patients’ and carers’ ability to attend appointments is taken into account and additional support is offered, where necessary.
- Patients are communicated with clearly, understand the information provided, and are given additional support, such as access to a clinical nurse specialist (CNS) or navigator, psychological support, buddy system, where necessary.
For clinicians
- Using a nationally agreed and clinically endorsed pathway to support quality improvement and reconfiguration of lung cancer diagnostic services.
- The use of predetermined diagnostic algorithms and standards of care to streamline clinical decision-making and reduce delays for multidisciplinary team (MDT) discussion.
- Improved ability to meet increasing demand and ensure best utilisation of the highly skilled workforce.
28-day best practice timed pathway
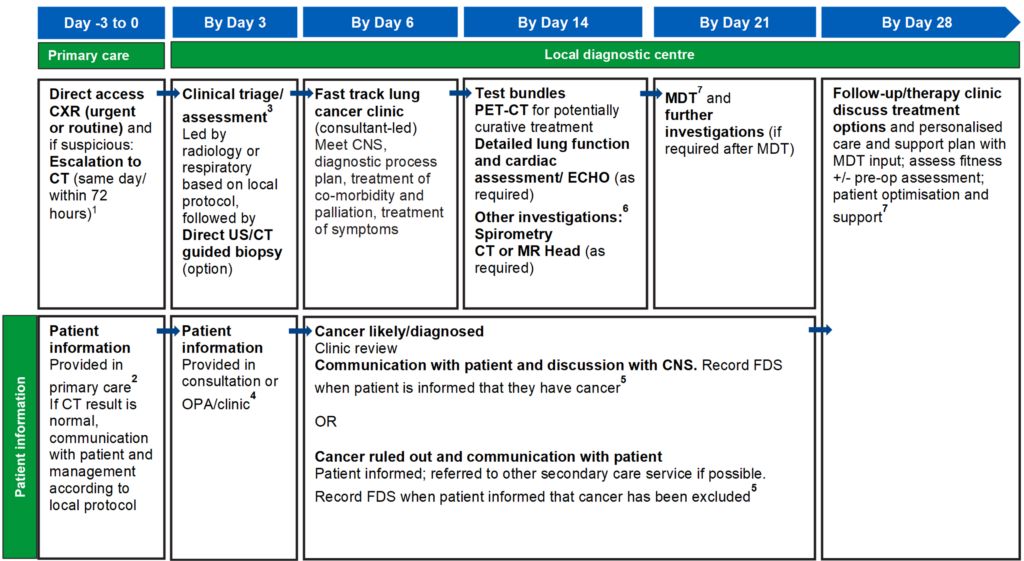
For footnotes/references, see ‘detailed information’ section on pages 10-12.
Detailed information
1. This pathway represents a high-level view of the referral to diagnosis section of the NHS England National Optimal Lung Cancer Pathway (NOLCP). Please follow the link for more information on this pathway and how to implement it in full. Note particularly the new Diagnostic Standards of Care and Treatment sections in version 3.
2. Urgent GP referral pathway should be used for patients who meet NG12 criteria for suspected cancer pathway referrals. The National Cancer Waiting Times Monitoring Dataset Guidance 0 sets out consultant upgrade rules, including from non-GP scenarios such as A&E and acute settings. Cancer alliances may agree local arrangements to facilitate patient self-referral, community diagnostic hub and other referral routes, to access this pathway.
It is noted with the implementation of community diagnostic centres that referral pathways may be subject to change. Primary care should inform the patient that they are being referred for an urgent suspected cancer pathway, although stating that vast majority of referrals result in non-cancer diagnoses. Primary care should also make the patient aware of their responsibilities to make themselves available for the first four weeks for full diagnostic testing.
3. Clinical triage/assessment can be done by a suitably experienced radiologist or lung physician using the available referral information and CT results. Patients should have same day investigations to reduce repeat visits and improve patient experience. Preparation for any tests can be communicated to patients at this stage.
4. Patients and care-givers should be asked what information they require about the pathway, provided with standard leaflets about investigations when sending confirmation of appointment, confirmation of next step(s) and any patient needs required to prepare for the day (for example, can they eat and drink before), and whether they have any disabilities or language barriers.
Preferences for amount of information and when it is provided will vary, and therefore it will help to provide caseworker/navigator telephone contact details to provide support throughout the pathway and outside of clinic times, provide signposting to charities and support services, provide information about care-givers attending appointments, and offer follow-up if patients do not receive confirmation of appointment in expected timescale. Where possible, continuity of caseworker/navigator should be provided to enable familiar contact and to build trust. Patients should also be informed that they may receive one or more procedures and/or diagnostic tests on the same day, at the first face-to-face appointment.
5. Patients should be informed about cancer being diagnosed at the earliest face-to-face opportunity, unless the patient has expressed an alternative method to speed up communication. In this timed pathway, this can be done as part of the clinical assessment process, follow-up testing or results outpatient appointment. Early consideration of patient’s fitness for radical therapy and requirements for prehabilitation should be addressed as soon as possible in the pathway to minimise delays in expediting treatment.
All patients diagnosed with cancer should have a referral to relevant allied health professionals, including a specialist dietitian and speech and language therapist within seven calendar days of diagnosis, and where required, will also be involved during treatment planning. Local protocols and initiatives should be developed in collaboration with perioperative medicine, elderly care and specialist dietitians. Where lung cancer is ruled out, letters can be sent to the patient and GP informing them of the non-cancer diagnosis and next steps on a non-urgent pathway or discharge back to GP. Cancer waiting time rules (including ‘clock start’, ‘adjustments’ and ‘clock stop’) are set out in the National Cancer Waiting Times Monitoring Dataset Guidance v11.0.
6. Following tissue sampling results, confirmed cancer tumours should be tested for all molecular markers required to determine onward management.
7. The core roles at the full MDT (to be carried out following cancer diagnosis) are lead clinician, radiologist, pathologist, oncologist, CNS and relevant AHP, to review investigation results with a pathway navigator. All lung cancer MDTs should have a named clinical lead for the service, with job planned time for the role to allow for service development and management.
An oncologist with an interest in lung cancer and a radiologist with an established lung interest should also be present at the full MDT. The capacity required to deliver these core roles should be reflected in job plans. National guidance on how to maximise effectiveness of MDT meetings is available.
8. Personalised care and support planning should be based on the patient and clinician(s) completing an holistic needs assessment (HNA), usually soon after diagnosis. The HNA ensures conversations focus on what matters to the patient, considering wider health, wellbeing, practical issues and support in addition to clinical needs and fitness. This enables shared decision-making regarding treatment and care options.
Additional information
Audit tool
Can be used to undertake a baseline audit of services being delivered and whether sufficient capacity is in place to routinely deliver, identify areas for improvement, select measurements for improvement, and conduct re-audits as part of continuous improvement.
| Dau | Pathway step | Service in place? | Capacity in place? |
|---|---|---|---|
|
-3 to 0 |
Direct access to urgent or routine CXR from primary care – arrangement should be in place for hot reporting (or within 24 hours), action on abnormal result is secondary care responsibility. | ||
|
Escalation from CXR to CT (same day/within 72 hours) – ‘straight-to-CT’ arrangement in place as described in NOLCP implementation guide. | |||
|
Direct access to CT (same day/within 72 hours) – Arrangements can be put in place with primary care for patients with normal CXR but when clinical symptoms and risk factors continue to cause concern. Timeframes should be the same as for those with abnormal CT. | |||
|
3 |
Triage by radiologist or lung physician – local protocol developed to facilitate streamlined triage process. If lung cancer can be ruled out there is no need to see patient in a cancer clinic. Local arrangements to be made with primary care over redirection to respiratory clinic or alternative non-urgent pathway. | ||
|
Direct biopsy option – for when initial triage suggests cancer but patient unlikely to be suitable for curative treatment, consider developing local protocol. | |||
|
6 |
Fast track lung cancer clinic – meet lung cancer nurse specialist (diagnostic process plan, diagnostic planning meeting prior to clinic, treatment of co-morbidity and palliation/ treatment of symptoms). | ||
|
14 |
Curative Intent Management pathway – test bundle requested at first outpatient appointment including at least PET-CT spirometry, with lung function and cardiac assessment/ ECHO as required. Consider local arrangement/networking to reduce PET-CT delays at this stage. | ||
|
21 |
MDT with some patients on clear and agreed cancer pathways being discussed more briefly either at the beginning, or end, of the MDT and discussion of treatment options. | ||
|
28 |
Follow up in lung cancer clinic: cancer confirmed and treatment options discussed, or if no cancer diagnosis then manage/discharge (this should be at earliest opportunity: eg by day 1-6 stage if CT excludes cancer). Any further investigations following MDT will have been completed by day 28. |
Guidance from the NHS England Clinical Expert Group on lung cancer
- The National Optimal Lung Cancer Pathway (NOLCP) provides a detailed roadmap from referral to initiation of treatment.
- The NOLCP Implementation Guide provides practical examples of how service reconfiguration can facilitate NOLCP implementation.
- Clinical Advice for the Provision of Lung Cancer Services provides cancer alliances and commissioners with the knowledge they require to commission high quality lung cancer services.
Other resources for lung cancer pathway improvement
- The Greater Manchester RAPID Pathway / GM Optimal Lung Cancer Pathway was developed and tested as a variation on the NOLCP. Resources that are available include evidence-based MDT algorithms, a final report, and case for change.
- The Improving Lung Cancer Outcomes Project (ILCOP) at the Royal College of Physicians.
- The ACE Programme report, ‘Improving diagnostic pathways for patients with suspected lung cancer’ and wave 3 programme to reduce variation.
NHS England resources
- The Change Model is a framework for any project or programme seeking to achieve transformational, sustainable change (refreshed on 4 April 2018).
- The Improvement Hub provides a number of useful resources that can support service improvement including guidance, modelling tools, and webinars.
- The Delivering Cancer Waiting Times – A Good Practice Guide sets out good practices to achieve and sustain CWT performance.
Cancer alliance workspace
Cancer alliances access this workspace for national guidance, resources, and to share learning. Please use this space to upload materials you have developed locally and that you think would be useful for colleagues implementing this pathway across the country.
Acknowledgements
This guidance was developed by the NHS Cancer Programme (with Arun Takhar as former Clinical Fellow and Professor Christopher Harrison as former National Clinical Director) and builds on the experience and expertise provided by the Clinical Expert Group for Lung Cancer (Professor David Baldwin as Chair, Professor Sam Janes as Vice Chair, and the Roy Castle Foundation as secretariat), and the National Cancer Vanguard (in particular, Dr Richard Booton, Simon Evans, Dr Matthew Evison, Nicola Hunt, Katie Morris, Dr Neal Navani, Dr Thomas Newsom-Davies, Claire O’Rourke, Prof Kathy Pritchard-Jones, Mr David Shackley, and Dr Nicholas van As).
Publication reference: PRN1346

