1. Diagnosis
Clinical diagnosis
- Measles is highly infectious and correct triage is essential. Ensure receptionists and or triage teams ask patients, parents and carers about any fever and rash, and ensure that all staff know that non-immunised individuals should be isolated on arrival at the care area.
- Measles is a clinical diagnosis and alternative diagnoses including streptococcal and meningococcal infections and Kawasaki disease should be considered and excluded.
- Consider a measles diagnosis in children or unvaccinated adults, including pregnant women, presenting with a measles-like rash, fever, and other symptoms suggestive of measles, including:
- A high temperature
- A runny or blocked nose
- Sneezing
- A cough
- Red, sore, and watery eyes
A timeline of these symptoms is available online.
- Measles can be seen in children, as well as adults, including pregnant women, and may be more severe in young adults compared to children.
- Check immunisation history and whether appropriately vaccinated for their age and whether they have previously had measles. One dose of the measles, mumps and rubella (MMR) vaccine is at least 95% effective in preventing measles and with two doses, 99% of individuals will be protected. People who have previously been vaccinated against measles (with two doses of a measles containing vaccine) in countries other than the UK are likely to have protection.
- Determine any significant contact with a suspected case of measles. Significant contact is defined as close contact face to face or for more than 15 minutes in an enclosed space within the infectious period, four days either side of the rash developing e.g. being in a room in the same house as or a child in the same class with a confirmed measles case is considered to be a close contact.
- Ask about, and look for, the presence of typical features of measles:
- Fever of 39ºC or more without taking paracetamol or ibuprofen, a rash which goes away with pressure and at least one of the following: cough, runny nose, and conjunctivitis.
- Initial symptoms: raised fever, runny or blocked nose, sneezing general discomfort, cough, conjunctivitis.
- A measles-like rash consisting of red spots that are usually not itchy, sometimes raised and joined together to form blotchy patches. The rash starts three to four days after the initial symptoms behind the ears, then the face, then spreads to trunk of body, and then limbs.
- Small white spots inside the cheeks and on the inner part of the lips.
- If there is any suspicion of measles infection, notify the local Health Protection Team (HPT) by telephone immediately.
- All staff working in proximity to patients should have two documented MMR vaccinations or be IgG positive for measles. Staff who do not have immunity should not be involved in the assessment or management of patients with suspected measles.
- In cases of breakthrough or modified measles (for example after Immunoglobulin treatment), symptoms may be mild, of a shorter duration, and there may not be the typical rash). Breakthrough measles is uncommon and occurs in those who are immune, typically at least six years after full vaccination (two doses of MMR). It may occur in healthcare workers exposed to a high viral load but tends to be much less infectious with risk of transmission only to the immunosuppressed.
Note:
- Measles infected immunosuppressed individuals may not present with a rash.
- Not all individuals presenting with a rash, even in areas of high prevalence, will have measles.
Differential diagnosis
Consider a different cause for the rash if the individual is likely to have immunity to measles, their clinical features are atypical, they have no history of contact with measles or travel to measles-endemic countries, and there are no local outbreaks.
Other causes of a rash are much more likely in individuals who have previously had measles or who have been fully immunised. Differential diagnoses include, but are not limited to:
- Scarlet fever/streptococcal infection: palpable (sandpaper) maculopapular rash on the abdomen which spreads to the back and limbs 12-48 hours following symptom onset. Sore throat and a swollen, bumpy, red tongue (‘strawberry tongue’). Note that strawberry tongue can also appear with Toxic Shock Syndrome.
- Kawasaki disease: high fever (with the case definition requiring it to be present for five days or longer), a widespread and non-specific rash, bilateral dry conjunctivitis, inflammation of the lips and mouth (there may be a ‘strawberry tongue’), red and swollen hands and feet, leading to peeling skin, and cervical lymphadenopathy. Note that strawberry tongue can also appear with Toxic Shock Syndrome.
- Early meningococcal disease: may present with a maculopapular rash, but becomes petechial evolving to being purpuric in later stages, and the rash does not fade when a glass is pressed against it.
- Parvovirus B19: symptoms include bright red rash on the cheeks, red, lacy rash on the rest of the body but no Koplik’s spots.
- Rubella: typically, mild and presents with a non-specific or maculopapular rash that is not confluent. Rash usually starts behind the ears and on the face, and spreads down the body (similar to measles). Infection is generally mild, if fever is present, and it rarely occurs after the first day of the rash. Post-auricular and sub-occipital lymphadenopathy may occur, and Koplik’s spots are not visible.
- Infectious mononucleosis (glandular fever): typically presents with a sore throat, malaise, and fever. A rash occurs in around 8% of Epstein Barr virus diagnoses.
- Herpes virus type 6 (roseola infantum): may be asymptomatic and the fever can last three to five days after which a maculopapular rash appears (when clinical improvement has occurred).
Note:
- Drug reactions may also cause a rash which can appear similar to the rash associated with measles.
- Unvaccinated individuals who have been given antibiotics by a GP for high fever/cough, who then present with a rash, during an outbreak of measles are more likely to have measles than an allergic reaction and should be triaged and managed appropriately.
2. Assessment and management
Individual with suspected measles in primary and community care
- Place the patient in a room, away from other patients on arrival.
- If the patient with suspected measles is assessed in a face-to-face setting, the assessment should be done in an isolated room.
- Wear appropriate personal protective equipment (PPE). Refer to the National infection prevention and control manual (NIPCM) sections 1.4, 2.4 and appendices 5b and 6 for further information of the use of PPE. NHS England’s Infection Prevention and Control guidance on PPE is available online.
- Measles is a clinical diagnosis and isolation should not be delayed awaiting PCR results where there is clinical suspicion.
- Immediately notify the local Health Protection Team (HPT): Contact details for HPTs are online on the GOV.UK website. They will advise on public health measures including contact tracing to identify vulnerable individuals and arrange surveillance testing.
- The large majority of children and young people will not need admission and should not be referred for confirmatory testing or review unless there is a specific concern with patient acuity.
- Family members should be immediately vaccinated against measles as per national guidance.
- Seek immediate advice on management from local paediatric services through locally agreed rapid access routes if the patient has a measles-like rash and is in one of the following high-risk groups:
- Younger than one year of age
- Immunocompromised (regardless of immunisation status)
- pregnant
- If the patient presents with or reports symptoms of a serious complication:
- Pneumonia
- Neurological problems
- fever and immunocompromised
- Where admission is planned, contact the hospital regarding isolation before admission.
- Advise on rest, fluids, and paracetamol and ibuprofen for symptomatic relief (children younger than 16 years old should avoid aspirin).
- Vitamin A should not be prescribed in suspected or confirmed cases of measles when the patient (adult or child) has not been hospitalised. Vitamin A is not without harm if prescribed inappropriately.
- Adults and children with measles should stay away from nursery, school, or work for at least four days after the initial development of the rash.
- For individuals with immunosuppression, discuss management with clinicians managing their immunosuppression as they are at more risk of severe disease and complications, and may be infectious for longer.
- Provide written advice about measles to parents and carers. The QR code for NHS measles guidance is in Appendix 3.
- Individuals vaccinated against measles outside the UK are considered to be vaccinated against measles, but should be encouraged to have the MMR vaccination if their doses are undocumented.
Individuals who have been in contact with suspected measles
- Determine immunisation status and whether there has been significant contact with a suspected case, or is immunocompromised.
- Contact the local HPT immediately if the individual is:
- Immunocompromised
- Younger than one year of age
- Pregnant
- Susceptible to measles infection but the MMR vaccine is contraindicated
The risk assessment for the post exposure prophylaxis of susceptible vulnerable close contacts is detailed in the national measles guidance.
- For immunocompromised patients, intravenous immunoglobulin is recommended.
- For pregnant women and infants, HNIG is recommended.
Please refer to national guidance for further information and work with you local HPT who will support risk assessment.
| Table 1. Assessment and treatment of infants in contact with measles (UKHSA) See national guidance for further information | ||
| Infants under 6 months | Assume susceptible and administer intramuscular Human Normal Immunoglobulin (HNIG), ideally within 72 hours but up to 6 days, regardless of maternal status. | |
| Infants aged 6 to 8 months | For household exposure, administer HNIG, ideally within 72 hours but up to 6 days if necessary. | For exposures outside of the household, administer MMR, ideally within 72 hours. |
| Infants 9 months and older | Administer MMR vaccine, ideally within 72 hours of exposure. | |
- For children more than one year of age, not immunocompromised, and with no other contraindications to the MMR vaccine:
- Offer immediate vaccination
- The MMR vaccine should ideally be given within three days of contact with a possible case and repeated after an interval of at least one month
- For children younger than 15 months old when they receive their second dose, another routine (third) dose should be given after 18 months for full protection.
- For children younger than 12 months old when they receive their first dose, two further doses will be required at the normal ages in accordance with the Childhood Immunisation Programme.
- Provide written advice about measles to parents and/or carers. If the information is required in a language other than English, advice is available on the NHS website.
Individuals with confirmed measles in hospital
- Ensure triage in place and that suspected cases are isolated on arrival.
- Clinicians should work with their local HPT for risk assessment of close contacts.
- Measles is diagnosed clinically, and PCR testing should be used for confirmation of diagnosis.
- The management of unwell children, particularly those under two years of age, malnourished or with underlying health conditions should be discussed with the regional paediatric infectious diseases team.
- For a child likely to be in hospital for two or more days, aged under two years, malnourished and diarrheal, the use of high-dose unlicensed vitamin A can be considered. These cases should be discussed with your regional paediatric infectious diseases team. Vitamin A (Provepharm) 100,000 international units per 2ml ampoules is an unlicensed medicine imported from France. It is available to import via the usual UK unlicensed medicines importers. Please contact your hospital pharmacy procurement team. There is no dosage currently listed in the BNFC or other commonly used reference sources.
- There is limited evidence of benefit of anti-viral treatments in children and young people with severe measles infection and they are not routinely recommended. There may be some benefit for a subset of children with significant immunocompromise and overwhelming infection, but this should be agreed on a case-by-case basis at an MDT including paediatric infectious diseases specialists.
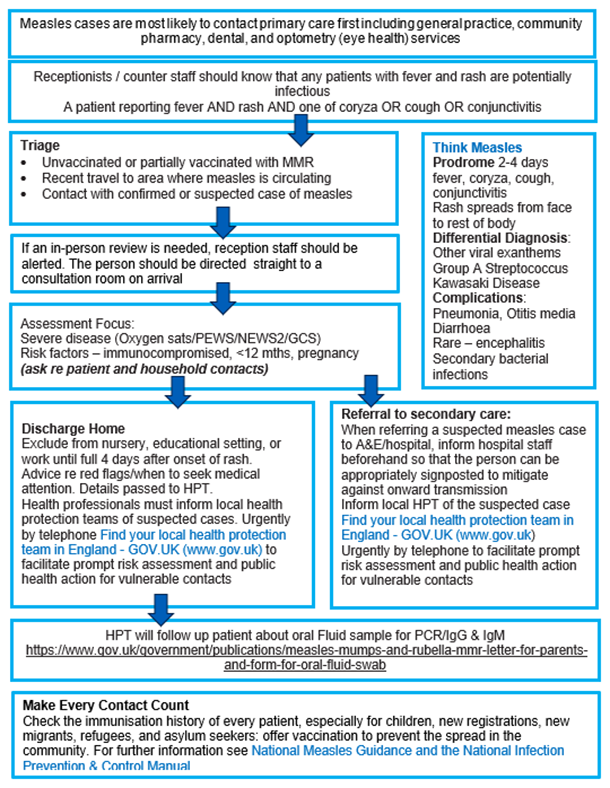
Appendix 1: Managing patients with measles: summary flow chart
NHS England Infection Prevention and Control guidance on how patients with suspected measles should be assessed and managed is available online.
Appendix 2: Prescribing and supply of immunoglobulin for measles post-exposure prophylaxis (PEP) following risk assessment
The NHS England commissioning criteria policy for the use of therapeutic Ig includes the indication for use following exposure to measles available in the Commissioning criteria policy for the use of therapeutic immunoglobulin (Ig) England, 2021.
Use for PEP of pregnant women and infants should follow a risk assessment supported by the local Health Protection Team, sharing patient details and agreeing the indication for Ig. If eligibility criteria are fulfilled, approval from the Sub-Regional Immunoglobulin Assessment Panel (SRIAP) is not required on a case-by-case basis.
Trusts are requested to use local stocks of subcutaneous Ig (SCIg) for PEP of infants and pregnant women where possible, which will also support timely administration within PEP windows. This is most effective if given within 72 hours of exposure but may still be effective if given within 6 days of exposure.
| Indication | Eligibility criteria | Position of immunoglobulin, taking into account alternative therapies | Recommended dose | Prior panel approval required |
|---|---|---|---|---|
| Immune-suppressed individuals | Immunosuppressed individuals (Group A and Group B based on level of immunosuppression) who have had a significant exposure to measles and are known to be susceptible (based on vaccine history and /or IgG testing). | For immunosuppressed contacts IVIg is mainstay management | 0.15g/kg of IVIg recommended ideally within 72 hours of exposure although can be given up to 6 days. Where exposure recognised late or found to be antibody negative between 6 and 18 days after exposure, IVIg may be considered following discussion with specialist clinician. | Prior approval is via discussion with UKHSA health protection team. Find your local protection team here: https://www.gov.uk/health-protection-team |
| Pregnant women and infants | Pregnant women who have identified as susceptible based on vaccine history and /or antibody testing who have had a significant exposure to measles. Infants under 9 months of age with a significant exposure to measles. Advice is available at: National measles guidelines – GOV.UK (www.gov.uk) | For pregnant contacts, immunoglobulin is mainstay management for PEP For infants below 6 months immunoglobulin is mainstay treatment; For infants aged between 6-8 months, MMR vaccine can be offered if exposure occurred outside household setting AND ideally should be given within 72 hours. | For pregnant contacts, approximately 3000mg of human normal immunoglobulin (HNIG) Infants 0.6ml/kg up to a maximum of 1000mg of HNIG HNIG to be given within 6 days of exposure in pregnant women and infants. | Prior approval is via discussion with UKHSA health protection team. Find your local protection team |
Appendix 3: QR code for NHS.UK measles webpage
QR code web address: https://www.nhs.uk/conditions/measles/
Publications reference: PRN01256