Equality and health inequalities statement
Promoting equality and addressing health inequalities are at the heart of NHS England and NHS Improvement’s values. Throughout the development of the service specifications and processes cited in this document, we have:
- given due regard to the need to eliminate discrimination, harassment and victimisation, to advance equality of opportunity, and to foster good relations between people who share a relevant protected characteristic (as cited under the Equality Act 2010) and those who do not share it; and
- given regard to the need to reduce inequalities between patients in access to, and outcomes from healthcare services and to ensure services are provided in an integrated way where this might reduce health inequalities.
Summary
1.Specialised services commissioned by NHS England support people with a range of conditions, including the rare and/or complex. The 149 services in the specialised portfolio range from the higher volume services such as renal dialysis, chemotherapy or secure inpatient mental health services, through to treatments for rare cancers and genetic disorders or complex medical or surgical interventions.
2. NHS England is responsible for setting the national standards that organisations commissioned to provide specialised services need to meet. The national standards for specialised services have two components:
- Service specifications setting out the standards for service providers.
- Clinical policies, setting out the eligibility criteria for service users to access a service/treatment.
3. The existing NHS England service specifications can be found here: NHS England » Service specifications. The clinical policies can be found here: NHS England » Policies: Routinely commissioned and are subject to a separate development and approval process which is detailed here: NHS England » Methods: national clinical policies. The specifications and clinical policies operate alongside the NHS Standard Contract which is used to commission all healthcare services other than primary care.
4. There are around 300 service specifications in total which are reviewed and updated periodically by NHS England to ensure they reflect the latest in best practice and treatment innovations. There are two processes for updating a specification:
- New service specifications or substantial changes to existing specifications that may impact on the way that patients access and/or experience care will follow the Service Specification Methods process. Specifications progressed via this process go through a rigorous, transparent and evidence-based development and sign-off process involving a range of different stakeholders, internal and external to NHS England. It can take on average 6-12 months for specifications to go through the process.
- Minor and incremental changes to existing specifications may follow the Change Process (either the Expanded Change or Minor Change Process). This is a shorter process which is proportionate to the less substantial nature of the changes being made. It can take on average 1-3 months for specifications to go through this process.
Methods process
This process has three key stages:
A: Prioritisation – where the initial proposal to develop/revise a specification by the Clinical Reference Group (or other party) is developed and a priority determined by the national Specialised Commissioning governance structures.
B: Clinical Build – where the detailed work on the draft specification and supporting documents takes place. The proposals are tested with key stakeholders and revised accordingly.
C: Final Approval, Publication and Implementation – where the final draft service specification and supporting documentation is progressed through the NHS England governance structures before being published on the NHS England website.
Further detail on the process is set out in the table at Appendix 1 and in the diagram at Appendix 2.
In very exceptional circumstances, for patient safety reasons or to ensure continuity of service provision, it may be necessary for a specification which may impact on the way that patients access and/or experience care to be rapidly developed or amended and follow a shorter governance process prior to publication. These specifications will be published as ‘interim’ specifications with the expectation that the specification will be taken through the full Methods process and published in final form as soon as practicable. NHS England will ensure full consideration of the need for stakeholder engagement in developing the interim service specification, having regard to its statutory responsibilities to make arrangements to involve the public in commissioning.
It should be noted that due to differences in the governance arrangements for specific National Programmes of Care (NPoC), there may be some minor variation in the stakeholders involved and the process followed.
Once the specification is approved, the following documentation is published on the NHS England website:
- Service specification
- Engagement report
- Equality and health inequalities impact assessment.
Change process
There are two types of change process:
Minor formatting change process
A minor formatting change constitutes changes within service specifications, such as updating terminology or updating references/links to external documents and websites e.g. reflecting updated Royal College or NICE guidelines.
Once the specification is approved, the following documentation is published on the NHSE website:
- Service specification
- A change form setting out the changes that have been made to the specification
Expanded change process
The expanded change process involves minor changes to a service specification, but these go beyond just minor formatting changes.
The type and extent of these changes can vary, but the changes should not impact on the way that patients access or experience care or have unallocated financial implications.
Once the specification is approved, the following documentation is published on the NHSE website:
- Service specification
- A change form setting out the changes that have been made to the specification
- Equality and health inequalities impact assessment.
Both change process methods follow broadly the same process but with differing levels of assurance. A specification working group may be formed in some but not all cases and this is dependent on the nature of the changes being made.
The particular steps of the change process are set out in the table at Appendix 3.
Note: External stakeholder engagement is not usually undertaken in relation to the change process as the changes being proposed should not have a material impact on patients and the public.
Glossary – key groups/people in service specification development
Clinical Reference Groups (CRGs) – groups of clinicians, commissioners, public health experts, patient and public voice (PPV) representatives and professional associations which provide clinical advice and leadership to NHS England on the best ways that specialised services should be provided. CRGs (via specification working groups) also take a lead role in the development of the specifications.
National Programmes of Care (NPoC) – the specialised services have been grouped into six National Programmes of Care and each NPoC has several CRGs to provide clinical advice and leadership. The NPoCs bring together clinical and commissioning leadership, an empowered patient and public voice, and policy expertise to contribute to the development and delivery of strategy and policy objectives and support the commissioning of specialised services.
Patient and Public Voice Advisory Group (PPVAG) wasestablished in June 2014 in order to act as a critical friend to NHS England in the delivery of specialised commissioning and provide assurance that plans to involve people and communities are reasonable and proportionate. The group is made up of PPV partner representatives across all NPoCs, as well as independent members who do not have direct experience or interests in specialised services.
Clinical Panel – a panel of clinical advisors, PPV representatives, and commissioners that considers clinical evidence informing the development of national service specifications and clinical policies and makes recommendations accordingly.
Clinical Priorities Advisory Group (CPAG) is a non-executive advisory committee established by the Board to make recommendations to NHS England about which new treatments, services and technologies should be prioritised for inclusion in prescribed specialised services routine commissioning and could be funded by potential discretionary spend within the annual Specialised Commissioning budget allocation. CPAG provides assurance that the draft clinical commissioning policy or service specification propositions have been developed through the correct published process.
Specification Working Groups lead the work to develop/review the service specification. The membership includes CRG clinicians, the lead NHS England commissioner for the service, a PPV representative and NHS England public health, quality and nursing, finance and information leads.
Senior Management Team – National Director-level senior managers responsible for the commissioning of Specialised Services.
National Commissioning Group/Delegated Commissioning Group (NCG/DCG) oversee the commissioning of specialised services and includes senior NHS England Director-level representatives from national and local commissioning teams as well as PPV representation.
Rare Diseases Advisory Group (RDAG) makes recommendations to NHS England and the devolved administrations of NHS Scotland, NHS Wales and NHS Northern Ireland on developing and implementing the strategy for rare diseases and highly specialised services.
Further information
For further information about service specifications and the NHS England processes described within this document, contact england.acuteprogrammes@nhs.net
Appendix 1 – methods process flowchart
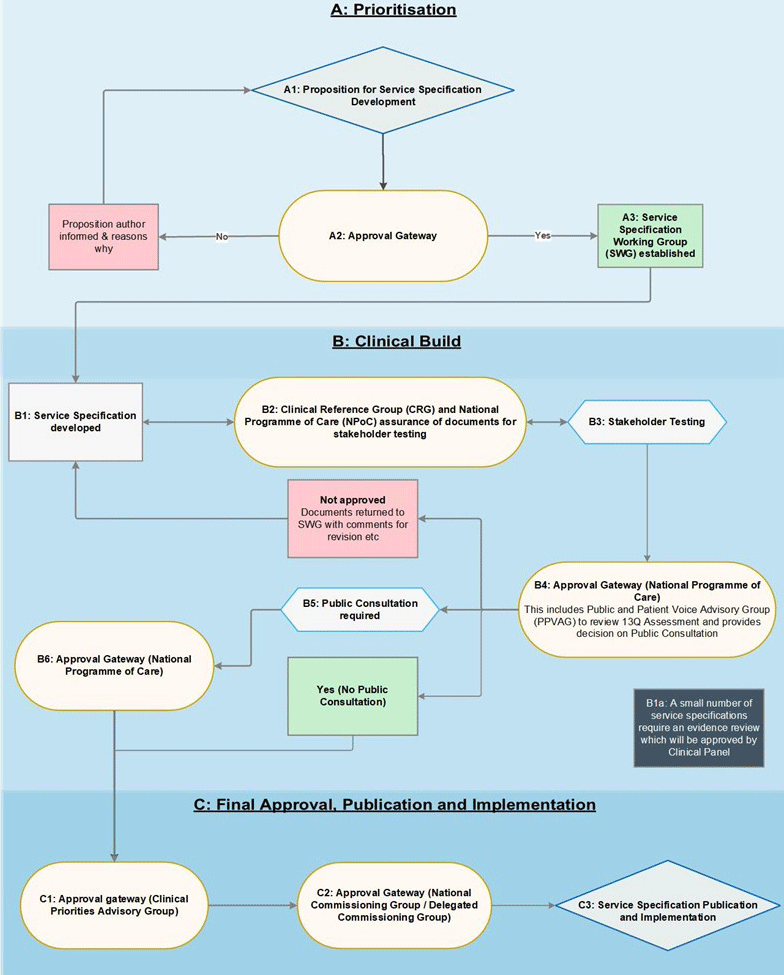
Appendix 2 – methods process
A. Prioritisation
A1: Proposition for Service Specification Development
Preliminary proposals for developing or reviewing/amending specifications are developed by CRGs and co-ordinated through the NPoC. (There are a small number of specialised services which do not come within the scope of an existing NPOC. In these circumstances, development of the specification may be managed through the governance structures relating to that service). The list of service specifications to be worked on within the NPoC will be agreed at the start of each financial year as part of a process to agree the priorities and associated work programme of each CRG/NPoC. However, there may be exceptional circumstances where the CRG/NPoC wishes to propose ‘in-year’ additional service specifications to be added to the work programme.
A2: Approval Gateway
Where an ‘in-year’ additional service specification is being proposed by the CRG, this is considered by the NPoC and then submitted to the Specialised Commissioning Senior Management Team (SMT) for a decision. If the SMT approves the proposal, the specification is added to the CRG/NPoC work programme.
A3: Specification Working Group established
A specification working group (SWG) is formed for each prioritised specification.
B. Clinical build
B1: Service Specification Developed
The SWG begins to populate a draft service specification template.
B1a: Evidence review and Clinical Panel assurance
Note: This step only applies to a very small number of specifications
The SWG decides whether an evidence review is required to inform the specification development. The service specification, the evidence review and evidence report are submitted to the Clinical Panel for consideration.
B2: Clinical Reference Group and National Programme of Care assurance of documents for stakeholder testing
The draft service specification and equalities and health inequalities impact assessment are approved by the CRG and NPoC to go out for stakeholder testing.
B3: Stakeholder testing
At this early stage, stakeholders are invited to comment on the draft specification.
The draft service specification, and an equalities and health inequalities impact assessment (EHIA) are shared with stakeholders, alongside other relevant documents such as an evidence review, if undertaken.
Stakeholders usually have two weeks to share their feedback, but this timescale can vary. Responses are collated into an engagement report, which explains the SWG response to comments and outlines where amendments have been made to reflect the feedback.
A 13q assessment will be completed by the SWG following stakeholder testing to determine whether further public consultation is required or would be helpful, and if so whether a 30-, 60- or 90-day consultation is proposed.
B4: Approval Gateway (National Programme of Care)
The NPoC considers the service specification’s readiness for progression to public consultation or the next approval stage if public consultation is not required.
The 13q assessment form, is also reviewed by the Patient and Public Assurance Group (PPVAG). PPVAG considers if the specification presents any impacts to how patients receive care andwhether issues raised through stakeholder testing suggest that more formal public consultation is needed and assures the consultation length.
B5: Public Consultation
Note: step not applicable if no public consultation undertaken
If our 13q duties are triggered, or it is felt further engagement would be beneficial, the draft specification will be subject to public consultation.
Consultation documents published on the NHS England website include:
- Draft service specification
- Evidence review (where applicable)
- Evidence report (where applicable)
- Clinical panel report(s) relating to evidence review (where applicable)
- Integrated Impact Assessment
- Engagement report
- EHIA report
Following consultation, the SWG reviews the responses and:
- updates the draft service specification and supporting documentation as required.
- updates the Engagement Report to summarise consultation responses received and actions taken.
B6: National Programme of Care sign-off
Note: step not applicable if no public consultation undertaken
A complete set of paperwork is collated and submitted for consideration by the NPoC.
For Highly Specialised Services only – following NPoC approval, the documents are submitted to the Rare Diseases Advisory Group (RDAG) for a view and then to the Highly Specialised Services Oversight Group.
C. Final approval, publication and implementation
C1: Approval Gateway (Clinical Priorities Advisory Group)
Clinical Priorities Advisory Group (CPAG) receives the draft service specification and supporting documents and makes a recommendation on their adoption after assuring itself they have been developed through the approved development process.
C2: Approval Gateway (National/Delegated Commissioning Group)
The appropriate Specialised Commissioning Group will consider the draft service specification, the recommendation from CPAG and the Commissioning Guidance. NCG/DCG and makes a decision as to whether the specification should be approved and adopted.
C3: Service Specification Publication and Implementation
The specification and accompanying documents are submitted for NHS England Director/Chief Executive sign-off prior to publication.
The final service specification, an engagement report and EHIA and other supporting documents are published on the NHS England website.
NHS England, ICB commissioners and commissioned providers are notified that the specification has been published.
Appendix 3 – change process
First Approval Gateway
The amended specification and accompanying documentation are signed off by the CRG chair and appropriate NPoC commissioners who will ensure the appropriate documentation has been completed, the correct people involved and that the change process is the correct process to follow.
Final Approval Gateway
The completed documentation is signed off by the appropriate National Specialised Commissioning senior managers as a final approval. Clinical Priorities Advisory Group (CPAG) is notified that the amended specification has been approved.
Publication
The updated specification is subsequently published on the NHS England Service Specifications page as well as the relevant CRG page. Regional and local commissioning teams as well as commissioned providers are notified of the publication.
Publication reference: PR1541

