Context
1. As medicine advances, health needs change and society develops, the NHS must evolve. NHS commercial activity plays a pivotal role in supporting this evolution, ensuring patient access to clinically and cost-effective new treatments and technologies. It maximises health outcomes for patients in England and value for money for taxpayers.
2. As set out in paragraph 1.8 of the 2024 Voluntary Scheme for Branded Medicines Pricing, Access and Growth (‘the Voluntary Scheme’), the NHS in England is committed to supporting the introduction of the most clinically and cost-effective medicines. NHS England has already expanded the commercial flexibility offered to industry for the best value new treatments, delivering the greatest clinical benefits at the lowest cost, and we expect this to benefit patients, the NHS, individual companies and the life sciences sector more broadly.
3. The Voluntary Scheme notes that these enhanced commercial arrangements may include complex confidential commercial arrangements, where we deem these appropriate and reserve them for companies aspiring to deliver greater health gain relative to cost. to It aims to deliver value for money for the NHS by securing the provision of safe and effective medicines at reasonable prices, and to encourage the efficient development and competitive supply of medicines.
4. It also emphasises that such arrangements would normally be for medicines expected to have value propositions at or below the lower end of the standard National Institute for Health and Care Excellence (NICE) cost-effectiveness threshold range, with greater flexibilities made available for value propositions at even greater levels of cost-effectiveness.
5. The Voluntary Scheme highlights the benefit of open and regular dialogue with industry representatives, and of providing the opportunity for earlier engagement, advice and signposting on the development of new products. The aim of this advice and support is to enable more appropriate uptake of clinically and cost-effective products to improve patient outcomes, with benefit for companies and patients through the acceleration of access and uptake of medicines.
6. Given this context and our role working alongside NICE and industry to support the introduction of clinically and cost-effective medicines, this NHS commercial framework for new medicines (the framework) sets out the NHS’s approach to commercial activity in relation to branded medicines. While it focuses on new medicines, we will continue to consider the case for developing a related framework for best value biologics (biosimilars) and generic medicines. In the meantime, Section 3, which details roles, responsibilities and engagement, will be of interest to all companies.
7. We will review and update this framework over Further details are provided in Section 6.
1. Aims and purpose
8. Supporting improvements in the quality of life and health outcomes for all patients is the overarching purpose of the framework and this underpins all NHS commercial medicines activity. It sets out how we will work with NICE and industry on commercial medicines activity that supports the introduction of clinically and cost-effective treatments into the NHS and maximises value for money for taxpayers.
9. The framework has 4 sections:
- Core objectives and principles: setting out the core objectives and principles on which NHS commercial medicines activity will be based.
- Roles, responsibilities and engagement: defining the roles and responsibilities of those involved in commercial medicines activity and detailing how companies can engage with the NHS.
- Routes to commissioning: clarifying the routes to routine commissioning in the NHS and where commercial activity can occur in those routes.
- Commercial options: outlining the types of commercial options that exist as well as commercial flexibilities, and the circumstances where they could be considered.
10. The framework, and the activity it enables, will support our and NICE’s ambition to deliver patient access to proven, affordable and transformative medicines in a financially sustainable way. For industry, it will encourage faster market entry for new treatments and support uptake and adoption where these medicines are priced fairly and responsibly.
2. Core objectives and principles
11. This section sets out the core objectives of NHS England’s commercial medicines activity and the principles underpinning this.
Objectives
12. The framework has 3 core objectives:
- To facilitate timelier and more streamlined discussions about value, affordability and transactability so that technology evaluation decisions, and ultimately patient access, are not unnecessarily delayed, ensuring early and fast access to new medicines.
- To drive earlier and more purposeful engagement between us, industry and NICE, to enable better planning at both individual company and system levels.
- To clarify the commercial flexibilities that may be available to companies where appropriate. This will ensure that all companies – in particular, smaller and specialist companies with less experience –understand the full range of commercial options available to them.
Principles
13. 6 principles guide and underpin our commercial activity. They support the achievement of the above objectives and ensure:
- treatments are clinically and cost-effective and represent the best use of NHS resources
- the NHS can afford to introduce clinically and cost-effective treatments now and in the future
- any commercial arrangements are transactable within the NHS so that their value is realised and the administrative burden on the NHS and frontline staff is minimised
14. Principle 1: NHS England’s commercial medicines activity serves to support NICE’s technology evaluation process, rather than acting as a substitute for or alternative to it.
NICE plays a critical role in ensuring equitable patient access to new medicines and treatments through assessing the clinical and cost effectiveness of health technologies in a transparent and consistent way. Its recommendations are based on a review of clinical evidence (which demonstrates how well the medicine or treatment works) and economic evidence (which shows how well the medicine or treatment works relative to how much it costs the NHS, and from this whether it represents value for money for taxpayers).
Where issues arise in relation to the economic evidence, whether for cost-effectiveness or affordability, NHS England considers whether commercial arrangements can help resolve these.
The relative roles and responsibilities of the organisations are described in more detail in Section 3.
15. Principle 2: NHS England and NICE will collaborate to provide a joined-up way for companies to engage with the NHS regarding technology evaluations.
NHS England recognises that companies want and need to have aligned and integrated conversations with us and with NICE to address issues concerning value, affordability and transactability, to achieve the fastest possible access for patients to clinically and cost-effective treatments.
NICE assesses value through its technology evaluation process. NICE also assesses affordability by considering the net budget impact of a medicine and, where that is expected to exceed £40 million per year in any of the first 3 years of its use in the NHS, NHS England engages in commercial discussions to manage the affordability. Transactability is a key element informing any commercial arrangement between the company and us.
The process of engagement and routes to commissioning are described in Sections 3 and 4 respectively.
16. Principle 3: Commercial arrangements must be as simple as possible, minimising the burden on the NHS and frontline staff.
All commercial arrangements must be transactable within the NHS, both to realise the value of products to the NHS and to minimise the administrative burden on frontline staff. Complex arrangements will only be considered once simple discounts alone (which facilitate fastest access) have been fully demonstrated to be unsuitable. Where there is a case for more complex schemes, NHS England will need to carefully scrutinise these to ensure complex monitoring or burdensome data collection and disproportionate additional costs are avoided, and that they are consistent with NHS financial flows, accounting rules and commissioning arrangements.
Further details are given in Section 5.
17. Principle 4: Confidential complex commercial arrangements are expected to be considered only for products that represent value at or below the lower end of the standard NICE threshold or other applicable thresholds.
The standard cost-effectiveness threshold NICE uses will be retained at the current range (£20,000 to £30,000 per quality adjusted life year [QALY]) for the duration of the Voluntary Scheme.
Enhanced commercial arrangements would normally be reserved for medicines expected to have value propositions at or below the lower end of the standard NICE cost-effectiveness threshold range, with greater flexibilities made available for value propositions at even greater levels of cost-effectiveness, taking into account any applicable QALY weightings. This refers to the value proposition once any commercial arrangements have been applied.
Further details are given in Section 5.
18. Principle 5: Bespoke commercial arrangements (commercial flexibilities) will be considered on a case-by-case basis.
Although this framework describes the types of commercial arrangements that may be available to companies, the different challenges facing different treatments (value, uncertainty, affordability) demand consideration on a case-by-case basis. No previous deals are an indicator of future deals and discretion on whether an acceptable offer has been made and to agree a commercial arrangement ultimately rests with NHS England. We will take steps to ensure that a fair and consistent approach is applied.
The types of commercial flexibilities and the circumstances in which they may be available are described in Section 5.
19. Principle 6: Commercially sensitive information will be kept confidential at all times.
NHS England recognises the critical importance of this to industry and, in turn, the benefit to the NHS in terms of securing the best possible deals and value for money for taxpayers. Notwithstanding this principle, and consistent with the Voluntary Scheme, we will work with companies and the devolved administrations to confidentially share, wherever possible, commercial arrangements, recognising the reach of the NHS across the UK and the interests of UK taxpayers. We work in accordance with the Freedom of Information Act (FOI). Where it is in the public interest to withhold commercially sensitive information, such as confidential pricing, requested via FOI, this is exempt from disclosure under FOI.
3. Roles, responsibilities and engagement
20. Objectives 1 and 2 of the framework are to:
- facilitate timelier and more streamlined discussions about value, affordability and transactability so that technology evaluation decisions, and ultimately patient access, are not unnecessarily delayed, ensuring early and fast access to new medicines for patients
- drive earlier and more purposeful engagement between us, industry and NICE, to enable better planning at both individual company and system levels
21. This section sets out NHS England’s and NICE’s respective roles and responsibilities and how we work together to enable companies to engage with the NHS about introducing their new medicines and building partnerships. This engagement can happen in different ways across the product lifecycle, and this section clarifies the timing, opportunities and nature of the advice available at different points.
Roles and responsibilities
National Institute for Health and Care Excellence
22. NICE uses its internationally respected methods to produce independent evidence-based guidance and advice for the NHS on the use of medicines. This is an important mechanism for ensuring the medicines used in the NHS are both clinically and cost-effective (value) for patients and taxpayers.
23. As stated in the Voluntary Scheme, all new active substances in their first indication, and extensions to their marketing authorisation to add a significant new therapeutic indication, are expected to undergo an appropriate NICE evaluation unless there is a clear rationale not to do so. Topics that were previously considered to be out of NICE’s remit (specifically HIV and haemophilia treatments) can now be considered for NICE technology evaluation. Therapeutic vaccinations will be considered for NICE technology evaluation, whereas prophylactic vaccinations remain under the remit of the Joint Committee on Vaccination and Immunisation (JCVI).
24. NICE has published a health technology evaluations manual outlining the methods it uses in its technology evaluation
25. NICE also has a key role in enabling engagement with the life sciences industry, both by providing direct advice and directing enquiries to other appropriate functions within NICE and NHS England.
26. The NICE Advice service offers a range of support to companies at different stages of their product development, as a fee-based service. The complementary offers through NICE Advice operate outside NICE’s guidance-producing programmes.
27. In particular, the NICE Advice health system engagement and insight service helps companies engage with NICE, system partners and wider NHS stakeholders. Companies can engage at any stage of product development to inform their market access strategy, including to:
- identify the most appropriate route to NHS access
- understand the changing healthcare landscape
- explore their value proposition
28. NICE Advice can also provide confidential strategic advice on clinical and economic development plans and system engagement, as an additional fee-based service.
29. NICE assesses new medicines through its technology evaluation process. During that process, there are opportunities for commercial engagement between the company and NHS England. NICE has an important role in helping companies understand the process and timeline for the technology evaluation and facilitating the start of commercial discussions by signposting them to NHS England’s Medicines Value and Access (MVA) directorate.
30. NICE is committed to working closely with NHS England to provide timely information that supports the planning of commercial activity and enables timely commercial discussions between NHS England and the company.
31. NHS England’s partnership with NICE is key to ensuring patient access to cost-effective and affordable technologies. In achieving this common purpose, NHS England and NICE will share appropriate information in line with each organisation’s own standard principles, respecting confidentiality on all and any matters relating to the information in question.
32. NICE’s Commercial Liaison Team (CLT) is responsible for briefing NHS England about all types of technologies undergoing NICE technology evaluation, to inform our commercial discussions. The CLT advise NHS England on the feasibility, transactability and expected operational success of patient access schemes (PASs). In addition, they project manage the Budget Impact Test (BIT) process at NICE.
33. A company interested in submitting a PAS or a commercial access agreement proposal should first contact the NICE CLT; it must also consult with the MVA directorate at NHS England.
34. NICE’s Managed Access Team (MAT) focuses on data collection activities for technologies in managed access. The MAT also work on the early identification of technologies that potentially require a managed access option.
NHS England
35. NHS England sets the overall commissioning strategy and clinical priorities for the NHS. We directly commission specialised services as set out in the Prescribed Specialised Services Manual. The commissioning of primary care services sits with integrated care boards (ICBs) under delegation from NHS England.
36. NHS England has an important role in ensuring the best use of public resources and securing the greatest possible health gain for patients from every pound spent. One of the ways we achieve this is through undertaking commercial activities for medicines.
37. Since 2016, NHS England has built on NICE’s work by negotiating time-limited managed access agreements (MAAs) for oncology drugs (using the Cancer Drugs Fund [CDF]) and non-oncology drugs (using the Innovative Medicines Fund [IMF]), bespoke commercial agreements to support routine commissioning and products which meet the BIT, to address uncertainty, value, affordability and risk.
38. NHS England considers commercial arrangements in a range of circumstances (for example, to address both cost-effectiveness and affordability issues) where a company offers a value proposition at or below the lower end of the standard NICE cost-effectiveness threshold range. Several examples demonstrate this flexibility.
39. NHS England works with NICE and industry to:
- encourage companies to engage effectively with horizon scanning activity
- create more opportunities for early engagement
- have more planned and consistent engagement with the industry representative bodies
- enable timely and structured commercial discussions where relevant and appropriate
40. NHS England’s Medicines Value and Access (MVA) directorate aims to balance access and value across the product lifecycle. The team seek to engage with the relevant commissioning functions to prepare the relevant NHS services for the implementation of new medicines, which is particularly important given the increased requirement for corresponding clinical pathway transformation.
41. The scope of the MVA’s activities is:
- medicines negotiation and managed access: negotiations and contracting for commercial or innovative agreements, operation of managed access funds and strategic engagement with industry
- medicines procurement and supply chain: medicines tendering and frameworks, strategic category management, Homecare and supply chain management
- medicines policy and strategy: policy to support access to and uptake of medicines, optimisation of the use of medicines and the NHS’s net zero ambitions
- pharmacy and clinical support: providing clinical and pharmacy input to the work of the MVA, aligning clinical and commercial opportunities
42. In addition, NHS England hosts the Accelerated Access Collaborative (AAC). This is responsible for co-ordinating activities across AAC partner organisations with the aim of making the UK one of the most pro-innovation healthcare systems in the world. This includes:
- supporting the development of innovative products and overcoming system-wide barriers to access
- driving the uptake and spread of the best value innovations across the NHS
- identifying and supporting innovations that will deliver the greatest benefits for patients
Process of engagement
43. Improved and proactive engagement with companies will enable all parties to build trusting relationships, understand the opportunities for aligning strategic objectives and seek opportunities of mutual benefit. Early engagement with stakeholders will also promote better understanding of available routes to NHS access, identify value and affordability challenges upfront, and increase the focus on operational planning and system preparedness for the introduction of new medicines into the NHS, for the benefit of patients.
44. NHS England and NICE have established processes for providing advice to companies across the product lifecycle. This framework enables closer alignment of these existing approaches, providing clarity on these opportunities, their timing and the nature of the advice available.
These engagement opportunities are fully explained below but are also schematically summarised in Figure 1.
Engagement with NICE
45. NICE provides early engagement opportunities for companies through NICE Advice. These fee-based services sit outside NICE’s formal guidance-producing processes and operate on a not-for-profit basis and in accordance with government requirements for managing public money. Companies frequently engage with all NICE Advice services as they offer different types of insights that are beneficial for both market access and navigating the reimbursement pathway.
46. NICE Advice helps companies engage with NICE, system partners and wider NHS stakeholders. Company queries are systematically ‘unpacked’ through preparatory discussions to facilitate a structured and extended ‘safe harbour’ engagement meeting across multiple stakeholders. This enables confidential, free-flowing, peer-to-peer conversations that can act as a basis for ongoing engagement with stakeholders.
47. While companies can engage with NICE at any stage of product development to inform the development of their ongoing market access strategy, the NICE Advice health system engagement and insight offer is of maximum benefit when taken early in the market access and reimbursement process. NHS England is a key participant in these engagement meetings.
Figure 1: Engagement opportunities with NICE and NHS England
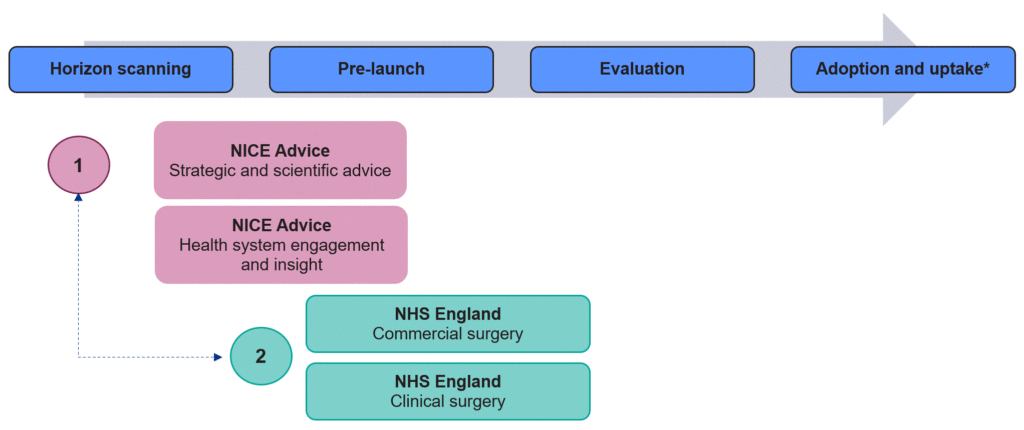
Alignment between NICE and NHS England to ensure triaging of requests to appropriate service**
Broadly there is a 2-step process for engaging with NICE and NHS England
Step 1:
- Initial engagement on emerging market access challenges for individual and group treatments
- Structured multi-stakeholder interaction enabling shared understanding and initiating solution-focused co-working
- Comprehensive scientific advice on evidence generation
Step 2:
- Targeted discussion on commercial and clinical issues
- Surgeries tend to be closer to evaluation and focus on practical, commissioning-related issues, including the potential requirement for complex commercial arrangements
* NHS England and NICE both offer advice at later stages of the product lifecycle. Requests are triaged appropriately.
** Acknowledging that confidentiality plays a role in what information can be shared.
48. The NICE Advice strategic and scientific advice offer can provide views on technical questions at key points in the technology development process, including trial design, early modelling plans, additional data collection and model validation.
49. NICE Advice services can be used in the ‘product development to adoption’ journey. In the rare instances where a company considers there is a barrier to accessing these services, NHS England will strongly encourage it to contact NICE Advice to discuss how the relevant service could be tailored to resolve this.
50. Companies should use this contact form to engage with NICE.
Engagement with NHS England
51. NHS England offers free commercial surgeries to discuss a variety of commercial issues companies may face (for example, commercial access arrangements, managed access arrangements, budget impact mitigation agreements). These meetings should be scheduled downstream of NICE’s strategic and scientific advice and are often most beneficial after the company Health Technology Assessment (HTA) submission but well in advance of the first NICE committee meeting. This enables surgeries to focus on specific commercial issues arising from the submission, while providing enough lead time for companies to deliver on any solutions proposed during the surgery.
52. Commercial surgeries bring together the relevant expertise and skill mix from NHS England and NICE to discuss the issues raised in the brief, ahead of the meeting. For commercial surgeries to be as useful as possible, NHS England asks companies to complete the required template and give 4 weeks’ notice of when they are requesting a surgery.
53. Companies and patient groups can also engage with NHS England, from a commissioning perspective, on clinical matters through clinical stakeholder surgeries. Established in 2014, these surgeries are a method for companies and patient groups to gain in-depth understanding of particular therapeutic areas from key opinion leaders and NHS system stakeholders, and where a new treatment might sit in existing care pathways.
54. Requests for commercial and clinical stakeholder surgeries are managed via the triage function (see the Getting in contact section below.
Horizon scanning
55. Effective horizon scanning is essential for the NHS to understand the products it will likely be using and give an indication of their likely impact for patients and on existing pathways, services and budgets. It also provides an indication of the future commercial environment so that the NHS can be ready to respond as markets evolve.
56. Companies are strongly encouraged to make information available at all stages of product development, to inform horizon scanning across the product lifecycle. These stages are:
- Therapeutic trends and scientific discovery:
- market dynamics and drug discovery trends
- Commercial product development and evidence signals:
- trial results and evidence generation
- clinical development plans and regulatory status
- service requirements
- Market evolution and patent expiry:
- business development
- timelines for therapeutic competition, biosimilars and generics
57. UK PharmaScan is the single national database repository of information about all new medicines and significant new indications to be launched in the UK. It is used by us, NICE and devolved national health technology assessment bodies to inform their horizon scanning activities.
58. Companies are strongly encouraged to submit timely, accurate and comprehensive information to the fullest extent possible on all medicines in development using UK PharmaScan, with the aim of a UK PharmaScan record being created at least 3 years prior to marketing authorisation. Companies are expected to include plans and timings for regulatory submission routes in the information they provide and to update information if their plans change.
59. Information on patent expiry and potential timing of competition entry, such as for biosimilars or generics, is also vital and the NHS will seek clarity from companies on this.
60. Companies should ensure they are registered to use UK PharmaScan, have appointed leads for keeping the system up to date and respond to requests about horizon scanning information in a timely For further information about registering or using UK PharmaScan, please use www.ukpharmascan.org.uk/login or email contactus@ukpharmascan.org.uk.
61. UK PharmaScan is being further developed and enhanced to meet the evolving horizon scanning requirements of the system. This will ensure it remains the primary source of information for all relevant agencies to drive improved financial, clinical, assessment and service planning around the introduction of new medicines.
62. NHS England and NICE also acknowledge the vital role patient groups and organisations such as medical research charities and pharmacy play in horizon scanning across the product lifecycle.
63. NHS England and NICE will draw on all available information, including that requested through participation in clinical and commercial surgeries. Access to this accurate and timely information will enable rapid commercial decisions from us and facilitate commissioning as efficiently as possible.
64. NHS England supports the ongoing development of a single, shared approach to ensure accurate horizon scanning and will engage in the UK-wide cross-government working group to ensure our processes remain interconnected with emerging regulatory pathways, as set out in the Voluntary Scheme.
Getting in contact
65. Companies can contact both NHS England and NICE directly.
66. NHS England provides a single point of contact for all commercial queries but also a triage function to enable faster and more consistent engagement between the NHS and companies.
67. The triage function allows us to:
- respond to enquires and requests in a timely fashion, with helpful and relevant advice
- arrange a meeting or teleconference where appropriate
- signpost and redirect enquiries outside the MVA directorate when necessary
68. A summary of the way the triage function operates is:
- company sends enquiry or meeting request to the dedicated MVA inbox – the single point of contact: england.commercialmedicines@nhs.net
- NHS England acknowledges receipt
- NHS England triages the enquiry or request
- NHS England send the company a rapid response to its enquiry giving advice, signposts it to advice or organises a meeting
69. Typical outcomes for companies engaging with NHS England through the triage function are: resolution of their query, advice on who to engage with or the arrangement of a tailored commercial or clinical surgery arranged.
4. Routes to commissioning
70. As set out in Section 3, the main route for commissioning medicines is usually via a NICE technology evaluation. This is the standard route for medicines being recommended for use in the NHS in England. In very limited circumstances, medicines may go through other routes to become routinely commissioned in the NHS. These routes are described in full below but are also schematically summarised in Figure 2 below.
Regulatory approval and early engagement
71. The route to commissioning begins with companies providing horizon scanning information through UK PharmaScan. Companies can then engage early with NHS England and NICE, as set out Section 3.
72. NICE selects the topics for which it will produce technology evaluation guidance. NICE manages this process on behalf of the Department of Health and Social Care (DHSC). While it recommends the selected topics to the Secretary of State for Health and Social Care for formal referral, it can only begin to evaluate a technology once it has been formally referred to it. NICE provides further information on this process it is manual on NICE-wide topic prioritisation.
73. The Early Access to Medicines Scheme (EAMS) aims to give patients with life-threatening or seriously debilitating conditions access to medicines that do not yet have marketing authorisation, when there is a clear unmet clinical need. The EAMS operates within the current regulatory structure and is voluntary and non-statutory.
74. Aligned to the regulatory process, NICE (and equivalent bodies in the devolved administrations) will evaluate medicines for routine use that have been through the EAMS. Companies can use the evidence collected as part of the EAMS to support the evaluation.
Figure 2: Routes to commissioning new medicines or indications in the NHS in England*
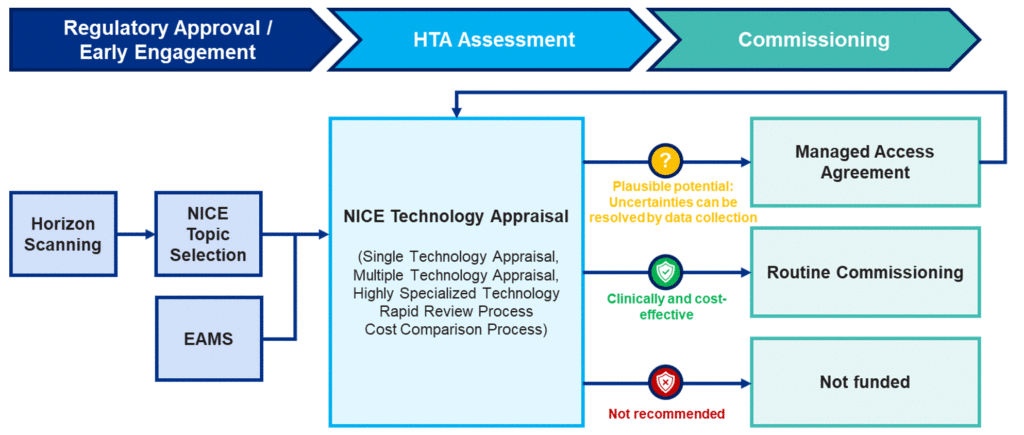
Evaluation
75. All new active substances in their first indication and extensions to their marketing authorisation to add a significant new therapeutic indication are expected to undergo an appropriate NICE evaluation unless there is a clear rationale not to do so. Medicines selected for NICE evaluation can be routed through one of the following processes:
- single technology appraisal (STA) – this technology evaluation assesses a single drug or treatment
- multiple technology appraisal (MTA) – this technology evaluation assesses several drugs or treatments used for one condition
- cost comparison process – shorter than the STA and does not formally use the technical engagement step
- rapid review process – can be requested within 16 weeks of the final guidance publication to consider new PAS or commercial access agreement proposals
- highly specialised technologies (HST) – this technology evaluation assesses a single drug or treatment for very rare conditions
Further information on the NICE technology evaluation processes can be found in the NICE health technology evaluations manual.
76. There may be instances where topics are not prioritised for a NICE technology evaluation or are off-label. In such circumstances, it will be for the relevant commissioning body (NHS England or individual ICBs) to consider developing a policy position on whether the treatment will be routinely commissioned or not. In the case of NHS England for specialised services, we will follow the published service development policy and methods for specialised commissioning.
77. Note that the framework does not advocate the use of off-label indications or suggest companies promote off-label use of their The Medicines and Healthcare Regulatory Agency (MHRA) provides further information.
Commissioning services
78. NHS England and ICBs (depending on which is the responsible commissioner) are legally obliged to fund and resource medicines recommended by NICE’s technology evaluations (the statutory funding requirement). This legal requirement is reaffirmed in the NHS Constitution, which states that patients have the right to drugs and treatments that NICE has recommended for use in the NHS, if their doctor believes they are clinically appropriate.
79. When NICE recommends a treatment for routine commissioning, the NHS must make sure it is available ‘as an option’ within 90 calendar days (30 calendar days for EAMS products) of its final guidance being published, unless otherwise specified in the guidance. This means that if a patient has a disease or condition and the patient and doctor responsible for their care thinks that the technology is the right treatment, it should be available for use as ‘an option’ alongside other potential treatments, in line with NICE’s recommendations.
80. In certain circumstances where there is outstanding clinical and consequently financial uncertainty, and these can be plausibly addressed by data collection, a managed access approach via a managed access agreement (MAA) may be appropriate. Any MAA must be formally agreed between us, NICE and the relevant company. If and when agreement is reached, commissioning in line with the terms of the MAA will start (interim funding for CDF and IMF medicines; otherwise, it will usually be within 90 days after final guidance). The statutory funding requirement does not apply to MAAs.
Further details on this and other commercial options are set out in Section 5.
81. Although the NHS commissions most licensed treatments available to it, some will not be available routinely as NICE has not recommended them as clinically and cost- effective and, therefore, they do not represent an appropriate use of NHS resources.
82. However, a clinician may believe that their patient’s clinical situation is so different from that of others with the same condition that they should have their treatment paid for when others would not. In such cases, NHS clinicians can ask the relevant commissioner (NHS England or an ICB) on behalf of a patient to fund a treatment that would not usually be provided by the NHS for that patient. Such a request is called an individual funding request (IFR).
5. Commercial options
83. This section sets out the commercial options that may be available to companies and that NHS England and NICE may pursue in particular circumstances. The fastest (and preferred) route to market in England is for companies to propose simple discounts via a PAS.
84. This section fulfils objective 3 of the framework: to clarify the commercial flexibilities that may be available to companies where appropriate. This will ensure that all companies – in particular, smaller and specialist ones with less experience – understand the full range of commercial options available to them.
85. The framework of potential commercial options:
- supports companies in presenting a value proposition to NICE that is considered clinically and cost-effective within the relevant threshold
- offers companies the opportunity to enter into complex and confidential agreements beyond a simple discount, under specific circumstances and where an enhanced value proposition is presented to NICE that is at or below the lower end of the relevant clinical and standard cost-effectiveness threshold range
- provides companies with a confidential commercial mechanism where NICE believes there is significant uncertainty surrounding the clinical and consequently cost-effectiveness of a treatment and where there is plausible potential for that treatment to be clinically and cost-effective
- supports companies and NHS England to work together to identify confidential commercial solutions for NHS affordability challenges that may arise from otherwise clinically- and cost-effective treatments
86. A single commercial solution may be needed to address more than one of the points set out The commercial options that can be considered fall into 4 broad categories:
- patient access schemes (PASs); simple and complex
- commercial access agreements (CAA)
- managed access agreements (MAA)
- budget impact schemes
Detail for each of these is given below.
Patient access schemes
87. PASs are the preferred option for companies to consider when developing their value proposition for evaluation by NICE. They provide a mechanism for companies to improve the cost-effectiveness of a treatment under evaluation beyond that driven by its list price.
88. Unless a treatment is to be considered by NICE at list price, companies should always include a PAS in their initial evidence submission to NICE, to ensure sufficient time for full consideration in advance of the NICE evaluation committee meeting.
89. NHS England has responsibility for agreeing PASs. However, it is important to note that our agreement to a PAS should not be seen as a willingness to pay at the proposed level of discount.
90. The NICE CLT advises NHS England on the feasibility of a PAS as set out in its Procedure for the review of patient access scheme proposals. This advice informs the final decision by NHS England on whether the proposed PAS can be considered as part of a NICE technology evaluation. It is for NICE to determine whether the level of discount being offered by the company represents a clinically and cost-effective use of NHS resources.
91. In addition to the principles underpinning all commercial activities set out in Section 2, companies should apply the following operational guidance to PAS:
- the full costs to the NHS of any such arrangements should be included in the costs considered by the NICE evaluation committee
- the PAS should be clinically robust, clinically plausible, appropriate and monitorable (for example, if it is a responder scheme, there must be a relatively straightforward way to measure a patient’s clinical response)
- any PAS should be operationally manageable for the NHS without unduly complex monitoring, disproportionate additional costs and bureaucracy. Any burden for the NHS should be proportionate to the benefits of the PAS for the NHS and patients. It is reasonable for NHS England to take operational management into account when considering the viability of individual PAS proposals, and we will likely give priority to PAS proposals that deliver the greatest benefits to patients (for example, by enabling the NHS to address a previously unmet need)
- clarity is also required on the exact duration of any agreement and the circumstances in which it might be terminated
- a PAS should be consistent with existing financial flows in the NHS and with commissioning arrangements (for example, payers must be able to calculate the effective price for their patient population, so the costs and savings accrue to those services making commissioning and treatment decisions)
- the NHS in England and Wales must be consulted on PAS proposals, and particularly where these involve data collection beyond that associated with the conventional purchase of medicines (for example, in relation to patient numbers, or the monitoring and recording of a patient’s condition over and above that for the normal management of a patient). The CLT process includes arrangements for consultation with the NHS
92. There are 2 types of PAS:
- simple PAS (confidential)
- complex PAS (transparent)
Simple patient access schemes
93. Simple PASs are confidential and provide a fixed price or percentage discount on the list price that is applied at source. These are always the preferred option, in line with principle 3 of the framework, as they require less monitoring by all parties and minimise the administrative burden on NHS organisations. They also ensure that where VAT is incurred it applies at the lowest level of the effective net price.
94. As the review for a simple PAS is more rapid than that for a complex PAS, both the NICE CLT’s advice and our approval are
Complex patient access schemes
95. A complex PAS is not confidential. By definition, it will involve a more complex reimbursement proposal (see Table 1) that, in turn, will be more complex to administer in the NHS. The requirement for transparency is to ensure the administrative burden and cost to the NHS of implementing such schemes is minimised, which in turn helps ensure the value of the treatment, as determined by NICE, is achieved.
96. If a company chooses to propose a complex scheme, it needs to justify this with a strong rationale for the complex scheme and give an indication of how the associated risks will be shared equitably between the NHS and the company. VAT consequences associated with the proposed scheme must also be accounted for in the proposal to ensure the full financial benefits of the scheme are realised by the NHS.
97. NICE CLT’s advice on the feasibility of implementing a proposed complex PAS will inevitably be a more involved process, including consultation with the NHS and a feasibility review of commercial arrangements to ensure the full benefit can be realised.
Potential timing of proposals for patient access schemes
98. While PASs are intended to help secure access for NHS patients to medicines that might otherwise not be deemed cost-effective, it is important that arrangements for proposing and agreeing PASs do not jeopardise the timeliness of NICE evaluation guidance, which is in itself designed to provide guidance to the NHS on the cost-effective provision of treatment to patients.
99. Where companies choose to bring forward PAS proposals in the context of a NICE evaluation, they should do so at 1 of 2 stages in the process:
- at the outset, when making their initial evidence submission to NICE. This implies that any submission to NHS England should precede the company’s submission to NICE
- at the end of the evaluation process, once any appeals have been heard and NICE’s final guidance has been issued to the NHS, under the rapid review facility
100. In exceptional circumstances, a PAS proposal assessed as meeting the simple discount criteria may be accepted at additional times in the NICE process, but this is not be possible for those that do not.
101. Where a company submits a PAS proposal through the rapid review facility following an MTA, other companies whose products were evaluated in the same evaluation will also have a single opportunity to propose a PAS.
Transparency
102. Commercial confidentiality of all PAS proposals will be respected while they are being considered by NHS England and NICE CLT. However, the arrangements applicable at the relevant time for consultation and disclosure will apply to PAS proposals when they are considered as part of the NICE evaluation process. In particular, when NICE consults on draft evaluation guidance, this must provide stakeholders with an opportunity to comment on the full content of the evaluation, including information on any proposed PAS.
103. The decision on whether to submit PAS proposals is a commercial decision for the company concerned. It is not therefore appropriate, where a company makes a PAS proposal, for NHS England or NICE to issue an alert to other company stakeholders before a PAS proposal is referred to NICE for consideration as part of a technology evaluation.
104. PAS proposals submitted through the rapid review facility will remain confidential until such time as NICE needs to consult, as set out in NICE’s health technology evaluation manual.
105. The details of PASs agreed with the purchasing authority in one UK country will be made available on a confidential basis to purchasing authorities in the other UK countries. Where a discount level as part of a simple discount PAS is not published in final NICE guidance, the NHS must have access to the discount price so providers and commissioners can properly account for the PAS. Where agreed, this will be within appropriate arrangements to safeguard confidentiality.
106. In publishing guidance, NICE must be satisfied that the information it can communicate to stakeholders is sufficient to explain an evaluation In this regard, what constitutes a sufficient level of transparency is a matter for NICE to determine in developing its guidance.
107. It is important that these arrangements do not have a perverse effect on the evaluation of other technologies. Where a medicine recommended by NICE with a PAS is used as a comparator for the evaluation of another treatment, NICE must be able to make relevant details of that PAS available to relevant consultees and commentators.
Arrangements offered outside or prior to NICE evaluations
108. Provided no MVA framework, contract or national rebate mechanism exists for a medicine, then companies may continue to offer local discounts or other arrangements to the local NHS outside NICE evaluations; but decisions on whether to participate in such arrangements and the terms on which they are offered are matters for the company and the local NHS. Only a scheme that is included in final NICE evaluation guidance should be described as a PAS. A different term should be used for arrangements offered outside NICE evaluations.
Monitoring and review of operational PASs
109. To maintain confidence in PASs, realisation of their full value to patients and the NHS is essential, and their individual and cumulative impact on the NHS must remain manageable.
110. When submitting a proposal for a new PAS, companies should set out information on its expected level(s) of uptake, and the data they would share with NHS England, NICE and appropriate NHS bodies to enable periodic monitoring of the PAS’s operation (including in relation to uptake). The expectation is that companies will make such information available.
111. Where periodic monitoring indicates problems with the operation of a PAS, including greater than expected administrative burdens or under-delivery of benefits, the company should work with the NHS to urgently identify the underlying causes and, in discussion with NHS England – and where appropriate NICE – make proposals to address them. These may include measures that are consistent with existing financial flows to ensure that the NHS realises the full expected financial benefits of the PAS.
112. NICE’s health technology evaluations manual gives a timeframe in which NICE will consider whether to review the guidance. Where guidance includes a PAS, this provides an opportunity to review how the PAS is operating and consider whether it would be appropriate to make any changes to the PAS to simplify and improve its operation. Any changes to an operational PAS are subject to discussion with and agreement by NHS England.
113. The expectation is that an operational PAS will remain in place for the lifetime of the relevant NICE guidance, and that normally there should be no changes that fundamentally alter the nature of the PAS once it is operational. Where significant changes to an operational PAS are proposed (for example, change of scheme type or extension to a new indication), a submission will need to be made to NHS England, for consideration in the same way as for a new PAS proposal.
114. PASs extant as of 31 December 2023 are maintained in accordance with their terms as per the 2019 Voluntary Scheme for Branded Medicines Pricing and Access (VPAS).
Commercial access agreements
115. CAAs are confidential. In line with the principles set out in Section 2, CAAs are at the discretion of NHS England, with the default arrangement of offering a PAS (simple or complex) always being available to companies.
116. NHS England encourages companies to engage early on commercial options, particularly where they believe they will require a CAA, to mitigate delays later in the evaluation Early discussions through commercial surgeries, as set out in Section 3, can allow for discussions on the commercial case including any data requirements and provide an initial steer on the potential flexibilities available.
117. Table 1 gives example formats for CAAs (or complex PASs) but each CAA will be considered on a case-by-case basis.
Table 1: Example formats for CAA arrangements or complex PAS*
| Scheme type | Description |
|---|---|
| Budget cap | Maximum budget impact for a product (or products) beyond which a central rebate is payable. |
| Price/volume agreement | Price agreed for set volume of patients and then reductions staged based on additional patient numbers, or company pays back the full amount (similar to budget cap). |
| Cost sharing | Company funds initial cost of therapies such as by offering the first month for free. |
| Stop/start criteria | Rules on eligibility criteria for when patients should start and stop therapy. |
| Outcomes-based agreement and payment by results | Discount or rebate applied if a product does not perform as expected or for non-responders. |
| Indication-specific pricing | Differential prices for a given medicine based on the differences in clinical and cost-effectiveness across indications. |
* Note: This list is not exhaustive and a combination of schemes can be applied. The difference between a CCA and complex PAS relates to the transparency of the agreement.
118. Some specific circumstances may make the launch of a product particularly challenging or commercially unviable on the basis of a PAS alone. NHS England will consider a CAA in these circumstances where the company proposes an enhanced value offer aligned to principle 4 of the framework and consistent with paragraphs 3.29 and 3.30 of the Voluntary Scheme:
“The NHS England Commercial Framework for New Medicines sets out the purpose and principles on which NHS commercial medicines activities are based. This includes clarification of commercial flexibilities that may be available to companies where deemed appropriate by NHS England, reserved for companies aspiring to deliver greater health gain relative to cost.” (paragraph 3.29)
“The arrangements detailed in paragraph 3.29 would normally correspond to medicines that would be expected to have value propositions at or below the lower end of the standard NICE cost effectiveness threshold range, with greater flexibilities made available for value propositions at even greater levels of cost effectiveness, plus any applicable QALY weighting.” (paragraph 3.30)
Indication-specific pricing
119. Increasingly, new medicines are found to provide clinical benefits to different groups of patients (indications) and this can be the case across several therapeutic areas. Medicines can show differing levels of clinical effectiveness across these different indications and as a result, in evaluation, the cost-effective price across indications for the same medicine can vary.
120. As an example of the circumstances referenced in paragraph 118, the preference for a simple PAS, with a uniform price across all indications, could make it commercially unviable for companies to launch new indications in some cases, particularly smaller indications, which require a larger discount to be cost-effective.
121. As an alternative to uniform pricing, indication-specific pricing allows for the price of a medicine to reflect the differences in clinical and cost-effectiveness across indications.
122. In line with principle 5 of this framework, indication-specific pricing will be considered when each of the following criteria are met:
- The medicine for the indication under consideration meets an unmet clinical need:
- Medicines can meet an unmet clinical need by providing a therapeutic benefit over existing treatment options, for example greater efficacy or fewer side effects, or through demonstrable health system productivity benefit enabling more patients to be treated, for example by requiring fewer healthcare interactions or a less time-intensive mode of administration.
- The company can demonstrate with a high degree of confidence that uniform pricing would reduce the total revenue for a medicine across all indications:
- Companies should present a case for revenue loss to NHS England and share their indication-specific volume forecasts (generally over a period of 3 years) and anticipated cost-effective price for each indication at the earliest appropriate opportunity for validation and assessment.
- The revenue forecast should be limited to the indication under consideration and any existing indications in routine commissioning. Revenue from existing indications in managed access or potential future indications can be considered in circumstances where there is a high degree of confidence of regulatory approval, of a positive NICE recommendation, of forecast volumes, and of price.
- Sufficient data is available within existing NHS systems to make such arrangements operationally feasible:
- High quality, complete data is needed to monitor how many patients are treated across each of the different indications for a given medicine. NHS England’s prior approval system data will support the transaction of indication-specific pricing arrangements.
- Where prior approval system data is not available, NHS England will consider alternative data sources where they are proven to be of comparable quality and completeness to prior approval system data, and where the data can be provided to NHS England in a timely manner to transact indication-specific pricing arrangements.
- The cost-effective price is highly differentiated for all indications under consideration:
- Where the level of differentiation between indications is deemed to be low, companies will be expected to maintain a uniform price across all indications.
123. Where an indication-specific arrangement is agreed for a medicine, the indication-specific price under this arrangement will then be the price used in any subsequent evaluations for the same indication where the medicine is used as a comparator.
124. There may be specific circumstances beyond those considered here that may justify bespoke commercial flexibilities, and these will be looked at on a case-by-case basis.
Managed access agreements
125. There are situations where uncertainty exacerbates the challenge for NICE in its technology evaluations. In general, there are 2 main sources of outstanding uncertainty at the time of evaluation:
- clinical uncertainty
- (and as a consequence) financial uncertainty
126. Where such uncertainty exists, NICE can recommend that NHS England and the company explore an MAA. This will only happen when there is plausible potential for a medicine to satisfy the criteria for routine commissioning but uncertainty surrounds the clinical data and consequently the cost-effectiveness estimates on which to make such a recommendation.
127. MAAs have 2 key components:
- a data collection agreement to mitigate clinical uncertainty (as defined by the NICE evaluation committee)
- either a commercial access agreement or a PAS (simple or complex)
128. MAAs are an interim commissioning position with a future date committed to for re-evaluation, which may result in routine commissioning; they are therefore time-limited.
129. MAAs require the company’s agreement to offer the treatment at a cost-effective price for the duration of the MAA. Exit clauses are in place in place as part of each MAA, including the obligation to maintain funding and existing patient access should any reassessment result in a negative decision. It should be noted (as with the CDF and IMF) that it is possible when exiting the MAA for the price of the product to be higher or lower, depending on the re-evaluation outcome.
130. To date, MAAs have been most frequently used in the context of the CDF and for NICE HST guidance where very small patient numbers can mean significant uncertainty in the clinical evidence being presented. This is not an exclusive position and NICE may recommend their use in a broader set of circumstances.
131. One of the key constraints to overcome when considering the possibility of a MAA is whether data collection has the potential to collect data on the relevant health outcomes and resolve the uncertainties.
132. Further detailed information is available in relation to the operation of the CDF and IMF, and managed access more generally.
Budget impact schemes
133. A technology that NICE evaluates as cost-effective may still have a high budget impact.
134. The BIT helps manage the impact of introduction of NICE recommended medicines on wider NHS services. For technologies with a projected net budget impact that exceeds £40 million in any of the first 3 years of implementation, NHS England can engage in commercial discussions with the company to mitigate the affordability challenge of immediately funding the technology for other NHS services.
135. The degree of additional value expected from application of the BIT will take into account 2 main dimensions:
- overall cost impact to the NHS in each of the first 3 years
- any likely direct competition to or external impact on that market that may mitigate any spend by the NHS
136. NHS England can also apply to NICE to vary the statutory funding requirement, allowing increased time to implement a new technology recommendation and therefore delaying the cost implications.
Combination therapies
137. Combination therapies combine 2 or more component medicines into a single treatment. Combination therapies can comprise of one or more backbone medicines and one or more add-on medicine, where:
- The backbone medicine, or medicines, is already available to NHS patients as a standalone medicine, prior to it being used in a combination
- The add-on medicine, or medicines, is added to an existing backbone medicine to create a combination therapy. The add-on medicine may also be available as a standalone medicine or may have been developed specifically to be used in a combination therapy
138. Using drugs in combination, rather than as monotherapies, has the potential to provide additional clinical benefits for patients, and NHS England recognises the growing importance of these therapies in many therapy areas.
139. However, where the components of the combination therapy are provided by different companies, a pricing discussion between the companies is required, potentially creating competition law concerns.
140. The Competition and Markets Authority (CMA), as the sole competent authority for the UK, has published a prioritisation statement on combination therapies following engagement with NHS England, NICE and the Association of the British Pharmaceutical Industry (ABPI).
141. The CMA’s statement on combination therapies sets out that “the CMA will not prioritise the investigation of commercial negotiations and any subsequent agreements that are carried out according to the negotiation framework, where particular market features are present and provided certain conditions are met.” The market features and conditions referenced are stated in paragraphs 2.7, 2.9 and 5.2 of this statement.
142. Under the negotiation framework set out in the CMA statement, the add-on medicine company will submit information to NICE for the evaluation of the combination therapy. The companies supplying the component medicines of the combination can then negotiate a ‘contribution payment’, paid by the backbone medicine company to the add-on medicine company as compensation for its supply of the add-on medicines at a price that achieves cost-effectiveness for the combination therapy. The price for the backbone medicine in other indications remains unchanged.
143. NHS England and NICE support the CMA’s statement and will engage with companies in accordance with the negotiation framework to enable patient access to health improving combination therapies on the basis of this statement.
144. Companies are encouraged to reach a commercial agreement using data sources already available to them. Where this is not possible, NHS England will consider requests for data from companies that already have combination therapies in the NICE evaluation process.
145. On company request, NHS England will provide companies supplying components of a given combination therapy with data from the NHS England prior approval system on the number of new approvals, but no more frequently than on a quarterly basis. NHS England does not have access to this data for ICB or primary care commissioned drugs and indications.
146. Companies can request access to the Systemic Anti-Cancer Therapy (SACT) dataset via the Data Access Request Service for a fee. This allows companies to request record-level data for specific drug administrations from SACT data.
147. To maintain patient confidentiality, any data values for under 10 patients will be supressed from these data sources.
148. Data on a medicine provided by a company that is not party to the combination therapy agreement and data from commercially sensitive datasets, such as the minimum dataset or Pharmex data, will not be provided as per principle 6 of the framework.
149. Companies can also access Secondary Care Medicines Data (SCMD) at a product level nationally, as the finalised data is published by the NHS Business Services Authority on an annual basis.
150. Requests for data that is required for commercial agreements for combination therapies should be directed to the MVA triage function at NHS England, as set out in Section 3.
Escalation
151. If, in commercial discussion between NHS England and a company under the terms of this framework, NHS England or the company considers a failure to reach agreement could be escalated to reach a mutually acceptable commercial arrangement, either NHS England or the company may escalate the disagreement as described below.
152. Initially, the level 1 negotiators (for us, the Director of the Medicines Negotiation and Managed Access team; and for the company, the most senior national manager or deputy with knowledge of the escalation issue) meet within 14 calendar days to discuss the escalation issue and seek agreement if possible, provided that the discussions (and all and any subsequent levels of escalation) are strictly limited to this escalation issue.
153. If no agreement is reached at level 1, NHS England or the company may refer the escalation issue to the level 2 negotiators (for us, the Medicines Value and Access Director; and for the company a senior independent company representative, for example at global level) not previously involved in the commercial discussion.
154. Level 2 negotiators meet within 14 calendar days to discuss the unresolved escalation issue and seek agreement if possible.
155. If NHS England cannot reach agreement with the company following the meeting of the level 2 negotiators, the relevant commercial discussion will be treated as having come to an end.
Reporting
156. NICE’s CLT publish data on the number of active simple PASs, complex PASs, CAAs and MAAs by technology evaluation.
6. Updating the framework
157. The framework is a national strategy document setting out the NHS’s approach for commercial activity in relation to new medicines. NHS England envisages that the framework will be discussed as part of the Voluntary Scheme operational review meetings between NHS England, the DHSC and the ABPI.
158. NHS England believes that it is essential that the framework has the stability of a long-term strategy but remains up-to-date to reflect key developments that impact on its implementation.
159. As per the VPAG agreement, NHS England will launch a further consultation on the framework in 2025, to align with updated regulatory and access pathways, ensuring good connectivity with value assessment and commercial processes.
References
The NHS Commercial Framework for New Medicines has been informed by the following:
- Competition and Markets Authority (2023): Prioritisation statement on combination therapies
- Department of Health and Social Care (2023) The 2024 Voluntary Scheme for Branded Medicines Pricing, Access and Growth
- Department of Health and Social Care (2018) The 2019 Voluntary Scheme for Branded Medicines Pricing and Access
- The National Archives (2000) Freedom of Information Act 2000
- National Institute for Health and Care Excellence (2022) NICE health technology evaluations: the manual
- National Institute for Health and Care Excellence (2018) Procedure for the review of patient access scheme proposals
- NHS England (2023) Prescribed specialised services manual
Acknowledgements
NHS England is grateful for the input and support from health system partners (NICE, DHSC and the Office for Life Sciences) and the industry trade bodies (Association of the British Pharmaceutical Industry, the Ethical Medicines Group, the BioIndustry Association and the British Generic Manufacturers Association) in developing the framework.
Publication reference: PRN01610

