1. Service background
1.1 The NHS Long Term Plan (LTP) Chapter 2 highlights the importance of NHS services complementing the action taken by local government to support the commissioning of sexual health services. The LTP also facilitates exploration of the future commissioning arrangements to widen access and create capacity where it is needed.
1.2 A Public Health England resource for commissioners (March 2019) also highlights the role community pharmacy can play in supporting ongoing contraception.
1.3 In response to this, and in line with the Community Pharmacy Contractual Framework (CPCF) 2019-2024 commitment to “test a range of prevention services’, a pharmacy contraception service has been designed.
1.4 This service specification covers initiation of oral contraception (OC) and routine monitoring and ongoing supply of OC via a patient group direction (PGD).
1.5 The aim of the Pharmacy Contraception Service (PCS) is to offer greater choice from where people can access contraception services and create additional capacity in primary care and sexual health clinics (or equivalent) to support meeting the demand for more complex assessments.
1.6 This service will support the important role community pharmacy teams can play to help address health inequalities by providing wider healthcare access in their communities and signposting service users to local sexual health services in line with NICE guideline NG 102.
1.7 The Delivery Plan for recovering access in primary care (May 2023) highlighted the ambition to expand the PCS to increase access to and convenience of contraception services in line with the Government’s Women’s Health Strategy for England (August 2022) that flagged community pharmacy had a part to play in increasing choice in the ways people in can access contraception.
2. Service objectives
2.1 The objectives of the service are:
- To provide a model for community pharmacy teams to initiate provision of OC, and to continue the provision of OC supplies initiated in primary care (including general practice and pharmacies) or sexual health clinics and equivalent. Both initiation and ongoing supply will be undertaken using PGDs to support the review and supply process.
- To establish an integrated pathway between existing services and community pharmacies that provides people with greater choice and access when considering starting or continuing their current form of OC.
3. Requirements for service provision
3.1 Prior to provision of the service, the pharmacy contractor must:
- Be satisfactorily complying with their obligations under Schedule 4 of the NHS (pharmaceutical and local pharmaceutical services) Regulations (Terms of Service of NHS pharmacists) in respect of the provision of Essential services and an acceptable system of clinical governance
- Notify NHS England that they intend to provide the service by completion of an electronic registration declaration through the NHS Business Services Authority (NHSBSA) Manage Your Service (MYS) portal.
3.2 The pharmacy contractor must offer consultations for both initiation of OC and for ongoing supply of OC.
3.3 For the purposes of this specification the following definitions will apply:
- Initiation: where a person wishes to start OC for the first time or needs to restart OC following a pill free break. A person who is being switched to an alternative pill following consultation can also be considered as an initiation.
- Ongoing supply: where a person has been supplied with OC by a primary care provider (including general practice and pharmacies) or a sexual health clinic (or equivalent) and a subsequent equivalent supply is needed. Their current supply of OC should still be in use.
3.4 The service should be provided by suitably trained and competent pharmacy staff. For the rest of this document, the term “pharmacy staff” will be used to denote pharmacists, pharmacy technicians and other non-registered members of the pharmacy team. The responsible pharmacist must ensure that delegated tasks are being undertaken safely by competent pharmacy staff. Supply under a PGD must be undertaken by a pharmacist.
3.5 The pharmacy contractor must have a standard operating procedure (SOP) in place covering the provision of the service. The SOP must include the process for escalation of issues identified, signposting details, equipment maintenance and validation, and staff training.
3.6 The pharmacy contractor must ensure that all pharmacy staff involved in the provision of the service, are familiar with and adhere to the SOP. The SOP should be reviewed regularly, including following any significant incident or change to the service.
3.7 Pharmacists delivering the service must have completed one of the recommended Safeguarding level 3 training materials (see 5.3) or have direct access to professional advice from someone who can advise on Safeguarding at Level 3.
3.8 Pharmacies must have a consultation room that will be used for the provision of the service which meets the requirements of the terms of service. Where a face-to-face consultation is the preferred access model for the person, these consultations must be delivered from the consultation room at the pharmacy.
3.9 Remote consultations are also permitted to be used to provide the service where assessed as clinically appropriate by the pharmacist. When undertaking remote consultations, the contractor must ensure that there are arrangements in place at the pharmacy which enable staff to communicate confidentially with the person receiving the service by telephone or another live audio link or a live video link. NHS Guidance to support community pharmacy teams can help to plan for this.
Equipment
3.10 Where blood pressure measurements are performed within the pharmacy (see 4.16) the pharmacy contractor must use equipment that is validated by the British and Irish Hypertension Society (BIHsoc), as recommended by NICE, to measure a person’s blood pressure. The clinic blood pressure monitor used must be listed on one of the following lists:
- BIHsoc – Validated blood pressure monitors for home or
- BIHsoc – Validated blood pressure monitors for specialist use.
3.11 The pharmacy contractor must have appropriate equipment to measure a person’s weight and height. The NHS website provides an online BMI calculator.
3.12 Contractors must utilise an IT solution that meets the minimum digital requirements of the service (as specified within the Community Pharmacy Clinical Services Standard) and that includes an application programming interface (API) to facilitate transfer of data into the NHSBSA MYS portal to support the PCS. This will ensure that data is captured consistently, that all elements of the service are completed where appropriate, and the contractor is correctly reimbursed.
4. Service description
4.1 The pharmacy contractor must ensure the service is accessible, appropriate, and sensitive to the needs of all service users. No eligible person shall be excluded or experience particular difficulty in accessing and effectively using this service due to their race, gender, disability, sexual orientation, religion or belief, gender reassignment, marriage or civil partnership status, pregnancy or maternity, or age.
4.2 People will access the service by one of the following routes:
- Identified as clinically suitable by the community pharmacist and accept the offer of the service;
- Self-refer to a community pharmacy;
- Referred by their general practice;
- Referred from a sexual health clinic (or equivalent); or
- Referred from other NHS service providers, e.g., urgent treatment centres or
NHS 111.
N.B. for the purposes of this service, a referral includes active signposting to attend the pharmacy to receive the service.
4.3 When a person attends the pharmacy to collect an NHS repeat prescription for OC, the service can be highlighted to them to consider when they need their next supply.
4.4 When a person attends the pharmacy or makes contact for other services, e.g., to access a supply of Emergency Contraception (EC), the service can be highlighted to them to consider their ongoing contraception requirements.
4.5 A member of the pharmacy team will agree with a referred person, the date and time of their consultation.
Inclusion criteria
4.6 To be eligible to access this service a person must:
- Be an individual seeking to be initiated on an OC, or seeking to obtain a further supply of their ongoing OC:
- Combined Oral Contraceptive (COC) – from menarche up to and including 49 years of age; or
- Progestogen Only Pill (POP) – from menarche up to and including 54 years of age.
Exclusion criteria
4.7 A person will not be eligible for this service if:
- They are considered clinically unsuitable, or are excluded for supply of OC according to the PGD protocols, including, but not limited to:
- Individuals under 16 years of age and assessed as not competent using Fraser Guidelines
- Individuals 16 years of age and over and assessed as lacking capacity to consent
Consultation
4.8 The pharmacy must respond to anybody requesting a supply of OC as soon as is reasonably possible. Following discussion, if the pharmacy is unable to offer a consultation within the time needed to meet the person’s contraception need, they should be signposted to an alternative pharmacy or other service for a
consultation.
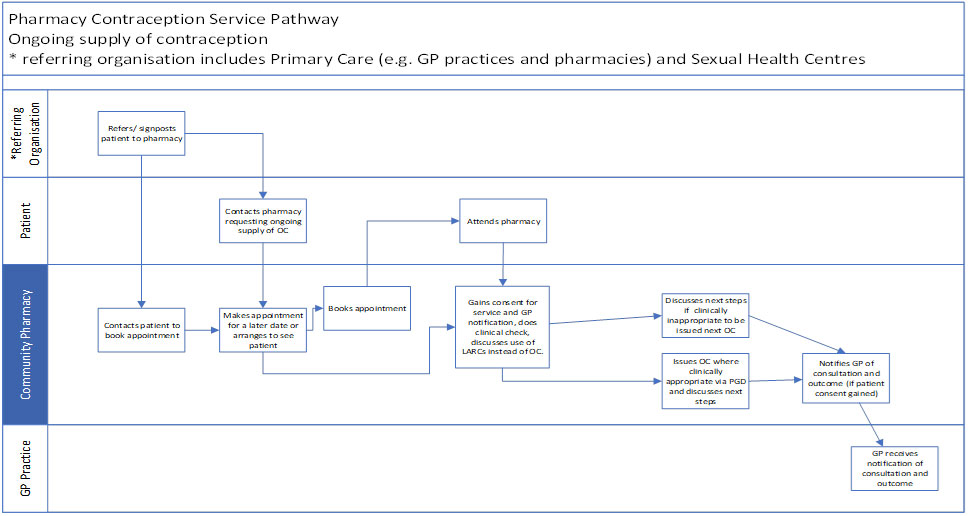
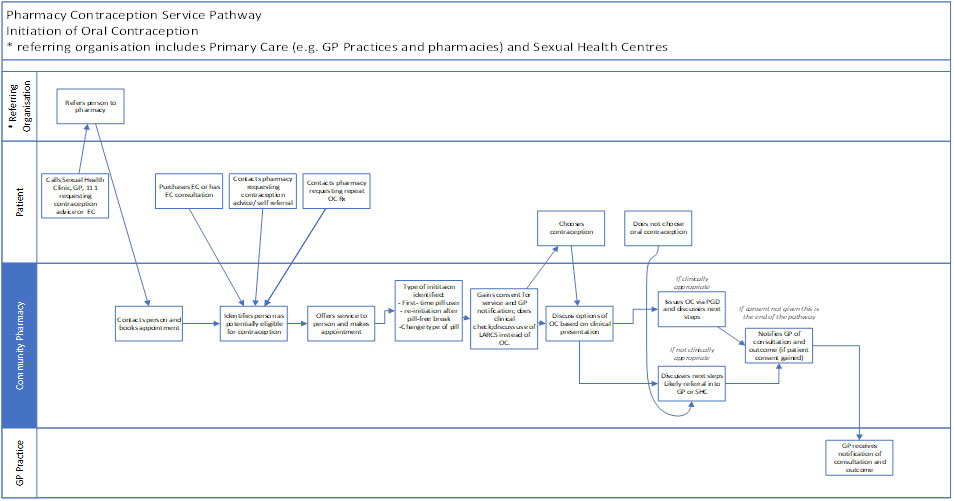
4.9 Please refer to Annex A for flow diagrams describing the service.
4.10 Verbal consent to receive the service must be sought from the person and recorded in the pharmacy’s clinical record for the service.
4.11 If the person provides consent to share the outcome of the consultation with their general practice, information relating to the consultation set out in Annex B will be shared with the person’s general practice. However, if the person does not consent to sharing information with their general practice or they are not registered with a general practice, the consultation can still proceed, and a notification to the practice will not need to be sent.
4.12 The person should also be advised of the following information sharing that will take place:
- The sharing of information about the service with NHS England as part of the service monitoring and evaluation; and
- The sharing of information about the service with the NHSBSA and NHS England for the purpose of contract management and as part of post-payment verification (PPV).
4.13 The clinical appropriateness of a supply of OC will be determined by the pharmacist, as part of a consultation with the person, following the guidelines in the PGDs..
4.14 During the consultation, if the pharmacist is concerned about a potential safeguarding issue, then appropriate action should be taken, where necessary, in line with local safeguarding processes.
4.15 Either party may request or offer a chaperone to be present during the consultation.
4.16 The consultation must include a conversation with the person regarding alternative and more effective forms of contraception, e.g., Long-acting reversible contraception.
4.17 Irrespective of the outcome of the consultation, it may be appropriate to also signpost the person accessing the service to another healthcare provider. In some cases, a person may require urgent escalation to another healthcare setting
4.18 For COC, a supply will require BMI and a blood pressure measurement to be taken in line with NICE guideline 136. A person accessing the service may also offer their own weight, height and blood pressure measurements. Any self-reported measurements that the pharmacist has deemed clinically suitable, will need to be recorded as such.
Outcomes and next steps
4.19 If the assessment criteria are met, supply of an OC can be made.
4.20 On initiation, the quantity of OC supplied should not exceed 3 months.
4.21 Following initiation, ongoing supplies of an OC can be made of up to 12 months duration. Unless there are reasons not to, such a duration of supply should be considered in line with the Faculty of Sexual and Reproductive Healthcare (FSRH) guidance. Restricting the length of supply could result in unwanted discontinuation of the method and an increased risk of pregnancy for the person.
4.22 Where a person is initiated on an OC, pharmacists should use their professional discretion as to the appropriate choice of product, from those included in the PGD. To help protect NHS resources, wherever practicable, pharmacy contractors should supply the best value product to meet the clinical need of the patient. Local formularies/restrictions should also be referred to and followed accordingly. Please refer to your local integrated care board (ICB) formulary for further information.
4.23 Ongoing supplies should be made in line with the person’s previous supply, e.g., in the instance that a branded product has been supplied for clinical reasons such as an allergy to product constituents, the ongoing supply should be made from an equivalent brand/generic equivalent of OC, that follows any medicines formulary requirements of the local ICB.
4.24 If a supply of an OC is not deemed clinically appropriate, the pharmacist should explain why this is the case to the person and refer them to their general practice or sexual health clinic (or equivalent).
4.25 The pharmacy is required to report any patient safety incidents in line with the Clinical Governance Approved Particulars for Pharmacies.
5. Clinical skills and knowledge
Competency requirements
5.1 Before commencement of the service, the pharmacy contractor must ensure that pharmacists and pharmacy staff providing the service are competent to do so in line with the specific skills and knowledge in paragraph 5.3 and with the relevant PGDs. This may involve completion of training.
Competency evidence
5.2 The pharmacy contractor must keep documentary evidence that pharmacy staff involved in the provision of the service are competent and remain up to date with regards to the specific skills and knowledge that are appropriate to their role, and to the aspects of the service they are delivering.
Recommended training modules
5.3 To deliver this service, the pharmacist should have evidence of competence in the clinical skills and knowledge required to deliver all aspects of the service. The appropriate clinical skills and knowledge are covered in the following training modules on the Centre for Pharmacy Postgraduate Education (CPPE) and/or the NHS England e-learning for healthcare (elfh) websites:
NB – packages that are highly recommended are indicated by an asterisk *
Safeguarding (see 3.7)
- Safeguarding Level 3 – – Safeguarding Children and Adults Level 3 for Community Pharmacists – video on elfh
or
- Safeguarding Level 3 Learning for Healthcare Safeguarding Children and Young People (SGC) – Safeguarding Children Level 3
Ongoing supply
- * CPPE Emergency contraception e-learning including emergency contraception e-assessment
- * CPPE Contraception e-learning including contraception e-assessment or the following four subsections of * module 3 – Contraceptive choices of the FSRH sexual and reproductive health e-learning (e-SRH) on elfh:
- 03_01: Mechanism of action, effectiveness and UKMEC
- 03_02: Choosing contraceptive methods
- 03_03: Combined hormonal contraception
- 03_04: Progestogen only methods (oral and injectable).
- * CPPE Sexual health in pharmacies e- learning and e-assessment or the following four subsections of * module 9 – STIs of the FSRH e-SRH on elfh:
- 09_01: Epidemiology and transmission of STIs
- 09_02: Sexually transmitted infection (STI) testing
- 09_03: STI management
- 09_04: Partner notification.
- and one subsection in the External resources module of the Sexual Health (PWP) e-learning on elfh:
- FSRH – Contraception counselling eLearning.
Initiation
- The following subsections of Module 2 of FSRH e-SRH on elfh :
- 02_01 Health history and risk assessment
- 02_02 Confidentiality, chaperones, and consent
- The following subsection of Module 3 of the FSRH e-SRH on elfh:
- * 03_07 Barrier contraceptives
- The following subsections of Module 5 of the FSRH e-SRH on elfh:
- 05_01 Managing bleeding problems in women using contraceptives
- 05_02 Managing contraceptive side-effects
- 05_03 Managing side-effects and complications of IUD and IUS
Other training to support clinical practice:
- CPPE Documenting in patient clinical records e-learning
- CPPE Remote consultation skills e-learning
- PGD e-learning on elfh
- CPPE consultation skills for pharmacy practice: taking a person-centred approach and e-assessment
5.4 Pharmacists must be familiar with at least one online shared decision-making contraception consultation tool. These tools will be used to support the pharmacist and should be shared with people to support their decision making. Examples are:
6. Data and information management
6.1 The pharmacy contractor must maintain appropriate records to ensure effective ongoing service delivery. The minimum requirements for the information which should be included in a contractor’s clinical record for the service are the mandatory sections indicated within the dataset which is set out in Annex B.
6.2 Where the patient consents, the pharmacy contractor will ensure that a notification of the provision of the service is sent to the patient’s general practice on the day of provision or on the following working day. A copy of the paperwork should be sent or emailed (via secure email) to the general practice.
6.3 Subject to patient consent, the information which must be sent to the patient’s general practice is set out in Annex B.
6.4 The data which is submitted to the MYS portal via the API will be used by the NHSBSA for payment and post-payment verification purposes. Some of this data, which has been anonymised, will be shared with NHS England for service evaluation and research purposes.
6.5 Records of the reimbursement data reported to the NHSBSA’s MYS portal should be retained for 3 years for PPV purposes.
6.6 All relevant records must be managed in line with the Records Management Code of Practice for Health and Social Care.
7. Payment arrangements
7.1 Data to populate a payment claim for this service will automatically be added to the MYS portal using the API between the approved service IT system and the NHSBSA. Contractors will need to submit the claim within the MYS portal, as part of the normal month end claims process.
7.2 Pharmacies providing this service will be eligible for the payments detailed in the Drug Tariff determination.
7.3 Reimbursement will be paid on the condition that the pharmacy has provided the service in accordance with the service specification.
7.4 If the pharmacy contractor is commissioned to deliver any related services e.g., the advanced Hypertension Case-Finding Service (incorporating BP clinic measurement), the contractor may not claim twice for the same activity.
7.5 The product price for the OC supplied will be reimbursed in accordance with the Drug Tariff determination. Any purchase margin by pharmacies relating to contraceptives supplied as part of this service would be included in the calculation of allowed purchase margin that forms a part of agreed NHS pharmacy funding.
7.6 Where a price concession has been granted for specific strengths of a product, this concession will apply to those specific strengths of products supplied as part of this service. Concessions will only apply to the month in which they are granted according to the usual Drug Tariff arrangements.
7.7 Prescription charges do not apply to products supplied in the provision of this service and an appropriate patient declaration is not required.
7.8 Claims for payment should be submitted within one month of, and no later than three months from the claim period for the chargeable activity provided. Claims which relate to work completed more than three months after the claim period in question, will not be paid.
8. Withdrawal from the service
8.1 If the pharmacy contractor wishes to stop providing the service, they must notify the Commissioner that they are no longer going to provide the service via the MYS portal, giving at least one month’s notice prior to the cessation of the service. Contractors may be asked for a reason as to why they wish to stop providing the service.
8.2 If the pharmacy contractor de-registers from the service or ceases trading within 30 days of registration, they will not qualify for the £400 set up fee. In this event, if the £400 fee has already been paid to the contractor, this money will be claimed back subject to contractor agreement.
9. Monitoring and post payment verification
Monitoring
9.1 In addition to meeting the Essential services requirements, the pharmacy contractor shall ensure the pharmacy has the following and that these are available for inspection should the local NHS England or integrated care system (ICS) primary care commissioning team undertake a site visit:
- A working and appropriately calibrated blood pressure monitor (see section 3.10).
- Sexual health promotional media or evidence of an ability to signpost.
- A suitable quantity of OC products to enable efficient and direct supply to the person attending and ensure continuation of supply.
Post payment verification
9.2 NHS England has a duty to be assured that where contractors make claims for payment for set up fees or activity in services, that they meet all the specified requirements of the service. NHS England will work with the NHSBSA Provider Assurance Team to undertake pre- and post-payment verification checks on claims made.
9.3 Additional evidence may be requested directly from contractors. The verification checks include comparing the information provided by contractors in their claims against datasets and evidence sources that are available to the NHSBSA Provider Assurance Team.
9.4 It is the contractor’s responsibility to be able to provide evidence of claims when requested by the NHSBSA for post-payment verification.
9.5 In cases where evidence is not available or does not demonstrate that the service activity was delivered, and so these claims cannot be verified, they may be referred to the Pharmaceutical Services Regulations Committee (PRSC) to decide whether an overpayment has been made.
9.6 In such cases, where the PSRC decides that an overpayment has been made, and will need to be recovered, contractors will be contacted by the NHSBSA and notified of the overpayment recovery process.
9.7 Any overpayment recovery would not prejudice any action that the NHS may also seek to take under the performance related sanctions and market exit powers within The National Health Service (Pharmaceutical and Local Pharmaceutical Services) Regulations 2013.
9.8 Accurate record keeping is an essential part of the service provision. The necessary records for reimbursement must be kept for a period of three years to demonstrate service delivery in accordance with the service specification, and to assist with post-payment assurance activities. These records must be provided by a contractor when requested by the NHSBSA Provider Assurance Team.
Annex A: service pathways
Initiation of Oral Contraception
 Ongoing supply of Oral Contraception
Ongoing supply of Oral Contraception
Annex B: data recording and data transfer
The following table sets out the fields which need to be collected in a pharmacy’s consultation record for the service, with further detail on why the individual datapoints are to be collected and which data may be shared with the patient’s general practice (where the patient consents) and with the NHSBSA.
The table is available in the PDF version of the service specification.