Classification: Official
Publication reference: PR00199
To:
- Primary care optometry contractors
- Secondary care ophthalmic service providers
- Integrated care board (ICB) primary care directors
- NHS regional heads of primary care
- Regional directors of public health
- NHS regional heads of primary care
cc.
- NHS regional directors
- NHS regional medical directors
Dear colleagues,
National patient safety alert: EyeCee One preloaded and EyeCee One Crystal preloaded intraocular lenses – guidance for commissioners, secondary care providers and primary care optometrists
Today the MHRA has issued a patient safety alert in respect of EyeCee One preloaded and EyeCee One Crystal preloaded intraocular lenses following the device safety information notification (DSI/2023/001) issued on 26 January 2023.
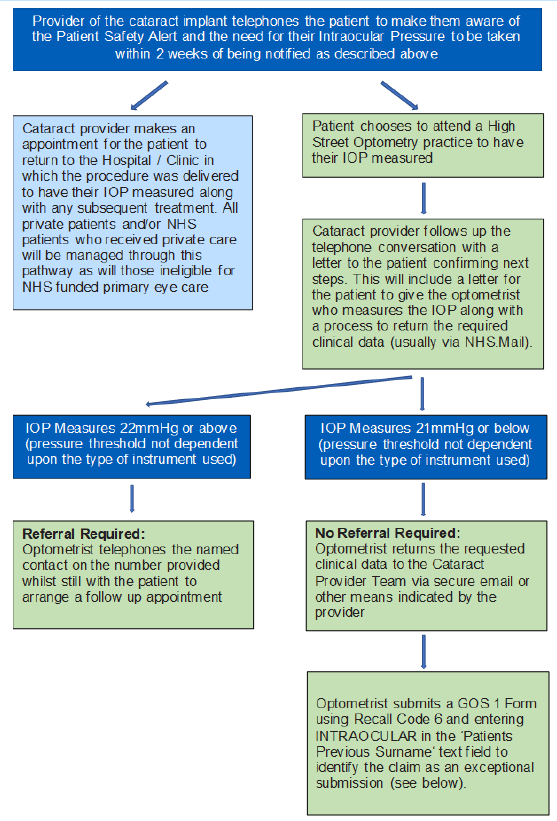
This alert requires that all patients who have been implanted with these devices since 1 October 2022 be contacted by telephone by their cataract care provider to advise them to have the pressure in their eye tested (within 2 weeks of notification). This impacts on both NHS and private providers of cataract care. Where the patient has received this care privately the default expectation is that this follow-up will be provided by the private provider. Where a patient has received NHS funded care this follow-up will be provided by the secondary care provider if the person is ineligible for an NHS funded sight test in primary care i.e. the patient is aged less than 60 years old. All other patients may present in primary care for this follow-up to be completed if they are unable to be seen in secondary care. Where this follow-up is provided under a General Ophthalmic Services contract the guidance below should be followed to ensure accuracy in claiming and subsequent payments.
All patients should wait to be contacted. The MHRA alert also notes that affected patients who are not being followed up by their cataract provider should be provided with a letter to take to their local optometrist confirming that they have been implanted with the affected devices.
This communication should also include details of how to inform the secondary care provider of the results of the test and a named contact should urgent referral be required. Regional and ICS Ophthalmic commissioning leads should work with their secondary care providers to confirm this information. However, it will be the responsibility of the secondary care provider to ensure that all patients are contacted and appropriately followed up.
Given the urgent nature of these tests, NHS England is allowing the following exceptions to the GOS contract only for patients within this cohort and in receipt of a referral letter from their cataract provider:
1. In cases where optometrists assess that a full sight test is not required following the Intraocular Pressure test, claims may still be made for a sight test via a GOS 1 submission (see below on how to make a claim).
2. Private patients should have been given an appointment by the provider of their cataract implant for their IOP to be measured. However, in the event that a private patient presents at a primary care practice for the test, the optometrist may conduct the test and claim payment for a sight test via a GOS 1 submission (see below on how to make a claim).
We recognise that there is still some uncertainty around the nature of the clinical risk associated with these lenses and the advice we can give to patients. We can confirm that, at this time, there are no specific equipment requirements for this screening. At present, we believe that only a small number of patients (2-4%) will be affected, however it is important to identify those at risk as quickly as possible so that we can understand the full extent of the complication. We are grateful to colleagues for prioritising these patients for intraocular pressure testing.
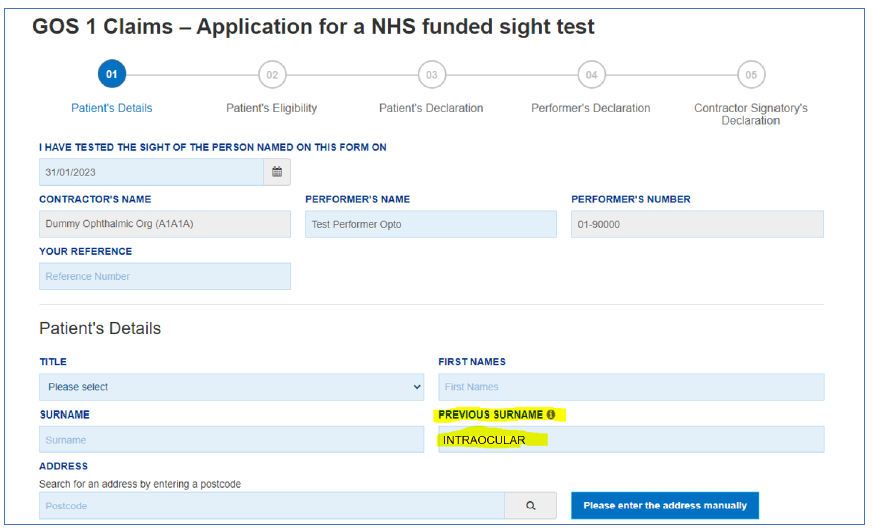
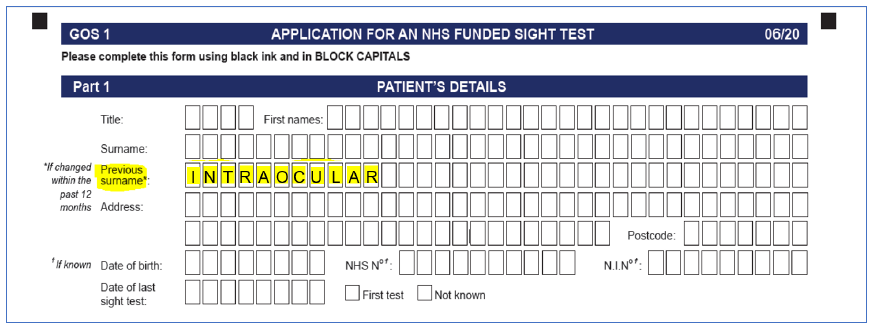
Optometrists should follow the instructions in the annex to this letter when making claims, and are reminded that the above exception applies only to patients who were implanted with the preloaded EyeCee IOLs and have received a referral letter from the provider of the implants (to be given to the optometrist). All claims must be submitted as detailed below. Colleagues are asked to note the requirement to annotate the GOS claim form with the word ‘intraocular’ in the previous surname field.
Thank you for your continued support to ensure the ongoing safety of our patients.
Yours sincerely,
Ali Sparke, Director for Dentistry, Community Pharmacy and Optometry, NHS England.
Louisa Wickham, National Clinical Director for Eye Care, NHS England.
Annex 1: Flow chart of process
Annex 2: Completion of GOS forms
Paper GOS 1 Forms and PCSE Online GOS 1 Forms
- In part 1 under Patient Details, the optometrist should enter INTRAOCULAR in the ‘Previous Surname’ text field (see screenshots below).
- In Part 4, the optometrist should use Recall Code 6.
For Private Patients only:
- In part 1 under Patients Details, patients should tick ‘I have been prescribed complex lenses under the NHS England Optical Voucher Scheme’.
- In part 1 under Evidence of Eligibility, the optometrist should tick ‘Not Seen’.