Purpose
- This document should be read in conjunction with the Framework for Commissioning Highly Specialised Services and the terms of reference for the Highly Specialised Services Oversight Group (HSSOG).
- Section 3B(1) of the National Health Service Act 2006 creates a power for the Secretary of State (SofS) to require NHS England to commission services. The SofS does this through regulations. The relevant regulations are the National Health Service Commissioning Board and Clinical Commissioning Groups (Responsibilities and Standing Rules) Regulations 2012. Regulation 11 requires NHS England to commission specified services for rare and very rare conditions. The full list of services is set out in Schedule 4 of the regulations.
- Within the list of c.150 services for rare and very rare conditions, there are around 80 that are considered to be ‘highly’ specialised. In general, these services:
- are delivered in a small number of expert centres, usually no more than three, and which have been designated by NHS England
- have small caseloads of patients, usually no more than 500
- are clinically distinct
- benefit from national coordination
- The Rare Diseases Advisory Group (RDAG) is responsible for providing strategic clinical advice and clinical leadership in discharging these duties in relation to highly specialised services (HSS).
- RDAG also has a broader role in implementing the UK Rare disease framework and links with the Department of Health and Social Care (DHSC)-led England Rare Diseases Framework Delivery Group in delivering the England rare diseases action plan. NHS England reports to this Delivery Group on its progress against the actions in the plan, with RDAG advising on how the actions should be implemented.
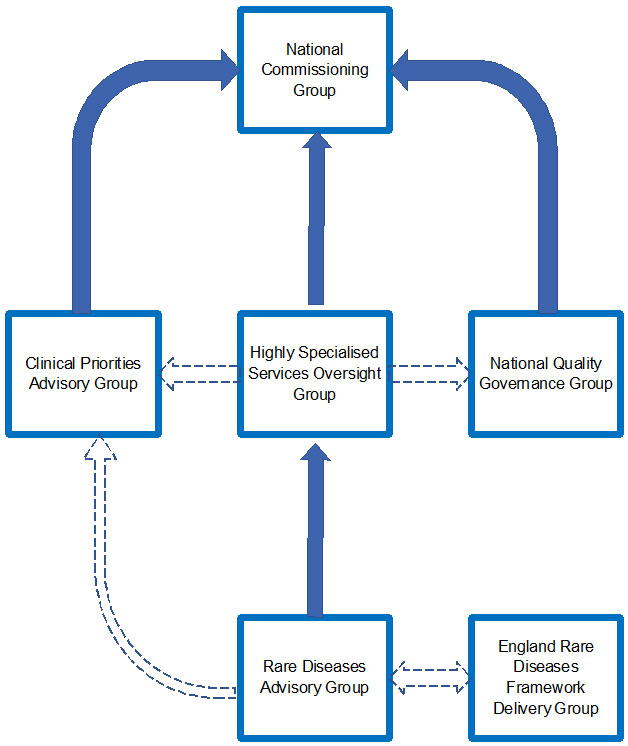
Governance structure (see also Appendix A)
- The Board of NHS England has established a forum known as the National Commissioning Group (NCG) for retained Specialised, Health and Justice and Armed Forces Services to support the discharge of the organisation’s duties, powers and responsibilities in respect of retained Specialised Services, Health and Justice Services and Armed Forces Services.
- The Highly Specialised Services Oversight Group (HSSOG) is the operational group that discharges these duties, powers and responsibilities in respect of HSS. The membership of the HSSOG is UK-wide.
- RDAG provides clinical advice and leadership to HSSOG on the implications of other relevant NHS England policy areas, for example, genomics.
- The HSSOG takes its strategic clinical advice and clinical leadership from RDAG, whose membership is UK-wide. NHS England also seeks advice about specific HSS from the individual clinicians leading the HSS. Clinical Reference Groups (CRGs) may also provide advice, for example, the Radiotherapy CRG may provide advice on the proton beam therapy service.
- RDAG also has a broader role in implementing the UK Rare disease framework and links with the DHSC-led England Rare Diseases Framework Delivery Group in delivering the England rare diseases action plan. NHS England reports to this delivery group on its progress against the actions in the Plan, with RDAG advising on how the actions should be implemented.
- The HSSOG makes recommendations to the Clinical Priorities Advisory Group (CPAG) on which technologies used in HSS should be prioritised for investment, alongside those technologies used in retained specialised (i.e. non-HSS) services. The HSSOG may also give advice to CPAG on technologies for patients with rare diseases where that rare disease is not managed within the HSS portfolio.
- The HSSOG will identify and raise any clinical quality and safety risks and issues, as appropriate, with the National Quality Governance Group. Where appropriate to the group’s scope, objectives and duties, they may mitigate and/or manage particular clinical quality and safety risks and issues.
- Whilst the business of the HSSOG is primarily concerned with those services for which NHS England has full commissioning and budgetary responsibility, it is essential that there is close working between the commissioners of HSS and those who commission services more locally.
Membership
- The following table details the membership of RDAG, the nomination arrangements and permitted deputies:
| Member | Nomination arrangements | Permitted deputy |
| Independent chair | Nominated by the NHS England national director of specialised commissioning | Vice chair |
| Representatives from the following Royal Colleges/professional organisations: – Royal College of Paediatrics and Child Health – Royal College of Pathologists – Royal College of Physicians – Royal College of Psychiatrists – Royal College of Surgeons – Royal College of General Practitioners – Joint Committee on Medical Genetics | Nominated by their respective president or equivalent
| Another nominee from the Royal College/professional organisation |
| A representative from the Specialised Commissioning Public Health Network | Nominated by the national medical director, specialised commissioning, NHS England | Another nominee from the network |
| Representatives from the three devolved nation governments | Nominated by their respective governments | Another nominee from the respective government |
| A representative from the Department of Health and Social Care | Nominated by the UK Government | Another nominee from the UK Government |
| Representatives from the three devolved nation NHS specialised commissioning or equivalent organisations | Nominated by respective devolved nation NHS organisations | Another nominee from the NHS organisation |
| Director, clinical commissioning, NHS England | Ex officio | Not permitted |
| National medical director specialised services, NHS England | Ex officio | Deputy national medical director |
A regional medical director, NHS England | Ex officio | Another regional medical director |
| A regional medical director (commissioning), NHS England | Ex officio | Another region medical director (commissioning) |
|
Four individuals responsible for delivering a highly specialised service, two of whom are from a medical background and two of whom are from a nursing background |
Nominated by the National Medical Director, Specialised Commissioning, NHS England |
Not permitted |
| A pharmacist, NHS England (ex officio) | Ex officio | Another NHS England pharmacist |
| A representative from the Quality and Nursing team, NHS England | Ex officio | Another representative from the Quality and Nursing team |
| Four patient and public voice members | Selected via a UK-wide public appointments process | Not permitted |
|
A health economist | Nominated by the National Medical Director, Specialised Commissioning, NHS England |
Not permitted |
| An ethicist | Nominated by the Institute of Medical Ethics | Another nominee from the Institute of Medical Ethics |
| A representative from NICE | Nominated by NICE | Another nominee from NICE |
- The following individuals are in attendance at all meetings:
- deputy director, clinical commissioning (highly specialised services), NHS England
- medical advisor, highly specialised services, NHS England
- senior project manager highly specialised service, NHS England (secretariat)
- head of innovative treatments, NHS England
- senior clinical lead quality, Quality and Nursing team, NHS England
- Other officers from NHS England attend meetings as necessary.
- The secretariat is provided by the deputy director, clinical commissioning (highly specialised services) or his/her nominee.
- Members of RDAG are nominated to ensure a fair geographical representation from across the UK.
- The chair and members of the committee are appointed for a period of up to three years, with the exception of those who sit on RDAG by virtue of their individual institutional roles. The specific period of appointment may vary for each member to allow a gradual renewal of the membership over time.
- The period of appointment may be extended by mutual agreement to a further term of up to three years and up to a maximum of six consecutive years. Appointments may be terminated at a member’s request or in the event of unsatisfactory attendance at meetings.
- The chair and the patient and public voice members have their expenses covered and be remunerated in line with NHS England policy. Other posts are not remunerated, although the Health Economist and Ethicist roles can claim travel allowances at the standard NHS rates (for those meetings held face-to-face).
- The chair of RDAG and the national medical director specialised services, NHS England nominate a vice chair from among the members. The vice chair is responsible for chairing meetings and providing leadership if the chair is unavoidably absent or is unable to chair the meeting due to conflict of interest for specific items on the agenda.
Meeting arrangements
- As RDAG is an advisory group, its role is to provide strategic clinical advice and clinical leadership to the HSSOG. This is in line with the governance structure set out in Appendix A and the decision framework set out in Appendix B.
- Meetings will generally be held virtually with papers circulated a week in advance.
- RDAG papers are marked:
- Confidential RDAG members only – for sensitive or confidential material; papers should not be shared or published without the express agreement of the secretariat.
- RDAG discussion – papers can be shared with a ‘colleague/s’ in order that a member can bring a considered perspective to the meeting; papers should not be published without the express agreement of the secretariat.
- No restrictions – paper can be freely shared and published
- The secretariat will minute the proceedings of all meetings of RDAG, including recording the names of those present and in attendance.
- Meetings will be held quarterly. Where there is a requirement for Chair’s action in between meetings, this will also need to be agreed by the Head of Highly Specialised Commissioning, NHS England.
Declarations of interest
- Members must submit any conflicts of interest on an annual basis and declare any additional conflicts of interest at the start of each meeting; they may be required to absent themselves from the relevant discussions.
Duties of RDAG
- RDAG is established to provide consistent strategic clinical advice and clinical leadership to NHS England and the devolved administrations. This includes providing advice to the NHS England Clinical Priorities Advisory Group (CPAG) on which HSS or technologies should be prioritised for investment. RDAG may also give advice to CPAG on technologies for patients with rare diseases where that rare disease is not commissioned in the HSS portfolio.
- RDAG provides clinical advice to NHS England and the devolved administrations on the most appropriate services to deliver those technologies that receive a positive appraisal determination from NICE through the Highly Specialised Technologies Programme, whether these services are in the Highly Specialised Services portfolio or not. RDAG may also provide advice on the most appropriate services to deliver technologies for patients with rare diseases and which are considered through the NICE Technology Appraisal Programme.
- RDAG provides clinical advice to NHS England and the devolved administrations on developing and implementing strategy for HSS.
- RDAG provides clinical advice to NHS England and the devolved administrations on how HSS should be commissioned. This includes advising on new or replacement providers of HSS through the Aspirant Market Entrants process and providing clinical advice on whether there are services that should be added to the HSS portfolio.
- RDAG advises NHS England on how the actions in the in the England Rare Diseases Plan are implemented, with NHS England reporting to DHSC on its progress against the actions in the Plan.
- RDAG considers the outcomes of the geographical access exercise in the HSS portfolio (undertaken every three years) and reports these to the HSSOG, making recommendations for action as required.
- RDAG considers the outcomes of the monitoring health inequalities in the HSS portfolio and reports these to the HSSOG on an annual basis, making recommendations for action as required.
- RDAG considers the outcomes of monitoring clinical outcomes in the HSS portfolio and reports these to the HSSOG on an annual basis, making recommendations for action as required.
- RDAG provides clinical advice on proposed new/refined service specifications for HSS.
- RDAG provides clinical advice to NHS England and the devolved administrations on agreeing and managing the Rare Disease Collaborative Networks.
Reporting responsibilities
- RDAG reports to the NHS England HSSOG (see Appendix A for governance structure).
- Appendix B sets out the decision framework across RDAG, the HSSOG and the National Commissioning Group.
Other matters
- RDAG will review on an annual basis its own performance and terms of reference to ensure that it is operating effectively.
Appendix A – governance structure
Image alternative text: The national commissioning group is supported by 3 groups: the clinical priorities advisory group, the highly specialised services oversight group and the national quality governance group. These groups are additionally supported by the rare diseases advisory group and the England rare diseases framework delivery group.
Appendix B – decision framework for highly specialised services and implementation of the Rare Disease Framework
| Type of business item | RDAG | HSSOG | NCG | Frequency |
| Management of the annual budget, including the management of significant financial under/overperformance | No | Yes | By exception | Six-monthly |
| Agreement of workplan | No | Yes | Yes – sign off | Annually |
| Management of workplan | No | Yes | By exception | Six-monthly |
| Management of individual service issues | By exception | Yes | By exception | As required |
| Management of risk register | No | Yes | By exception | Annually |
| Outcomes of Aspirant Market Entrants process | Yes | Yes | Yes – sign off | As required |
| Clinical commissioning policies | Yes – advice to CPAG | No | Yes – from CPAG | As required |
| Proposals for new services | Yes | Yes | Yes – sign off* | As required |
| New and revised service specifications | Yes | Yes | Yes – sign off | As required |
| Selection of new or replacement providers of HSS |
No |
Yes | Yes – sign off |
As required |
| Agreement of strategy and outcomes of reviews in relation to HSS | Yes | Yes | Yes – sign off |
As required |
| Quality of services | By exception | Yes | By exception | Quarterly |
| Activity in services | No | Yes | By exception | Annually |
| Geographical access to services | Yes | Yes | By exception | Around every three years |
| Clinical outcomes in services |
Yes |
Yes | By exception |
Annually |
| Health inequalities in services | Yes | Yes | By exception | Annually |
| Agreement and management of the Rare Disease Collaborative Networks | Yes | Yes – sign off | No | Annually |
| Implementation of the England Rare Diseases Action Plan | Yes | Yes | By exception | As required |
* These also require sign off by the Delegated Commissioning Group.
Dated: March 2023
Revised: July 2024