1. Background
This document uses the term chemical, biological, radiological, and nuclear (CBRN) to describe both accidental and deliberate releases of hazardous substances*.
In other sources, you may see the term ‘HazMat’ to refer specifically to accidental or non-weaponised releases, while ‘CBRN’ is reserved for deliberate releases carried out with malicious intent. In this document, these meanings are combined and described under the single term ‘CBRN’.
*Description from the NHS England guidance for the management of self-presenters from incidents involving hazardous materials or CBRN substances.
Organisations should consider the additional implications of a deliberate release. In these situations, they will need to work with the police or security services to support any criminal or forensic investigations
The UK Health Security Agency (UKHSA) and NHS Blood and Transplant (NHSBT) hold a selection of medications, in reserve stocks, for use in the response to incidents involving CBRN materials. These stocks are held in order to be distributed in an incident, for rapid response depending on the stock and holding location.
Any provider organisation with an emergency department (ED) may request that countermeasures are delivered to manage patients arriving with exposure symptoms requiring countermeasures.
As part of the incident response, NHS England will provide oversight and support to ensure that the trust receive timely access to medical countermeasures.
1.1 Purpose
This guidance describes how an NHS organisation should request and receive CBRN countermeasures.
Where NHS England is informed of the need for countermeasures they may, as part of incident response, in agreement with UKHSA, enable the release of countermeasures and inform the trust they are being dispatched before the trust is aware of the need to use them.
1.2 Definitions
Countermeasures are a group of medicines designed for use in specific scenarios, these are held in strategic locations, to enable the protection and treatment of the public in case of exposure to a CBRN material.
This guidance is specific for countermeasures for CBRN related incidents and does not apply to the distribution of pandemic stockpiles (infectious diseases) held by UKHSA on behalf of the NHS.
2. Roles, accountabilities and responsibilities
This section describes the roles, accountabilities and responsibilities of those functions required to deliver a response under the NHS England Incident Response Plan (national) (IRP(N)).
2.1 NHS England
2.1.1 NHS England national
The national emergency preparedness, resilience and response (EPRR) duty officer, in conjunction with the EPRR tactical advisor/strategic advisor and the second on-call will:
- assess the situation and activate the IRP(N), as required, including the nomination of an incident director (national)
- notify the UKHSA duty officer (National Response Centre) and the Department of Health and Social Care (DHSC) duty officer, of a countermeasure request (for biological, radiological and nuclear (BRN) this will include ensuring the delivery required is requested from UKHSA)
- ensure all countermeasures requests go via the Incident Management team (IMT)
- confirm if any further countermeasures will need to be dispatched to organisations in conjunction with UKHSA’s National Response Centre (NRC).
2.1.2 NHS England regions
The NHS England regional on-call, will be responsible for:
- establishing the details relating to:
- the cause of the incident
- the population affected
- the countermeasures required
- the quantity of the countermeasures required
- the delivery location(s)
- the contact at the delivery location(s)
- any other pertinent information
- confirming the need for countermeasures from the receiving NHS trust (if the person requesting countermeasures is unknown to the regional on-call, they should contact the requesting organisation via their on-call routes)
- requesting stock dispatch from either NHSBT and/or the NHS England National EPRR duty officer
- identifying and initiating any regional coordination arrangements required to support the response and distribution of countermeasures
2.2 The UK Health Security Agency (UKHSA)
The UKHSA will enable release of the countermeasures stock from the stockpiles relating to those not held by NHSBT.
2.3 Provider (requesting) organisation
Any provider organisation with an emergency department may request that countermeasures are delivered to manage patients arriving with exposure symptoms requiring countermeasures.
Provider organisations are responsible for:
- ensuring that once the countermeasures request has been actioned, the relevant integrated care board (ICB) is informed of the request and that existing deployment plans are utilised in a joint regional and ICB response, as appropriate
- requesting countermeasures from their NHS England regional on-call, with all the required information – see section 3.1
- ensuring arrangements are put in place for the safe and secure receipt of any countermeasures, including pharmacy support, within the specified timeframes for the countermeasures – see section 2.4
- ensuring arrangements are in place for the distribution of the countermeasures to patients, in a timely manner, given the amount of countermeasure required
2.4 Countermeasures stock
2.4.1 UK Health Security Agency (UKHSA) stock
UKHSA can authorise deployment of stocks for several countermeasures within the contracted timeframe to a previously accredited delivery location. Follow up stock can be delivered within 24 hours as agreed at the time.
2.4.2 NHS Blood and Transplant (NHSBT) stock
NHSBT will deliver stocks of chemical countermeasures within 2 hours of the request being made to a suitably qualified person at the requesting organisation.
3. Countermeasure requests
Requesting countermeasures is the first step in a multi-organisational chain to ensure that the correct countermeasures reach patients in a reasonable timeframe for use.
It is therefore important that organisations making a request give clear and concise information for this to occur.
3.1 Requests
Providers should make their requests via the NHS England Regional on-call, the request should include the following information:
- name of caller
- requesting organisation name
- contact telephone number
- name and quantity of countermeasure(s) pods
- department name (where it will be delivered)
- full postal address of the receiving location(s)
- name and contact details of receiving individual(s)
An action card is provided for regions to ensure they capture the correct information; this will be shared with them separately from this document. The flow chart below shows the activities at each level of the request chain. Release from NHSBT will require the use of a code word by the NHS England regional on-call.
Figure 1: Countermeasures request flow chart
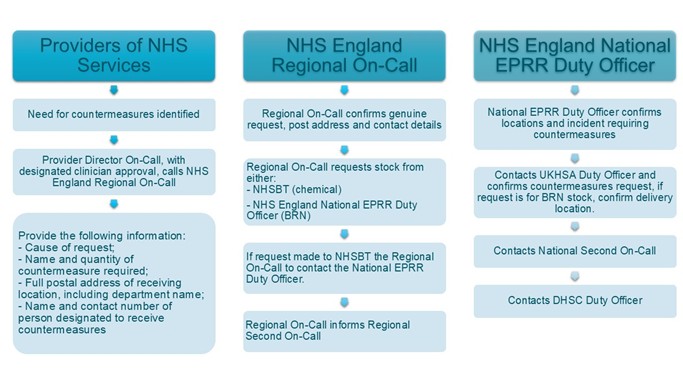
The flowchart above explains that providers of NHS services that identify a need for countermeasures should contact the provider director on-call with designated clinician approval. Then they should call NHS England regional EPRR on-call. They will need to provide the following information:
- cause of request
- name and quantity of countermeasure required
- full postal address of receiving location including department name
- name and contact number of person designated to receive the countermeasure
NHS England regional on-call should, confirm that it is a genuine request and that accurate postal and contact details are received. The on-call should then request stock from either NHS Blood and transplant (NHSBT) (for chemical) or NHS England national (for BRN). If the request is made to NHSBT the regional on-call should contact the national emergency preparedness, resilience and response (EPRR) duty officer. The regional on-call should also inform second on-call.
NHS England national EPRR duty officer should confirm locations and the details of the incident requiring countermeasures. They should then contact UK Health Security Agency’s (UKHSA’s) duty officer and confirm countermeasures request. If the request is for BRN stock they should confirm delivery location. They should also notify the second on-call as well as the Department of Health and Social Care’s (DHSC’s) duty officer.
3.2 Delivery
3.2.1 Chemical countermeasures
Chemical countermeasures supplied via NHSBT will usually be sent to an emergency department, and continued restock can be arranged but will need to be requested by the organisation, via the NHS England regional on-call. The region should also inform the NHS England national EPRR duty officer of this request.
Note: A trust requiring both atropine and pralidoxime will need to order a pod of each, the nerve agent pod has been discontinued.
3.2.2 Biological, radiological and nuclear (BRN) countermeasures
BRN countermeasures are dispatched by the UKHSA appointed holder and delivered to organisations, usually to goods receiving or pharmacy departments. Requesting organisations need to be absolutely clear on the address of the delivery location. Countermeasures in these categories may continue to be pushed to receiving locations until a stop is requested by UKHSA.
3.3 Receipt of countermeasures
The nominated department should be prepared to accept the countermeasures into a safe location and be able to break these down into suitable doses for those needing treatment. Trusts should therefore have a named individual to receipt the stock. A pharmacist should be present to take receipt and ensure appropriate management of stock is in place.
4. Patient group directions and protocols
Patient group directions (PGDs) and protocols have been produced by UKHSA to support the issuing of countermeasures to the public. PGDs for oral doxycycline and ciprofloxacin and protocols for potassium iodine are available on the NHS England website and can be used by trusts when issuing these countermeasures.
Publication reference: PRN00895_ii

