Introduction
1. Any provider or organisation that treats NHS patients and wants to purchase products at Medicines Procurement and Supply Chain (MPSU; formerly known as the Commercial Medicines Unit, CMU) framework (secondary care procurement) pricing must be registered as a purchasing point with NHS England and agree to the key conditions of access.
2. NHS England will grant purchasing points access to MPSC framework prices for the purpose of providing medicines for the treatment of NHS patients in England (although in some instances NHS England does also tender for Wales and Northern Ireland for treatment of NHS patients), for so long as the provider or organisation remains listed as a purchasing point.
3. Independent sector providers (for example, private hospitals or clinics), wholly owned NHS subsidiaries, homecare providers and outsourced pharmacies are not permitted to register as purchasing points. The exception being Prison Service, HIV, PrEP, and PEP providers contracted to support NHS England and/or local authority commissioned services. Access to MPSC framework prices for organisations out of scope for being a purchasing point (who are eligible) is covered in the National Pharmaceutical Supply Group guidance.
Key terms
4. For this document, the following key terms are defined as:
- purchasing point – a provider or organisation specified in accordance with NHS England’s policy position
- MPSC framework – an agreement between one or more contracting authorities and one or more economic operators, the purpose of which is to establish the terms governing contracts to be awarded during a given period, in particular with regard to price and, where appropriate, the quantity envisaged
- MPSC framework holder – a supplier that is awarded a framework following completion of a tender exercise
- third party – a provider or organisation that is sub-contracted to deliver a service on behalf of a host NHS provider or organisation
- NHS patient – where a patient is treated in an NHS organisation (host) or by a third-party (as above)
Key conditions of access
5. These are the key conditions a purchasing point is expected to abide by when granted access to medicines at MPSC framework prices. The purchasing point shall:
5.1 Commit to purchasing from a MPSC framework where one exists, and a clinically appropriate medicine is available (or from a defined list where full access has not been given).
5.2 Only purchase medicines at MPSC framework prices that are relevant to the services used to provide care to NHS patients.
5.3 Ensure local policies guarantee all confidential information is stored securely, and that relevant data governance procedures are adhered to. Under no circumstances should confidential information be disclosed without first obtaining written confirmation from the relevant framework supplier.
5.4 Provide medicines purchasing data monthly in such form as may be specified by NHS England (to include, without limitation, for the purposes of analysing public sector expenditure and planning future procurement activities), to NHS England by the 10th day of each calendar month.
Further behaviours expected of a purchasing point
6. In addition to the key conditions of access, there are several important related factors a purchasing point is expected to comply with:
6.1 Purchasing points are prohibited from the onward selling of medicines bought at MPSC framework prices to other organisations, including third-party wholesalers. The exceptions include but are not limited to:
6.1.1 Providers or organisations specified in accordance with NHS England’s policy position and only if assured that the use of any medicines sold will be solely for NHS patients.
6.1.2 Other organisations that meet the purchasing point criteria (but do not have a pharmacy) and have a service level agreement in place (with a registered purchasing point); for example, hospices, prisons, ambulance
6.1.3 National mutual aid arrangements for managing medicines shortages.
6.2 A pharmacy manufacturing unit that purchases and prepares medicines to treat NHS patients in a setting different from that provided by its own organisation (that is, over labelling medicines for use in a tertiary care centre) can sell (including an appropriate service level fee that incorporates on costs) to other providers or organisations treating NHS patients. Selling to organisations for which it cannot assure the medicines will only be used for NHS patients is not permitted.
6.3 While it is a key condition that medicines bought at MPSC framework prices are used to treat NHS patients only, NHS England acknowledges that a small number of private patients may be treated in NHS organisations with medicines bought at MPSC framework prices. Where this occurs, purchasing points must request approval to purchase at MPSC framework prices from the relevant framework holder in advance of treating private patients.
6.4 Third-party access to MPSC framework prices should be enacted in accordance with the National Pharmaceutical Supply Group guidance.
How to become a purchasing point
7. NHS England’s policy position sets out which providers or organisations are in scope for being a purchasing point and therefore able to access MPSC framework prices.
8. To assist NHS England with assessing the suitability of prospective purchasing points, a prequalification questionnaire can be found at Annex A.
9. As a final step before purchasing point status is granted, a signed Confidentiality Access Agreement will need to be signed and returned to NHS England – see Annex B.
Misuse of MPSC frameworks
10. An escalation process is in place for MPSC framework holders, and others, to report concerns over misuse of MPSC frameworks to NHS England for investigation – see Annex C.
11. If you work in an NHS provider or organisation and have concerns over the misuse of MPSC framework prices, please contact your local regional procurement pharmacist specialist or cmupharmacyteam@nhs.net for advice.
Queries relating to this document
12. If you have any queries relating to the content of this document, please contact MPSC via cmupharmacyteam@nhs.net.
Annex A: Prequalification questionnaire
NHS England assesses the suitability of those applying to be a purchasing point.
Please download the questionnaire and submit for review to england.CMUpharmacyteam@nhs.net.
Failure to answer all the questions will result in an application being rejected.
Download the prequalification questionnaire.
Annex B: Confidentiality Access Agreement
This Agreement is Dated: xx/xx/202x
Parties:
(1) NATIONAL HEALTH SERVICE COMMISSIONING BOARD, KNOWN AS NHS ENGLAND, whose principal office is at 7 and 8 Wellington Place, Leeds, LS1 4AP
and
(2) [NAME], a company incorporated and registered in England and Wales with company number [NUMBER], whose registered office is at [ADDRESS] (Provider),
- In consideration of the following, NHS England grants to the Provider / Organisation/ Organisation access to the Commercial Medicines Unit (MPSC) framework prices for the purpose of providing secondary care medicines for the benefit of National Health Service (NHS) patients within England for so long as the Provider / Organisation has a valid contract to deliver services to NHS England (the Purpose):
- the sum of £1, receipt of which NHS England expressly acknowledges;
- the provision of monthly medicines purchasing information; and
- each party’s mutual commitment to keep information (the Confidential Information as defined below) confidential, in accordance with the terms set out in this agreement.
- The parties acknowledge that access to the MPSC prices framework will allow the Provider / Organisation access to MPSC pricing for all medicines irrespective of whether the medicines are relevant to the services being provided to NHS patients The Provider / Organisation undertakes to purchase only those medicines that are relevant to the services the Provider / Organisation is providing to NHS patients and not to purchase medicines that are not relevant to the services being provided. If the Provider / Organisation purchases or NHS England believes that the Provider / Organisation shall purchase medicines via the MPSC prices framework that are not relevant to the services the Provider / Organisation is providing to NHS patients, NHS England may, at its sole discretion, immediately on giving notice, suspend, withhold or withdraw access to the MPSC framework prices.
- The Provider / Organisation shall provide monthly medicines purchasing information in such form as may be specified by NHS England and, where requested to do so the Provider / Organisation shall also provide such purchasing information to any competent authority whose role it is to analyse such purchasing information in accordance with UK government policy (to include, without limitation, for the purposes of analysing public sector expenditure and planning future procurement activities) to NHS England by the 10th day of each calendar month for the duration of this agreement commencing on the date of this agreement. If the Provider / Organisation fails to or is late providing the monthly medicines purchasing information to NHS England, NHS England may, at its sole discretion withhold, suspend or withdraw access to the MPSC framework prices.
- For the purposes of this agreement, Confidential Information includes any specific details of the negotiations between the parties (including the progress or status of any negotiations), the terms of this agreement, all confidential or proprietary information relating to the business and affairs of each party disclosed by a party during the course of negotiations including but not limited to any and all MPSC pricing and details of any MPSC framework agreements; financial or commercial proposals, mechanics or duration of proposed agreements, internal NHS England procedures or processes, possible indications and possible patient groups that may benefit from MPSC pricing and any other information that is identified as being of a confidential or proprietary nature. Notwithstanding the foregoing, each party shall, on the request from other sources be free to disclose the existence of such discussions and negotiations, and their general status only, provided that such disclosure does not contain Confidential Information.
- Each party to this agreement is referred to as ‘the Recipient’ when it receives or uses the Confidential Information disclosed by the other party and ‘the Discloser’ when it discloses or makes available Confidential Information to any other party.
- In consideration of the Discloser agreeing to disclose Confidential Information to the Recipient, the Recipient undertakes not to use the Confidential Information disclosed by the other party for any purpose except the Purpose, without first obtaining the written agreement of the other party.
- The Recipient undertakes to keep the Confidential Information disclosed by the other party secure and not to disclose it to any third party except to its employees and professional advisers who need to know the same for the Purpose, who know they owe a duty of confidence to the other party and who are bound by obligations equivalent to those in clause 6 above and this clause 7.
- The Provider / Organisation undertakes to procure that any sub-contractors to whom the Confidential Information has been disclosed, shall comply with the confidentiality obligations in clauses 6 and 7 above as if they were the Recipient and if NHS England so requests, procure that any of them enters into a confidentiality agreement with the NHS England on terms equivalent to those contained in this agreement.
- The Provider / Organisation acknowledges that NHS England follows the principles and guidance set out in the national document ‘Guidance for NHS Trusts (including Chief Pharmacists) prepared by the Commercial Medicines Unit on behalf of the National Pharmaceutical Supply Group’ which is attached to this agreement at Schedule 1 to determine the appropriateness for all third party service providers (including the Provider) to have access to the MPSC framework pricing.
- The Provider / Organisation shall be liable for the actions or omissions of its employees, professional advisers and sub-contractors in relation to the Confidential Information as if they were the actions or omissions of the Provider.
- The undertakings in clauses 6 and 7 apply to all of the information disclosed by each of the parties to the other, regardless of the way or form in which it is disclosed or recorded but they do not apply to:
- any information which is or in future comes into the public domain (unless as a result of the breach of this agreement);
- any information which is already known to the Recipient, and which was not subject to any obligation of confidence before it was disclosed to the Recipient by the other party; or
- any information contained in disclosures made to or engagements with any competent authority including but not limited to NICE, the Department of Health and Social Care; the Health Services Commissioner for England; and any applicable regulator of the services provided to NHS England.
- Nothing in this agreement will prevent the Recipient from making any disclosure of the Confidential Information required by law or by any competent authority. Notwithstanding the foregoing, NHS England, acting in accordance with the codes of practice issued and revised from time to time under both section 45 of Freedom of Information Act 2000 and regulation 16 of Environmental Information Regulations 2004, may disclose information concerning this agreement without consulting with the Provider.
- The Recipient will and will procure that its sub-contractors will as applicable, on request from the other party, return all copies and records of the Confidential Information disclosed by the other party to the Recipient or its sub-contractors as the case may be and will not retain any copies or records of the Confidential Information disclosed by the other party.
- Neither this agreement nor the supply of any information grants the Recipient or its sub-contractors if applicable any licence, interest or right in respect of any intellectual property rights of the other party except the right to copy the Confidential Information disclosed by the other party solely for the Purpose.
- The undertakings in clauses 6, 7, 8 and 13 shall continue in force for a period of 5 years from the date the Provider / Organisation ceases to have access to or use of the MPSC Framework prices for secondary care medicines, or for so long as NHS England reasonably determines that the Confidential Information has any commercial value whichever is the longer.
- If either party decides not to continue to be involved in the Purpose with the other party, it shall notify that party immediately, but shall in any event still be subject to the provisions of clause 15 above.
- The Provider / Organisation acknowledges and agrees that the Confidential Information given to the Provider / Organisation by NHS England may not be accurate or complete and NHS England makes no warranty or representation (whether express or implied) concerning the Confidential Information, or its accuracy or completeness.
- Without prejudice to any other rights or remedies that each party may have, each party acknowledges and agrees that damages alone would not be an adequate remedy for any breach of the terms of this agreement by the other party. Accordingly, each party shall be entitled to the remedies of injunctions, specific performance or other equitable relief for any threatened or actual breach of this agreement.
- This agreement is governed by and is to be construed in accordance with the laws of England and Wales.
- The English courts will have exclusive jurisdiction to deal with any dispute which has arisen or may arise out of, or in connection with, this agreement.
Schedule 1
This agreement has been entered into on the date stated at the beginning.
Signed on behalf of NHS ENGLAND by its duly authorised representative:
_____________________________
Signature
_____________________________
Name
_____________________________
Position
Signed on behalf of [PROVIDER / ORGANISATION NAME] by its duly authorised representative:
_____________________________
Signature
_____________________________
Name
_____________________________
Position
Annex C: Escalation process
To report misuse of Medicines Procurement and Supply Chain (MPSC; formerly known as Commercial Medicines Unit, CMU) framework concerns to NHS England.
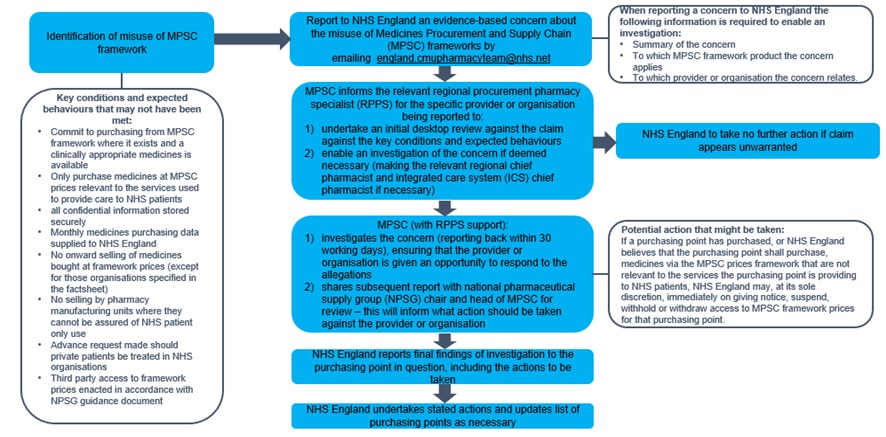
The stages of the escalation process to report concerns about the misuse of MPSC frameworks are set out below.
1. Identification of misuse of MPSC frameworks
Purchasing points are expected to comply with the key conditions of access and behaviours:
- commit to purchasing from a MPSC framework where it exists and a clinically appropriate medicines is available
- only purchase medicines at MPSC prices relevant to the services used to provide care to NHS patients
- all confidential information stored securely
- provide monthly medicines purchasing data to NHS England
- no onward selling of medicines bought at framework prices (except for those organisations specified in the factsheet)
- not selling by pharmacy manufacturing units where they cannot be assured of NHS patient only use
- advance request made should private patients be treated in NHS organisations
- third-party access to framework prices enacted in accordance with the National Pharmaceutical Supply Group (NPSG) guidance
When reporting a concern to NHS England the following information is required to enable an investigation:
- summary of the concern
- to which MPSC framework product the concern applies
- to which provider or organisation the concern relates
2. Escalation process
- step 1: report to NHS England an evidence-based concern about the misuse of MPSC frameworks by emailing cmupharmacyteam@nhs.net
- step 2: MPSC informs the relevant regional procurement pharmacy specialist (RPPS) for the specific provider or organisation being reported to:
- undertake an initial desktop review against the claim against the key conditions and expected behaviours
- enable an investigation of the concern if deemed necessary (making the relevant regional chief pharmacist and integrated care system (ICS) chief pharmacist if necessary)
NB: at this point NHS England will determine if further investigation is required or no further action needs to be taken as the claim appears unwarranted.
- step 3: MPSC (with RPPS support):
- investigates the concern (reporting back within 30 working days), ensuring that the provider or organisation is given an opportunity to respond to the allegations
- shares subsequent report with National Pharmaceutical Supply Group (NPSG) chair and head of MPSC for review – this will inform what action should be taken against the provider or organisation
- step 4: NHS England reports final findings of investigation to the purchasing point in question, including the actions to be taken
- step 5: NHS England undertakes stated actions and updates list of purchasing points as necessary
4. Potential action that might be taken
- if a purchasing point has purchased, or NHS England believes that the purchasing point shall purchase, medicines via the MPSC prices framework that are not relevant to the services the purchasing point is providing to NHS patients, NHS England may, at its sole discretion, immediately on giving notice, suspend, withhold or withdraw access to MPSC framework prices for that purchasing point
Publication reference: PRN1221ii

