Applicability
This NETB applies to all healthcare spaces with ventilation requirements.
Objective
To provide additional technical guidance and standards on the use of HEPA filter devices for air cleaning in healthcare spaces.
Status
The document forms an addendum to Health Technical Memorandum 03-01 Specialised Ventilation for Healthcare Premises (HTM 03-01).
Point of contact/feedback
Point of contact for any queries: england.estatesandfacilities@nhs.net
Executive summary
Ventilation* is an important line of defence for infection control in the healthcare environment. Its design and operation are described in Health Technical Memorandum (HTM-03-01). The current focus on ventilation has highlighted areas of high risk due to poorly performing and inadequate ventilation in hospitals and other healthcare settings. This may be due to change of room use, age, condition of air handling plant, lack of maintenance, challenges with effective use of natural ventilation or other. It is therefore important to bring these facilities up to the minimum specification of current standards, particularly recognising the challenges of COVID-19 and other infections.
Local HEPA filter-based air cleaners (also know as air scrubbers) are one option for improving and supplementing ventilation. The installation of a high efficiency particulate air (HEPA) filter air cleaner can reduce the risk of airborne transmission.
This guidance has been written as an interim specification to set the basic standard required for HEPA filter devices to be utilised in healthcare and patient-related settings. This edition is primarily aimed at portable and semi fixed (wall-mounted) devices. Devices relying on ultraviolet light (UVC) are the subject of a separate guidance document: Application of ultraviolet (UVC) devices for air cleaning in occupied healthcare spaces.
* Ventilation is the process by which ‘fresh’ air (normally outdoor air) is intentionally provided to a space and stale air is removed. This may be achieved by mechanical systems using ducts and fans, or natural ventilation most commonly provided through opening windows. The local redistribution of air may also be construed as ventilation.
1. Introduction
Ventilation is an important feature in the control of airborne infection. However, the emergence of SARS-CoV-2 as a highly contagious virus has demanded new and innovative solutions to safeguard patients, staff and visitors. Health Technical Memorandum 03-01 Specialised Ventilation for Healthcare Premises (HTM-03-01) is a robust standard for ventilation of higher risk clinical spaces based on high air change rates using outdoor air to continually flush indoor spaces. The COVID-19 pandemic has shown that greater attention must be paid to the improvement and maintenance of ventilation in healthcare settings.
The focus on ventilation has also highlighted areas of high risk due to poorly performing and inadequate ventilation, particularly in older hospitals and other healthcare settings such as primary care and dental suites, which increase risks of nosocomial infections.
In cases, where current ventilation does not meet HTM-03-01 standards, this may be due to age, condition of air handling plant, lack of maintenance or other design or operational issues. In the case of naturally ventilated spaces, there is a reliance on staff or patients opening windows. Weather conditions, external noise and air pollution and restricted window openings for safety affect the ability to open windows and means that ventilation in some settings can fall below recommended rates.
Local HEPA filter air cleaners are one option for improving and supplementing ventilation. The correct installation and operation of a HEPA filter air cleaner can reduce the risk of airborne transmission.
Healthcare trusts are under pressure to improve ventilation and in the meantime are considering options including filter-based air cleaning. This standard will assist trusts in selecting and implementing good quality, reliable equipment.
There is substantial evidence from laboratory studies and real-world settings that filtration is an effective technology for reducing airborne pathogens within room air and HVAC systems. A number of research studies have been carried out which indicate that measured levels of microorganisms in air are greatly reduced by air filters [R1-R5, R7]. There is also evidence which directly associates use of filter-based air cleaners with reductions in infection rates of environmentally-derived aspergillus [R8]. The potential of air scrubbers employing UVC or HEPA technology to mitigate SAR-CoV-2 risks is the subject of a rapid review (September 2022) [R.9]. Filter based air cleaners also remove other particulate matter and so can also reduce exposure to other air pollutants. However, air cleaners should not be used as a reason to reduce ventilation and care must be taken to ensure sufficient fresh air changes are provided for the dilution of medical gases and noxious odours, and the maintenance of appropriate oxygen and carbon dioxide levels to satisfy the Building Regulations Part F.
This document aims to serve as interim guidance and regulatory reference point for the design and correctly engineered deployment of HEPA filter devices in real-world settings with regard to effectivity and safety
It focuses on HEPA filter-based devices which can be positioned locally within a room; the document does not cover HEPA filters used within HVAC ducts. Local filter-based devices require fan assisted circulation to introduce the room air into the device, pass it through the filters and then to reintroduce the processed air into the room.
An important consideration regards the flow of the air which is induced, processed and distributed by the device external to the device itself. The design and placement of the device should promote efficient air distribution in the room space and avoid short-circuiting of air circulation relative to furniture, obstructions, and occupancy.
2. HEPA filter technology
HEPA filters comprise a porous structure of fibres or membrane which remove particles carried in an air stream. The mechanism by which particles are removed depends on the size of the particle. Larger particles are removed by impaction onto the filter while smaller particles <1 μm are removed through interception and diffusion. Interception occurs where the particle makes physical contact with the media fibres because particle inertia is not strong enough to enable the particle movement to continue. Diffusion is where random motion (Brownian motion) of the particle enables it to contact the media. These effects are enhanced by the electrostatic charges present on filters.
2.1 Selection of filters
Filter efficiency defines the fraction of particles removed and varies by size of particle. The most difficult size of particles to remove, known as the most penetrating particle size (MPPS), for the majority of filters is around 0.3 μm; particles larger or smaller than this size are captured more effectively. For healthcare applications it is recommended that devices should contain filters classified as High Efficiency Particulate Air Filters (HEPA) under BS EN 1822-1 or ISO 29463-1. HEPA filters have a filter efficiency of at least 99.95% (H13 filter) or 99.995% (H14 filter) for the MPPS, however the performance in situ is sometimes lower depending on the filter and device design and the air flow rate (section 5.1).
Microorganisms range in size from around 0.1 μm for the smallest viruses to several μm in diameter for larger bacteria and fungi. Some fungi and bacteria may be dispersed independent of other material, however, many pathogens will be released on or within another material and therefore the size of the particle that needs to be captured is larger than the pathogen itself. For example, respiratory and gastroenterology viruses will be released within liquid media that contains proteins, salts, surfactants, etc and evaporates to form particles that are larger than the virus itself. Similarly, many skin associated bacteria are released on skin squame which are larger than the bacteria.
Some filter-based air cleaning devices contain lower grades of filter. These devices may be appropriate in non-clinical areas, but as the filters have a lower performance for particles relevant to the size of airborne pathogens they are not recommended in settings with vulnerable patients.
It is common for HEPA filter-based devices to incorporate a coarse grade of filter (typically ISO ePM10 >50% under ISO 16890-1) to act as a dust filter. Some also include a carbon filter to manage odours and volatile organic compounds. Some devices contain several separate filters, while others incorporate the different stage filters into a single cartridge type unit.
2.2 Inclusion of other technologies
Devices which include germicidal ultraviolet (UVC) light alongside HEPA filters are likely to be effective [R4]. Where these devices are considered, this standard takes precedence in terms of clean air performance if the UVC lamp is located after the HEPA filter (i.e. the HEPA filter is the primary device for microbial removal). However, all the safety requirements pertaining to the UVC within that standard should also be complied with.
Devices which incorporate ionisation, photocatalytic oxidation, electrostatic precipitation or other similar technologies alongside filters are not currently recommended for healthcare use unless there is clear evidence for both effectiveness and safety. These devices can sometimes introduce, or create through secondary reactions, chemical by-products into a room which may themselves have an adverse health effect [R4, R11]. The independent research evidence that these products are any more effective at safely reducing microbial loads in air is still emerging.
3. Applications and sizing
Standalone, floor mounted devices can be positioned at any suitable location in a room. These devices are plugged into a standard electrical socket so do not require any installation, although location is important as detailed in sections 8.2 and 8.3.
Fixed devices are semi-permanently mounted to a wall or ceiling. These devices will normally be permanently wired into the room electrical systems rather than plugged into a wall socket. Some manufacturers offer local systems that can be interfaced with the ventilation system and are able to offer pressure differential control in a room.
Figure 1: Representation of typical air flows with respect to a recumbent patient in a regular room for two filter device locations: fixed, wall- or ceiling-mounted (left); mobile, floor-standing (right)
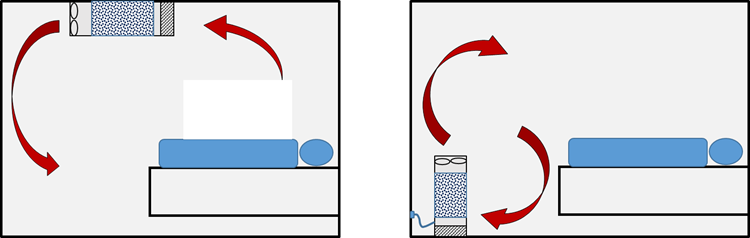
In rooms without natural or mechanical ventilation, or where the ventilation falls short of statutory requirements or regulatory advice, auxiliary devices may be deployed to enhance the equivalent air changes.
The installation of HEPA filter-based air cleaners can be considered to contribute additional ‘equivalent’ air changes (eACH). For example, a treatment room with 6 ACH could achieve the equivalent of 10 ACH by installing a local filtration unit which recirculated and cleaned the equivalent of 4 eACH. Hence, to meet the requirements that comply with HTM-03-01, the number of devices required will be dictated by the existing background levels of ventilation.
The high filter efficiency of HEPA filters means that the single pass efficiency of an air cleaning device for the MPPS should result in at least a 99% (2 log) reduction in the concentration of particles, including microorganisms, that pass through the device when in normal operation. However, the performance within a room depends on both the flow rate through the device and how it distributes the air in a room.
The performance of filter-based devices is described by some manufacturers in terms of a Clean Air Delivery Rate (CADR) which is usually expressed in metres cubed per hour (m3h-1) (some devices quote the CADR in cubic feet per minute, cfm). Where a CADR is given it should be derived from measurements of how well the device removes a defined size of particles in a test room environment; CADR is usually measured using particles rather than microorganisms. CADR is a function of the airflow rate through the device, the quality of the filter and the way the device distributes air in the test room.
Other manufacturers adopt different metrics such as the time to reduce particle concentrations in a room by a specific percentage.
The CADR or other metrics can be used, with care, for design purposes as they express how the device will perform in a standardised test room. However, it is important to note that the actual performance will depend on the particular location and operation of the device, including the room size, layout, background ventilation, device design and maintenance (section 8).
It is not recommended to use an air cleaning device with a lower grade of filter even if the quoted CADR is high, as the device may be less effective against the smallest pathogen carrying particles.
The CADR used for design purposes should be the rate applicable to the device setting at which the device is most likely to be operated and where the noise level is during operation is at a level of ≤50 dB measured at 3 m (dB3m) (section 5.3).
4. Engineering implementation
4.1 Regulatory and standards compliance
If selecting a device that incorporates both UVC and HEPA filters the device should also comply with Application of ultraviolet (UVC) devices for air cleaning in occupied healthcare spaces.
Standards are an integral part of product design and development and are important in medical applications. The Low Voltage Designated Standards should be followed implicitly as a minimum.
IEC 60601 is a series of technical standards which apply to medical electrical equipment and medical electrical systems for basic safety and essential performance. The basic scope of IEC 60601 is the safety of patients and users. It is recommended that the design of standalone HEPA filter devices should follow the principles of the 60601 Standard to ensure risks to patient and user safety within a medical environment are recognised and mitigated (section 4.1.2).
4.1.1 CE and UKCA marking
CE and UKCA marking are standards that appear on products traded on the extended single market in the European and UK economic areas. The marking signifies that the product has been assessed to meet high health, safety, and environmental requirements.
- Selling products in Europe:
- use of the CE-mark declares that the product meets the legal requirements for sale throughout the European Union. Note: note that some products are marked China Export (CE) which should not be confused with the EU standard.
- Selling products in the UK:
- the UKCA-mark is the product marking used for products being placed on the market in Great Britain (England, Scotland, and Wales)
- the UKCA-mark applies to most products previously subject to the CE- marking. The technical requirements (sometimes referred to as ‘essential requirements’) must be met.
4.1.2 Electrical safety
- Compliance with the Low Voltage Directive is mandated implicitly.
- Compliance with the IEC 60601standard is recommended.
- Class I (exposed metal components connected to earth):
- protective earth continuity <0.2 MΩ
- insulation tests: ≥50 MΩ
- earth leakage: ≤5 mA in normal condition (NC), ≤10 mA in SFC (single fault condition)
- enclosure leakage current: ≤1 mA in NC, ≤0.5 mA in SFC.
- Class II (double-insulated enclosure):
- insulation tests: ≥50 MΩ
- enclosure leakage current: ≤0.1 mA in NC, ≤0.5 mA in SFC.
Class III devices are not recommended.
4.1.3 Electrical wiring
Electrical wiring should be in accordance with IET Regulations BS 7671:2018 Requirements for Electrical Installations.
4.2 Ozone and other emissions
Devices which operate using filters only do not produce ozone or other chemical emissions. Devices which incorporate other technology alongside filters are not recommended, however, if they are used manufacturers are required to provide assurance that devices do not produce ozone levels or other chemical pollutants in excess of the Workplace Exposure Limits (UK Workplace Exposure Limit (WEL) for ozone of 0.2 ppm (15 minute reference period)).
5. Engineering design, specification and performance validation
5.1 Device verification
As the performance of a HEPA filter is determined by the size of particles rather than the species of microorganism, it is not necessary for a manufacturer to conduct validation tests using microorganisms. Performance and validation tests carried out by manufacturers can be carried out using inert particles of an appropriate size, usually in the 0.5–2 μm size range. -0
Manufacturers should provide evidence that the HEPA filter used within the device meets BS EN 1822-1/ISO 29463-1 or an equivalent standard, and that the air cleaning device with filters in situ has been tested to an appropriate protocol that demonstrates how the device is likely to perform in a typical healthcare setting. Performance data including airflow rate through the device, filter pressure drop and measured impact of the device on particle concentration in a suitable test environment should be provided for each operational fan speed and for the MPPS.
Device verification, as defined by the manufacturer, should be carried out on first installation to ensure filters are correctly installed and at every filter change. If filters are not correctly installed in devices, leakage around the edge of the filter can result in significant underperformance of the device. A verification check to ensure the device is operating correctly is also recommended if a device is moved to a different location within a hospital.
The verification test is designed to provide assurance that there is no unfiltered air bypassing the filter. This should be carried out by visual inspection to ensure the filter is intact and correctly seated, followed by appropriate measurement, usually through the pressure drop across the clean filter. Manufacturers should either provide a mechanism by which this is carried out in an automated way or by providing ports for a manual pressure drop measurement. Data on the expected pressure drop across the filters at each device flow rate should be provided and should be measured automatically within the device or manually by a qualified person at filter change. Where devices incorporate automated processes for measurement and calibration, manufacturers should provide evidence that this is robust and has been verified in a laboratory setting.
5.2 Filter life
Devices should be optimised to minimise filter replacement times and allow for a straight-forward replacement schedule. A pre filter typically of grade ISO ePM10>50% should be installed within the unit to maximise the life of the HEPA filter
In most healthcare environments devices should be selected such that filters should last around 12 months. Some may last longer than this, however, in environments which are more contaminated or at higher humidity filters may need replacing more frequently.
Devices should incorporate a dirty filter warning indicator or alarm for both the pre filter and the HEPA filter, to provide an easy visual indication to healthcare staff when a filter requires changing or when any other device maintenance is required. This should be in addition to the ability to measure the filter pressure drop for verification (section 5.1).
5.3 Noise considerations
Devices in occupied areas should normally operate at a sound level of ≤50 dB measured at 3 m (dB3m). Exceptionally, for operation at boost such that might be used to purge a room higher sound levels may be acceptable; this should be assessed based on the use of the room.
Noise is a particular consideration when devices are used in rooms where patients are sleeping, and lower sound levels than stated here may be required depending on local environmental conditions. Further guidance on wider considerations around acoustics in healthcare is given in HTM-08-01.
6. Competent persons
In the present context, competent persons (it should be noted that competent person may be defined differently in other documents, including in HTM03-01) are recognised as individuals who are suitably qualified and experienced with professional expertise in one or more of the following areas in the healthcare setting: the design and specification of HEPA filter-based systems (including with airflow assessment), the technical maintenance of HEPA filter devices and systems, and the implementation of schemes employing HEPA filter devices.
Competent persons should have training and familiarity with the HEPA filter-based devices used within the particular healthcare setting to be able to size, specify, operate and maintain devices effectively.
Further, involvement of appropriate people with particular expertise in infection prevention and control are essential during the process of specifying and deploying devices.
7. Engineering and operational considerations
7.1 Hazard, risk and operational delivery
A ventilation design incorporating HEPA filter-based air cleaners will require a hazard and operational study (HAZOP). This process will be convened by the local Ventilation Safety Group (VSG) (a group of individuals with recognised expertise in the design and operation of ventilation devices and systems responsible for the governance of the device deployments, as defined in HTM 03-01) which will include competent persons (section 6) including the Authorising Engineer (Ventilation) and representation from infection and prevention control, nursing and clinical engineering and/or estate management departments. The process will require considering infection control and health and safety aspects specific to the clinical requirements and patient groups within the particular setting and the safe installation of a portable electrical device.
7.2 Ventilation and device effectiveness
The Ventilation Safety Group will consider air flow strategies which achieve the most effective ventilation of occupied spaces. This requires that all factors such as air flow rate, mixing and distribution, dilution, thermal buoyancy and the impact of occupant movements and must be considered.
Airflow patterns and ventilation rates can be evaluated using measurements of air velocities, indoor air quality (IAQ) monitoring and visual methods such as smoke tracing. Computational Fluid Dynamics (CFD) modelling can also be a useful tool to assist the ventilation design engineer to assess airflow patterns in the rooms where HEPA filter devices are to be located. CFD, particle tracing and other forms of airflow assessment can be used to identify the optimal locations to place devices. CFD modelling requires specialist knowledge, and any simulations should be carried out by a competent person.
Airflow and particle/IAQ measurement, visualisation and CFD simulations can illustrate typical airflow patterns but unless carried out over a sustained period of time may not be able to capture all of the fluctuations that occur in real environments, particularly those that are naturally ventilated.
Air cleaner device performance depends on both the flow rate through the HEPA filter and the way the device distributes the air in a room, and both are important factors for ensuring devices are effective and properly positioned. Assessing how a device affects the air flow in a room using the approaches described above can give greater assurance that the device is sufficiently sized for the room and is positioned to be able to distribute air properly.
Although many devices are supplied as portable, they should be sized to the space where they are normally used. If a device is moved to a new location then it is recommended that a suitable risk assessment is undertaken by a competent person to ensure that the device is still likely to be effective.
7.3 Installation
The installation of any HEPA filter-based devices should comply with all building regulations and electrical guidance. A risk assessment should be undertaken by competent persons (section 6) including representation from infection and prevention control, nursing and clinical engineering and/or estates departments.
Units should be positioned so that they do not interfere with the provision of care or provide an obstruction. Floor standing devices can be a trip hazard in some locations and need to be positioned to ensure they or their cables do not pose a risk to patients and staff and do not impede access. This includes ensuring that power cables or other elements of the device do not pose a ligature risk. Consideration should include risks for people who have visual impairments or restrictions on their mobility.
Devices should consider the manufacturer’s recommendations around the best positioning to maximise the effectiveness alongside practical considerations around space available in a room and access to power supply, cable routes, etc.
Devices should ideally be positioned so that there is effective airflow into and out of the unit. Airflow inlet and exhaust panels on devices should not be blocked by furnishings and devices should be designed such that objects cannot be placed on top to cover the vents. Patient comfort should also be considered with devices positioned such that they do not create uncomfortable draughts.
Consideration should also be given to whether portable devices could be deliberately or accidentally moved or pushed over by patients or visitors. Device design should be stable and not easily toppled. In some settings it may be prudent to ensure there are design features that enable devices to be secured so that they cannot be moved. Devices which rely solely on remote controls or app-based controls are not recommended for healthcare settings. Remote controls tend to get lost and there may be privacy or Wi-Fi connectivity issues with app-based control. Devices which use voice activated controls linked to the internet (eg Alexa type systems) should not be used in healthcare settings as there are likely to be concerns around privacy.
7.4 Commissioning
Commissioning shall involve ‘acceptance testing’ according to local SOPs and include electrical safety testing to IEC 60601 (section 4.1.2). An audit of document compliance to the Low Voltage Directive is to be recorded. Where medical device classification is claimed, regulatory compliance with ISO 13485 Class 1 should be evidenced.
7.5 Verification and validation of performance
Manufacturers should evidence claims of engineering specifications (verification) and efficacy (validation) (section 5.1).
Devices should be checked every time the filter is changed (section 8.2) or the device is moved and periodically to ensure that performance is maintained. This can be accomplished by automated or manual measurement of the filter pressure drop under all of the device flow rate conditions as detailed in (section 5.1).
7.6 Training
Clinical and nursing staff in areas where HEPA filter-based air cleaning devices are located should receive training on operational and safety issues. A protocol should be in place such that staff can notify clinical engineering and/or estates management departments of suspected device malfunction. In a healthcare context, such training can often be manufacturer or supplier provided and might be included in staff mandatory training programmes.
7.7 Labelling
All HEPA filter devices should be labelled to inform users of operating procedures and potential hazards. Labels should serve to make users aware of how to interact with HEPA filter devices.
8. Maintenance
Day-to-day cleaning of devices and routine visual inspection (eg damage to casing, wear on cables, etc) can be carried out by healthcare or cleaning staff. Maintenance including filter replacement should only be conducted only by a designated competent person.
8.1 Cleaning
The outside surfaces of devices should be designed to be easily cleaned as part of standard cleaning regimes in the healthcare setting and should not have features which are prone to collecting dust and dirt. The device should be robust to cleaning materials routinely used in healthcare settings. Cleaning instructions should be provided by the manufacturer and easily visible to staff attending the unit.
8.2 Filter replacement
SOPs must be in place for both replacing and safe disposal of used filters. Evidence suggests that the hazards posed by filters are small (Mittal, 2011), but there could be potential risks from pathogens that have been trapped by the filter and hence risk assessments and guidance should be in place.
Filter changes should follow the manufacturer guidance regarding the process and internal cleaning of the device. Filters should not be changed in clinical areas due to the possible hazards of microorganism and dust dispersal during the procedure. Those carrying out filter changes should wear appropriate PPE as agreed with their infection control team.
Disposal of used filters requires a suitable risk assessment for safe bagging, handling and appropriate waste disposal for the used filter as it is potentially contaminated with pathogenic microorganisms.
When new filters are installed they must be correctly seated as per manufacturer guidance to ensure there are no airflow leaks around the filter. Verification tests should be carried out after the new filter is installed (section 5.1).
8.3 Annual checks
All devices should undergo at least annual checks to verify their continuing performance. These checks should include, but are not limited to, the following:
- visual inspection of external and internal
- electrical safety test (section 4.1.2)
- check alarms simulate failures
- check filter run times and replace if necessary (section 8.2)
- clean internals of the device.
- replacement and safe disposal of any filters (section 8.2)
- check and document air flow rate measurements at different fan speeds against manufacturer’s characteristic-specification (section 5.1)
- check and document noise levels against manufacturer’s characteristic-specification (section 5.3)
- for devices that also include UVC, ensure checks set out in Application of ultraviolet (UVC) devices for air cleaning in occupied healthcare spaces: guidance and standards, have also been completed
- apply annual check sticker.
9. Building Management System (BMS) module
The incorporation of a BMS (Building Management System) module into HEPA filter devices is recommended to afford the assurance of effective operation and to support maintenance scheduling. This can also be used to help identify any devices which have been inadvertently switched off, as well as the physical location of devices that are portable. Modules should be enabled with the Modbus or BACNet* open protocol for interfacing with existing an BMS.
*BACnet is a communication protocol for building automation and control (BAC) networks using the ASHRAE, ANSI and ISO 16484-5 standards protocol.
Annex 1 – Bibliography
Laboratory chamber studies demonstrating effectiveness of HEPA filter devices against particles and microorganisms
- [R1] Miller-Leiden S, Lohascio C, Nazaroff WW, Macher JM (1996) Effectiveness of in-room air filtration and dilution ventilation for tuberculosis infection control. Journal of the Air & Waste Management Association 46: 869–882. doi:10.1080/10473289.1996.10467523
- [R2] Offermann FJ. et al (1985) Control of respirable particles in indoor air with portable air cleaners. Atmospheric Environment 19: 1761–1771. doi:10.1016/0004-6981(85)90003-4
- [R3] Ueki H, Ujie M, Komori Y, Kato T, Imai M, Kawaoka Y (2022) Effectiveness of HEPA filters at removing infectious SARS-CoV-2 from the air. mSphere 7(4):e0008622. doi:10.1128/msphere.00086-22.
- [R4] Beswick A, Brookes J, Rosa I et al. 2022. Room based assessment of mobile air cleaning devices using a bioaerosol challenge. Applied Biosafety Journal. Published online Dec 2022. doi:10.1089/apb.2022.0028
- [R5] Lindsley WG et al (2021) Efficacy of portable air cleaners and masking for reducing indoor exposure to simulated exhaled SARS-CoV-2 Aerosols — United States, 2021. Morbidity and Mortality Weekly Report (MMWR) 70: 972–976. doi:10.15585/mmwr.mm7027e1
Testing approach for Clean Air Delivery Rate
- [R6] Foarde KK, Myers EA, Hanley JT, Ensor DS, Roessler PF (1999) Methodology to perform clean air delivery rate type determinations with microbiological aerosols. Aerosol Science and Technology 30: 235–245. doi:10.1080/713834074
Application of HEPA devices in healthcare setttings
- [R7] Conway Morris A, Sharrocks K, Bousfield R, et al, The Removal of Airborne Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Other Microbial Bioaerosols by Air Filtration on Coronavirus Disease 2019 (COVID-19) Surge Units, Clinical Infectious Diseases, Volume 75, Issue 1, 1 July 2022, Pages e97–e101, doi:10.1093/cid/ciab933
- [R8] Abdul Salam ZH, Karlin RB, Ling ML, Yang KS. The impact of portable high-efficiency particulate air filters on the incidence of invasive aspergillosis in a large acute tertiary-care hospital. American Journal of Infection Control. 2010 May;38(4):e1-7. doi:10.1016/j.ajic.2009.09.014.
- [R9] Bowles C, et al. A rapid review of supplementary air filtration systems in health service settings. September 2022. doi:10.1101/2022.10.25.22281493 medrxiv preprint
Wider reading on air cleaning applications
- [R10] Medical Advisory Secretariat. Air cleaning technologies: an evidence-based analysis. Ontario health technology assessment series vol. 5 (2005) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3382390/
- [R11] SAGE-EMG: Potential application of air cleaning devices and personal decontamination to manage transmission of COVID-19, 4 November 2020 https://www.gov.uk/government/publications/emg-potential-application-of-air-cleaning-devices-and-personal-decontamination-to-manage-transmission-of-covid-19-4-november-2020
Annex 2 – Acknowledgements
NHS England would like to thank the Institute of Mechanical Engineers (IMechE), the Chartered Institution of Building Services Engineers (CIBSE), the Institute of Physics and Engineering in Medicine (IPEM), the NHS Innovation Agency (AHSN NW Coast) and the Office of The Chief Scientific Office NHS England for their expertise in creating this document, and in particular our thanks go to the following colleagues who made expert contributions:
Authors
- Michael Ralph CEng FIMechE
- Professor Anthony Fisher MBE MD PhD FIET FInstP FIPEM FAHCS CEng CPhys CSci
- EUR ING Frank Mills BSc CEng FCIBSE FIMechE FASHRAE
- Paul Waldeck CEng MICE MIStructE MASHRAE MIMechE
- Professor Catherine Noakes OBE PhD CEng FREng FIMechE FIHEEM HonFCIBSE
- Mark Jackson TD VR MBA BEng(Hons) CEng FIMechE
- Stephen Clifford CEng BSc MCIBSE FIHEEM
Contributors
- Barry Paterson BSc CEng MIMechE
- Professor Fred Mendonça BSc MSc DIC ACGI
- Professor Chris Hopkins BSc MIET MIPEM FAHCS CEng
- EUR ING John Cashmore MCGI FIHEEM FIIRSM RSP MCIBSE MIET CBuild E MCABE MIGEM CEng CMgr MCIM
- Ian F Giles B Eng (Hons) C Eng MCIBSE
- Darren Sloof
- Andrew Carnegie SGA MIOD Dip.MPPM Dip.Th CAIHEEM BSA
- Dr Matthew J. Butler MBBS BSc MRCP (UK, Geriatrics) PGCert (Health Res.)
Annex 3 – Glossary
- Active operational life: A product’s operational life is the period for which a product is in use before it becomes obsolete, in terms of UVC lamps it is typically 70% of original efficacy.
- Air changes per hour: Air changes per hour (ACH) is the measurement at which air volume per hour is added to a room divided by the total volume of the room. It represents the number of complete air exchanges in one hour under perfect air circulation conditions. See also Equivalent air changes per hour.
- Air circulation: Mixing of the air from natural or mechanical ventilation sources inside an enclosure.
- Air circulation efficiency (%): A measure of the effectiveness of air circulation in a real enclosure with obstructions such as occupancy and furniture, compared with perfect mixing as quantified by ACH/eACH. CFD studies in hospital and high-street treatment rooms indicate that the air circulation efficiency can vary between 40% and 80% depending on the device placement and proximity of furniture, equipment and occupancy. Similar variance applies to AGP-clearance and therefore will affect fallow time.
- BMS (Building Management System): A computer-based control system installed in buildings that controls and monitors the building’s mechanical and electrical equipment such as ventilation, lighting, power systems, fire systems and security systems.
- Building regulations: Building regulations set standards for the design and construction of buildings to ensure the safety and health for people in or about those buildings. They also include requirements to ensure that fuel and power is conserved, and facilities are provided for people, including those with disabilities, to access and move around inside buildings. Current standards require that Health Care buildings conform to NHS standards. For ventilation NHS HTM-03 applies.
- CFD (computational fluid dynamics): Computer-based fluid dynamics modelling providing a means to simulate air flow combined with convective/buoyant/conductive/radiative heat transfer, particulate transport (aerosols and droplets) and turbulence.
- Characteristic specification (Characteristic verification): A measurable property of the device that can employed routinely by the user to provide assurance of device operation to the verification model. See Verification.
- Clean Air Delivery Rate (CADR): Experimentally derived data that expresses the performance of a filter-based air cleaning device in a test room. CADR is a function of the airflow rate through the device, the quality of the filter and the way the device distributes air in the test room.
- Clearance: The relative removal of a contaminant usually expressed as %. See Log reduction.
- Decontamination: Decontamination describes the reduction of pathogenic microorganisms to a safe level for human use. Technically, this means reduction by a minimum of 1 log step, meaning 90%.
- Disinfection: The term disinfection is not clearly defined in a technical sense. Generally, for the purposes of this standard, it means a reduction of pathogenic microorganisms by a minimum of 3 log steps Or 99.9%
- Equivalent air changes per hour, eACH: Equivalent air changes per hour, or eACH, is a measure of the ‘equivalent’ amount of air that is cleaned by a HEPA or UVC device as a ventilation rate of new outside-air changes would achieve in one hour. See ACH. Note that this applies to decontamination and does not obviate the need for meeting minimum fresh air standards.
- Electrical Safety Test (EST): Requirement of the Low Voltage Directive to demonstrate general electrical safety.
- Electrostatic precipitation (ESP): A method of removing particles from air by applying a charge to the particles (often through an ioniser) and then capturing onto a plate which has an opposite charge. Some filter-based air cleaners incorporate ESP.
- Fallow time: Time (s/min/hr) allocated to a treatment room without occupancy to allow for clearance of the room after a contamination event (eg an AGP) to recover safe levels for occupancy.
- Germicidal ultraviolet/germicidal ultraviolet irradiation: Referred to commonly as GUV and UVC. Both are one and the same in that they refer to ultraviolet C spectrum light that is germicidal.
- Hazard assessment: A hazard assessment is a thorough check of the occupational environment. The purpose of a hazard assessment is to identify potential risks and hazards in the area, as well as to identify appropriate safety measures to be used to mitigate, eliminate or control the identified hazards.
- HAZOP: [Hazard Analysis and Operational study] – a systematic way to identify hazards in a work process.
- HEPA: High Efficiency Particle Air Filter, used to describe a filter with a very high particle filtration efficiency with over 99.95% removal for the smallest particles (see MPPS).
- IAQ (Indoor Air Quality): A generic term used for air quality in enclosed spaces, usually referring to the combination of harmful gases (eg. CO2 and CO levels measured in parts-per-million, ppm), temperature (for thermal comfort), total volatile organic content (TVOCs measured in parts-per-billion, ppb), relative humidity (%) and particulate matter size (respiratory irritants/hazards) measured in micrograms/m3, eg. PM2.5, PM10.
- Infection: The process by which pathogens penetrate the body of an organism and multiply therein. Depending on the transmission route, we distinguish between contact infections and airborne infections.
- Infectiousness: Measure for describing the ability of a pathogen to cause actual infection in a host after transmission occurs.
- Ioniser: A device that uses a high voltage to electrically charge air molecules and particles in air. Ionisers are sometimes used as part of electrostatic precipitators or are used to emit ions into a room. There is evidence that ionisation of air can result in ozone generation.
- Ionising radiation: Ionising describes the type of radiation capable of permanently removing electrons from atoms or molecules. Note: UVC radiation has no ionising power.
- Log reduction: The reduction of a contaminant can be quantified in log stages. A Log reduction of ‘x number’ therefore means a reduction by ‘x number Log’ stages starting from a given population. The reduction by 1 log stage means a reduction of 90%, since only 10% have survived from the original population. See Clearance.
- Log stage (a.k.a. Log step): A log stage or log step describes the reductio n of a population by a (further) power of ten: in other words, 1 log stage = 90%, 2 log stages = 99%, 3 log stages = 99.9%, etc. See Log reduction.
- Microorganism (microbe): A microorganism is an organic structure so small that they can generally only be seen with the aid of a microscope and include viruses, bacteria and fungi. Such structures are usually single-celled, although they are occasionally multi-celled.
- MPPS: Most Penetrating Particle Size. The size of particle that leads to the lowest performance for a filter. For HEPA filters this is typically in the region 0.2-0.5 μm diameter particles.
- Nosocomial infection: An infection contracted in a hospital or care institution.
- Ozone: Represented as O3. Ozone is a gas with strong oxidation properties that is toxic in low concentrations. Ozone can result from the oxidation of O2 irradiated by far UVC.
- Pathogen: Pathogens are microorganisms capable of causing disease or illness in living creatures.
- Photocatalytic oxidation (PCO): Use of ultraviolet light with a catalyst (usually titanium dioxide) to generate hydroxyl radicals. These can potentially react with air pollutants to break them down, however they may also produce ozone or act to convert some pollutants into other chemicals.
- Sanitisation: The process of reducing microbiological contamination. See Clearance and log reduction.
- Single pass effectiveness: The percentage (or log) reduction in particles or microorganisms in the air that directly passes once through an air cleaning device. This is determined by the grade of the filter and the air flow rate through the device.
- SOP: (Standard operating procedure) A set of step-by-step instructions compiled by an organization to help workers carry out routine operations.
- Sound pressure level: dB3m: The acoustic output pressure represented by dB measured at 3 m from the source.
- Validation (bio-validation): The process to provide assurance that the device is effective as claimed by the manufacturer. For the purposes of this standard, assurance that particle removal or microorganism reduction is achieved as claimed.
- Verification: The process to provide assurance that the device performs to the manufacturer’s specification. For the purposes of this standard, assurance that air flow and filter performance are as claimed.
- Viruses: Viruses are particles or information carriers dependent for survival and replication upon the metabolism of a host cell since they themselves have no cytoplasm and are incapable of metabolism. Viruses are thus, de facto, not living organisms.
The National Estates and Facilities team at NHS England is responsible for producing Standards and Guidance for the NHS estate and ensuring that the information and guidance they contain remains up-to-date and relevant for users.
NHS Estates Technical Bulletins (NETBs) enable updated guidance to be passed to local systems, ensuring we maintain our focus on patient safety. NETBs contain technical guidance and standards which systems and organisations are required to consider and implement, where applicable. Boards are responsible for their assessment and application to their organisations.
Date of issue: 9 May 2023
NHS Estates reference: NETB 2023/01A
Publication reference: PR1324_ii

