Best practice timed diagnostic pathways
Best practice timed pathways support the ongoing improvement effort to shorten diagnosis pathways, reduce variation, improve experience of care, and meet the Faster Diagnosis Standard (FDS). This guidance will support cancer alliances and constituent organisations to adopt consistent, system-wide approaches to managing this diagnostic pathway.
This guidance sets out how diagnosis within 28-days can be achieved for the suspected breast cancer pathway. Alongside the pathway itself, resources are highlighted to support implementation of the pathway.
This breast pathway is part of a series, published since April 2018. From previous pathways implemented by cancer alliances, implementation guidance was shared in June 2021. This includes areas that are key to success, such as clinical and operational engagement, auditing pathways, allocating project management resources, ensuring leadership, analysing data, and sharing successes.
This guidance complements existing resources such as National Institute for Health and Care Excellence (NICE) guidelines (including NG12) and should therefore be read alongside such guidance.
While the guidance stipulates recommended clinical actions and timings, we recognise that this will not apply to all people in all circumstances, and responsibility for clinical decision making remains with local clinical teams with the knowledge and expertise to make appropriate decisions and policies.
The pathway in this document was developed by a multidisciplinary consensus group with clinical leaders from local and specialist services across England, expert advice from cancer alliances, and people with lived experience.
For any questions about this document please email england.cancerpolicy@nhs.net
Dame Cally Palmer, National Cancer Director, NHS England
Professor Peter Johnson, National Clinical Director for Cancer, NHS England
Dr Catherine Harper-Wynne, Chair of the Breast Task and Finish Group, NHS Cancer Programme
Executive summary
This best practice timed pathway is being published at a time of huge pressure on breast services. This pressure is partly driven by a significant increase in referrals, particularly in younger age groups with a low risk of cancer (see figure 1).
This best practice timed pathway specifically addresses the challenge of constrained capacity in one stop clinics and represents a departure from historic practice by mandating triage of all referrals and diversion of patients away from the one-stop clinics for those at low risk of a cancer diagnosis who do not have red flag symptoms.
This makes best use of the one-stop clinic for those who need examination, imaging, and potential biopsy. It also ensures that those at low risk are seen in an appropriate setting and avoid the unnecessary imaging that often occurs in the one stop clinic. Most of these patients can be seen by a single clinician, and do not need the multidisciplinary resource of the one-stop clinic.
This will ensure the best use of one-stop clinic resource, reduce unnecessary and inappropriate imaging, and improve patient care.
The Task and Finish Group recognises that the best practice timed pathway requires sufficient workforce to implement, and the group suggests that workforce requirements for the pathway should be assessed locally to ensure that long term workforce needs are met. NHS England recognises in the NHS Long Term Workforce Plan that workforce capacity and alignment with service planning is required to meet evolving challenges.
A cancer diagnosis (without receptor status) is no longer sufficient to manage patients with breast cancer and therefore tumour receptor status has been incorporated into the pathway to allow treatment planning by day 28, which goes further than achieving the Faster Diagnosis Standard.
The group recognises that localities will have differing practices with regard to ‘cancer not suspected.’ It is important, therefore, to understand that the timepoints and pathway suggestions in the pathway are best practice guidance to achieve the Faster Diagnosis Standard.
New pathways for management of patients with symptoms of breast pain should be evaluated to ensure safety and patient satisfaction. The Association of Breast Surgery is currently running a platform evaluation study – the Aspire Study – for evaluation of new pathways. The group recommends that providers join this study for pathway evaluation. The evaluation is open to participation from all providers and includes both new dedicated breast pain pathways and the traditional triple assessment model within the evaluation.
Figure 1: Volume of quarterly urgent suspected breast cancer referrals by age, in England
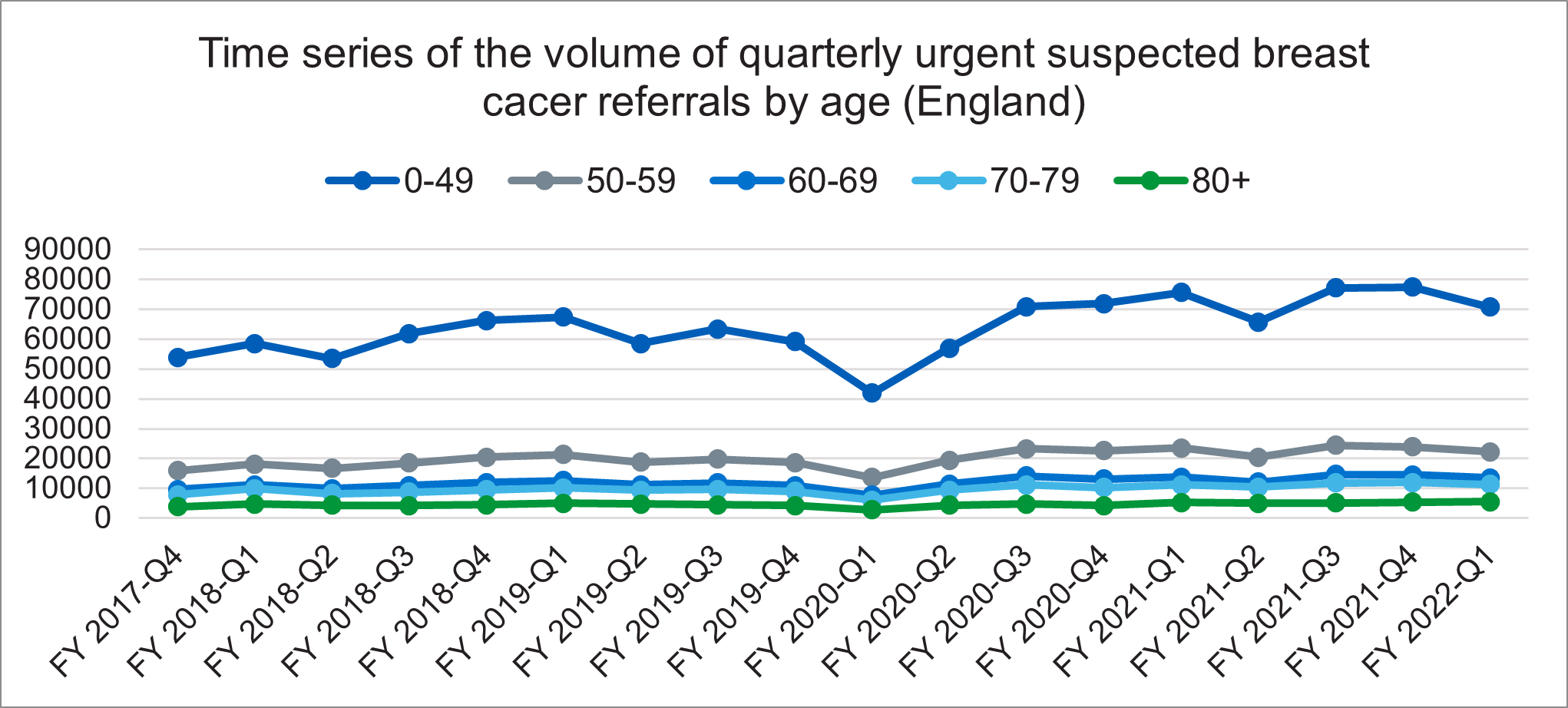
Figure 1 above shows the number of referrals made on a quarterly basis for urgent suspected breast cancer, split by age groups, zero to 49 years, 50 to 59 years, 60 to 69 years, 70 to 79 years, and 80 years plus. The graph shows that the age group zero to 49 years has increased more than the other age groups over the same period. The age group zero to 49 years has a lower overall risk of having breast cancer.
The Faster Diagnosis Standard
We committed in the NHS Long Term Plan to provide a faster diagnosis for people through the introduction of the Faster Diagnosis Standard (FDS). This standard will ensure people are told they have cancer, or that cancer is excluded, within a maximum of 28 days from referral. The new standard is intended to:
- Reduce the time between referral and diagnosis of cancer. The timed pathway sets the expectation that a breast cancer diagnosis includes the tumour receptor status, ie the oestrogen receptor (ER), the HER2 receptor, and in some centres, +/- the progesterone receptor (PR). It is recognised that if HER2 fluorescent in-situ hybridisation (FISH/ISH) is required, this may not be available for a further 7 days. In this case, a diagnosis should still be provided within 28 days without FISH/ISH if it is not available in time. Communicating the cancer or non-cancer diagnosis to the patient is required to stop the 28-day clock as specified within the Cancer Waiting Times guidance.
- Help to reduce anxiety for the cohort of people who will be diagnosed with cancer or have cancer ruled out.
- Reduce unwarranted variation in England by understanding how long it is taking people to receive a diagnosis or have cancer ruled out.
- Represent a significant improvement on the current two-week wait to first appointment target, and a more person-centred performance standard.
FDS performance data, including a breakdown by suspected cancer pathway, has been published since June 2021, and faster, more streamlined pathways will be a priority.
Those with suspected cancer should be seen in a one-stop clinic with same day access to clinical examination, mammography, ultrasound, and biopsy.
For those referred urgently with breast symptoms where cancer is not initially suspected, such as breast pain in the absence of any other symptoms, or gynaecomastia, each unit, working with their cancer alliance, should review their current practice and ensure it aligns with the guidance in this document. The purpose of the ‘cancer not suspected’ pathway is to reduce unnecessary and inappropriate imaging, to make most effective use of limited resources. A number of different models have been suggested, examples can be found in the Breast Getting It Right First Time Programme national speciality report. Any new models need robust evaluation to ensure they meet the needs of patients. The Association of Breast Surgery (ABS) have developed a platform to support the evaluation of different breast pain pathways.
As the key system-wide organisation for cancer services, cancer alliances will need to work across the local system to ensure that implementation is prioritised by senior stakeholders, clinical leaders, and operational colleagues, and that capacity is optimised to enable the standard to be delivered, including ensuring that providers are having due regard for monitoring differences in FDS performance across different patient cohorts and identify the underlying causes.
The FDS has been formally performance managed since October 2021, in line with cancer services recovery, with an initial threshold of 75%. Cancer alliances will need to ensure that they have plans to meet the threshold, which will need to be increased in subsequent years if we are to contribute to achieving the early diagnosis ambitions in the NHS Long Term Plan.
We are focused on increasing the delivery of the high-performing breast pathway, to see high performance on the FDS overall.
The case for change
Breast cancer is the most common cancer in England with suspicion of breast cancer being the most common suspected urgent referral type in England with more than 487,000 suspected breast cancer referrals a year, representing around 17% of all urgent suspected referrals in 2022/23.
Between April 2022 and March 2023, 87% of people received a communication of diagnosis of cancer or cancer ruled out within 28 days of referral. This relates to all Faster Diagnosis Standard (FDS) cohorts, suspected breast, breast screening, and breast symptomatic. Breast performance is higher than general performance across specialties, but there is some variation across cancer alliances with a range of 77% to 95%.
Between April 2020 and March 2021, 85% of people diagnosed with breast cancer on an urgent referral pathway commenced treatment within 62 days of referral. This guidance aims to support further improvement in referral to treatment performance for this cancer.
Creating streamlined and efficient pathways, including for those in whom breast cancer is not suspected, has the potential to help reduce overall waiting times and the considerable variation currently seen across the country. This pathway intends to reduce the pressure on one-stop clinics, with less inappropriate imaging.
Alongside adoption of the best practice timed pathway, cancer alliances must ensure appropriate resources and capacity are in place to deliver high-quality services to more people. This includes having sufficient capacity within pathology services (medical and scientific) to analyse and deliver diagnostic results, including information on tumour receptor status, in a timely way. This document does not prescribe exactly how all aspects of the service are delivered and cancer alliances should work with primary care and secondary care teams to deliver within their geographies.
It is important to consider workforce planning (across the screening and symptomatic pathways) especially within the breast service given its high reliance on diagnostics to meet increasing demand and ensure best utilisation of the highly skilled workforce. The aim of this document is to address the clinical pathway, however existing workstreams within NHS England continue to review workforce planning as part of ongoing work to deliver on the NHS Long Term Workforce Plan.
Figure 2: Breast 2 week wait referral to treatment Cancer Waiting Times data for England, 2018/19 quarter 1 to 2022/23 quarter 1
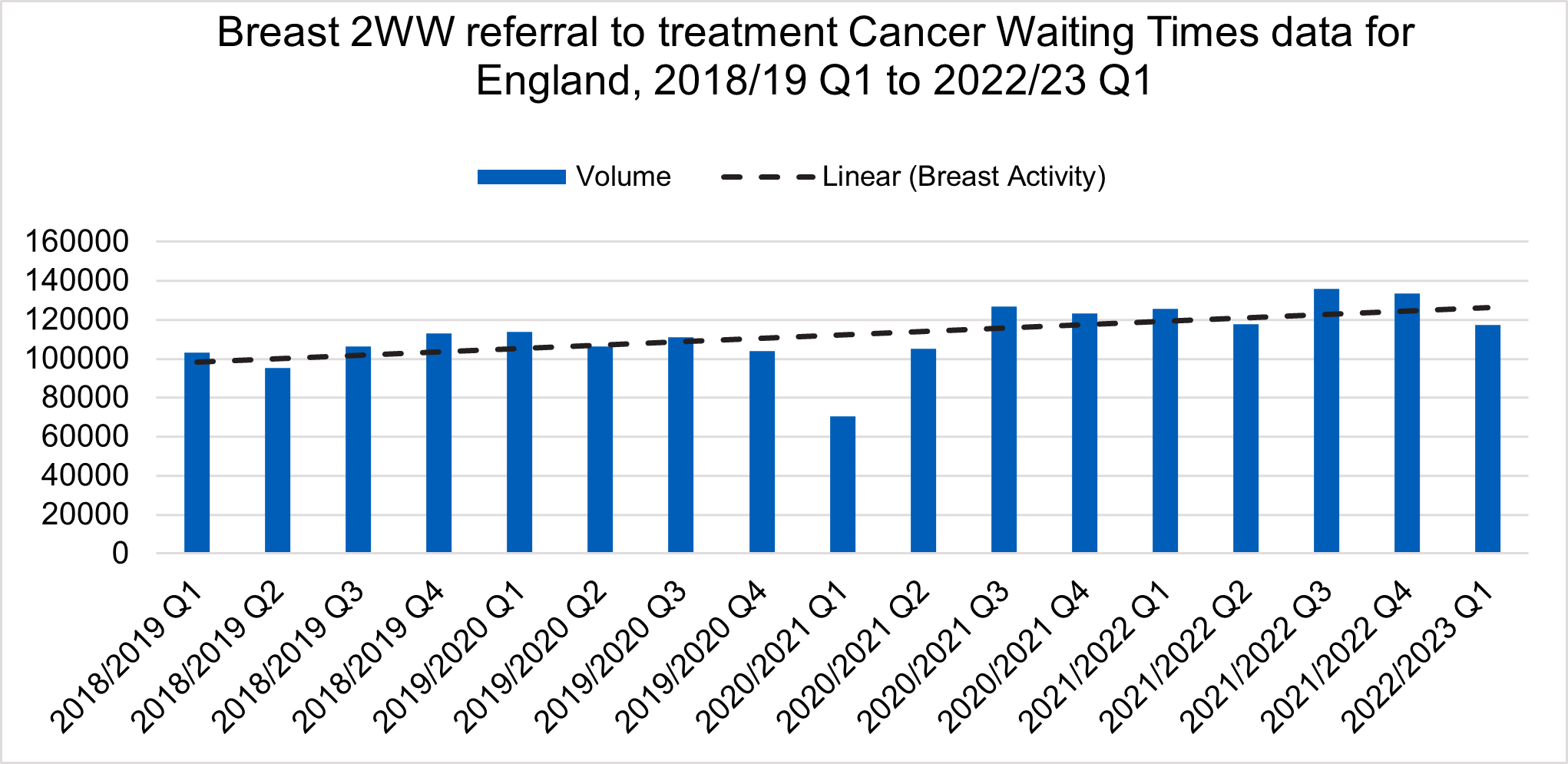
Figure 1 above shows the number of referrals made on a quarterly basis by primary care to secondary care services for urgent suspected breast cancer. When removing the effect of seasonality and variation of referral numbers, the graph shows a continuing upward trend of referral numbers, from the baseline of 100,000 per quarter in quarter one of 2018/19 to 125,000 per quarter in quarter one of 2022/23.
NHS England provides support, funding and guidance to help cancer alliances improve outcomes and reduce variation. The following support is available:
- funding and programme management to support delivery to achieve the FDS and best practice timed pathway milestones
- implementation guidance for achieving pathways
- collaboration and networking events to share best practice.
“The pathway for most people, from referral by the primary care clinician or from a screening service to diagnosis or the exclusion of breast cancer is straightforward. However, this document aims to improve the quality of cancer diagnosis as well as the time to exclude or diagnose cancer.
Large and increasing volumes of work mean that effective and robust capacity and demand analysis is required to ensure that resources are appropriate. Networked support should be made available to deliver and monitor this pathway.
Addressing workforce shortages nationally, and effective administration of the pathway, including excellent support for patients and timely access to clinical nurse specialist is also required”.
Dr Catherine Harper-Wynne, on behalf of the Breast Task and Finish Group, NHS Cancer Programme
“The inclusion of receptor status in the diagnosis is much more meaningful to the patient as it allows more information and an initial treatment plan to be discussed at the same time as being told you have cancer. Waiting, fear of the unknown, and lack of ability to plan ahead are incredibly difficult parts of waiting to hear about a cancer diagnosis. Therefore, the Breast FDS has been designed to minimise that as far as possible and ensure patients on this pathway can get the meaningful information they need as quickly as possible”.
Jo Chambers, Patient and Public Voice Forum Member, on behalf of the Breast Task and Finish Group, NHS Cancer Programme
“We need a better standard for supporting patients for quicker and more effective diagnosis. Our group have worked hard to make sure that patients are going to be better supported with a more rapid diagnostic system. Managing patient expectations and supporting patients with effective communication is key to the way forward”.
Jo Taylor, Patient and Public Voice Forum Member, on behalf of the Breast Task and Finish Group, NHS Cancer Programme
Benefits of pathway change
For patients and unpaid carers
- For those in whom cancer is suspected, faster access to the one-stop clinic with access to triple diagnostic assessment in a single hospital visit may reduce the anxiety and uncertainty of a possible cancer diagnosis, with less time between urgent referral and receiving the outcome of diagnostic tests.
- Improved patient experience with as few visits to the hospital as possible, ideally to specialist centres where available, and avoiding emergency admission.
- Appropriate management and support for those in whom breast cancer is not suspected within the same 28-day timeframe.
Experience of care
- Patients and carers know they are urgently referred for investigation of suspected cancer and should expect a diagnosis and to agree a management plan within 28 days. For some patients this may include further diagnostics (such as specialised biopsies or breast MRI), and these further tests should also be available in time to start optimal treatment within 62-days of initial urgent referral.
- Ensure that patients and carers’ ability to attend appointments is taken into account and additional support is offered to help with this, for example providing translation services, where necessary.
- Patients are communicated with clearly, understand the information provided, and are given additional support, such as access to a clinical nurse specialist or a patient navigator.
- Care and support that meets the needs of those in whom breast cancer is not suspected but are experiencing significant issues with their breasts, using evidence based clinically robust models of care delivery and subject to evaluation. See Association of Breast Surgery (ABS) position statement on breast pain.
For clinicians
- Access to appropriate pathways that better meet the needs of people referred with breast symptoms where cancer is not suspected and allow for imaging and pathology resources to be focused on urgent referrals where cancer is suspected.
- Allow clinicians to provide more information to breast cancer patients at the point of diagnosis and have an informed discussion about treatment options at an earlier stage of the pathway.
- Streamlining multidisciplinary team (MDT) cases and discussions, including potential use of pre-determined diagnostic algorithms. The MDT toolkit is available on the ABS website to support discussions.
For systems
- Effective management of patients who are not suspected to have cancer will reduce demand on imaging and pathology services.
- Optimised referral triage to ensure effective use of limited resources in the one-stop breast clinic.
- Improved quality, safety, and effectiveness of care with reduced variation and improvement in outcomes.
28-day best practice timed pathway (cancer suspected)
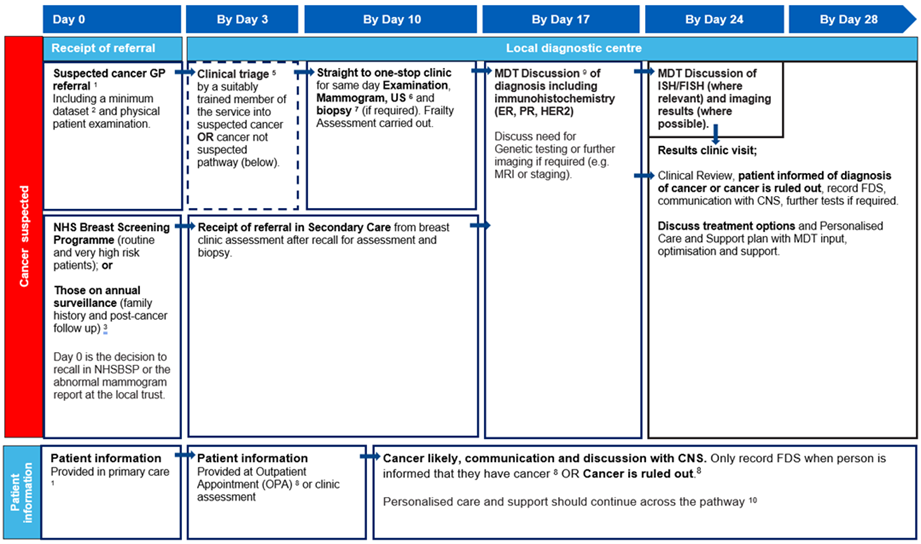
The pathway diagram above shows the key clinical and patient milestones within the breast best practice timed pathway from referral by day zero to decision to treat by day 28 for patients referred and triaged on a suspected breast cancer referral.
Timings shown in this pathway are recommendations only. See detailed information section for further details.
If cancer is suspected, refer straight to one-stop clinic/MDT on same day (parallel clinic) or next available.
These patients should still receive their cancer diagnosis as per FDS, by day 28.
28-day best practice timed pathway (cancer not suspected)
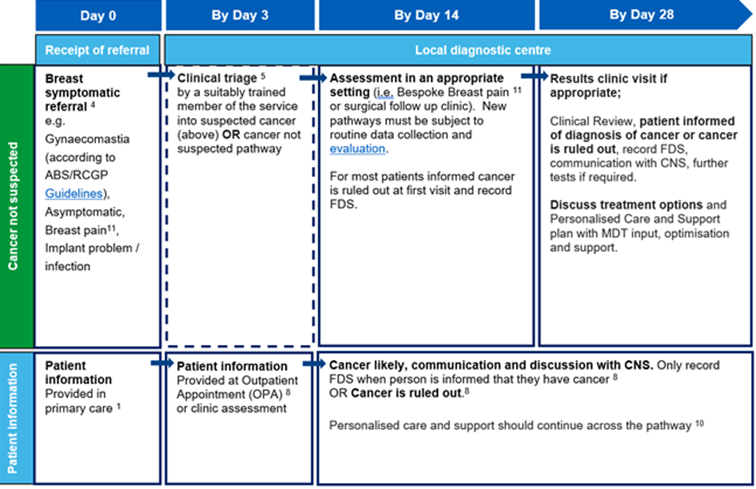
The pathway diagram above shows the key clinical and patient milestones within the breast best practice timed pathway from referral by day zero to decision to treat by day 28 for patients referred and triaged for a referral where breast cancer is not initially suspected.
Timings shown in this pathway are recommendations only. See detailed information section for further details.
Detailed information
1. Urgent GP referral pathway should be used for people who meet NG12 criteria for suspected cancer pathway referrals. The National Cancer Waiting Times Monitoring Dataset guidance sets out consultant upgrade rules, including from non-GP scenarios such as A&E and acute settings. Cancer Alliances may agree local arrangements to facilitate self-referral, community diagnostic centres and other referral routes, to access this pathway. Any new arrangements should be audited.
It is noted with the implementation of community diagnostic centres that referral pathways may be subject to change. Primary care should inform people that they are being referred for an urgent suspected cancer pathway and that a diagnosis will be provided within 28 days, although stating that the vast majority of referrals result in non-cancer diagnoses. Primary care should also make people aware of their responsibilities to make themselves available for the first 2 weeks for initial diagnostic testing (National Institute for Health and Care Excellence [NICE] Quality Standard 12).
‘Choose and book’ appointments are no longer widely used. Units still using ‘Choose and book’ should review patients going into these pathways. Patients that choose to delay an appointment even if beyond 28 days do not exempt themselves from the standards.
2. A minimum dataset should be agreed locally with GPs to accompany the referral and facilitate the clinical assessment. GPs should facilitate straight to clinic and immediate diagnostics, to be agreed locally, which should include description of referral reason in line with NG12 guidelines, patient demographics, relevant family history of cancer, performance status, co-morbidities, including diabetes status, dementia, mental health conditions, such as claustrophobia, body mass index, prescribed medication, allergies, family history of cancer, functional/frailty score (eg Rockwood) for patients above 70 uploaded to Cancer Outcomes and Services Dataset as recommended by the national audit of breast cancer in older people, presence of metal implants or pacemakers, need for interpreter, mental capacity to consent. Capacity will need to be considered for completing missing dataset tests in the first outpatient appointment or one-stop clinic, following referral from primary care.
3. A referral for breast screening assessment would only be made after there is consensus that the patient needs to be assessed, whether this is by the initial 2 readers or after arbitration.
Secondary/metastatic/advanced breast cancers – patients with suspected new advanced or locally advanced breast cancer can present in several ways. If this is the first presentation of breast cancer of any sort, ie ‘de novo’, or a suspected late relapse (beyond 5 years) from a previous breast cancer, they may present acutely to general practice, A&E, or via other sources. Any urgent GP referrals for suspected cancer that are diagnosed as metastatic disease with an unknown primary site are still covered by the Faster Diagnosis Standard (FDS).
It is envisaged that most patients that relapse within the 5-year follow up period will access surgical clinics via the breast cancer nurse. Secondary breast cancers that are diagnosed through routine follow up are not included in the FDS but are subject to the 31-day standard for starting treatment from when the treatment decision was made. These are classed as ‘subsequent treatments’ in Cancer Waiting Times data.
4. Breast symptomatic referral – if cancer is not initially suspected, there should be an urgent referral pathway for people with breast symptoms who cannot be managed in primary care. Although not on a suspected cancer pathway, these referrals should still be able to expect an outcome within 28 days of initial referral. Cancer alliances should ensure that any pathways set up to manage patients with breast pain, gynaecomastia and other non-suspected cancer referrals meet the needs of those patients and are delivered in the most clinically appropriate setting. Breast pain as a sole symptom is rarely a presenting feature of breast cancer, occurring in approximately 70% of women. It is not a sign of cancer but can take many months to resolve. In the presence of a normal examination patients can be reassured and may not need imaging. They should receive a link to information leaflets and videos. Non-suspected cancer pathways should maintain close links into one-stop clinic services and refer any patients on the pathway that present with red-flag symptoms. Pathways should be developed in line with existing models of care which have already been evaluated, as well as best practice more broadly.
5. Clinical triage can be done by a suitably trained member of the service. People who attend an outpatient appointment should have same day investigations to reduce repeat visits and improve experience. All those over 85 (or lower age if agreed locally) should have formal frailty assessment performed at the one-stop clinic when a cancer diagnosis is likely (such as National Audit of Breast Cancer in Older Patients frailty assessment or electronic frailty index).
6. Mammogram/ultrasound – patients in whom cancer is suspected should be seen initially in a one-stop clinic with access to triple diagnostic assessment in a single hospital visit including availability for same-day clinical examination, mammography, ultrasound, and biopsy. Imaging should comply with Royal College of Radiologists guidelines and recommendations.
Further imaging should be considered at the diagnostic multidisciplinary team (MDT) according to local protocols and in compliance with Royal College of Radiologists’ recommendations. Ring-fenced general cancer CT and MRI slots should be considered to ensure that capacity is available to deliver expected imaging within 8 calendar days of biopsies.
7. A core biopsy is a prerequisite for diagnosis and development of a management plan. Histopathology reports for tissue sampling should usually be available (including immunohistochemistry) in 7 calendar days. Reflex immunohistochemistry for all M5 and/or U5 biopsies should be considered. The time taken may be longer if ancillary tests are required to establish a primary diagnosis. In instances where fluorescent in-situ hybridisation(FISH/ISH is required to determine HER2 status, the expectation should be for these results to still be available within the 28-day pathway as best practice. The Task and Finish Group recognise workforce challenges currently faced by pathology departments in England (both at consultant and laboratory levels).
All histopathology should have a designated point of receipt, sign-off and management responsibility to ensure the chain of custody is not lost.
8. Patients and carers should be asked what information they require about the pathway, provided with standard information about investigations when sending confirmation of appointment, confirmation of next step(s) and anything required to prepare for the day, and whether they have any disabilities, language barriers or other factors which may need to be taken into account in regard to service accessibility. Preferences for amount of information and when it is provided will vary, and therefore it will help to provide caseworker/navigator telephone contact details to provide support throughout the pathway, provide signposting to charities and support services, provide information about carers attending appointments, and offer follow-up if people do not receive confirmation of an appointment in expected timescales. People should also be informed that it is likely they will receive one or more procedures and/or diagnostic tests on the same day, at the first face-to-face appointment. Patients should be given information on what the tests will involve and how long their appointment is likely to last. The one-stop clinic appointment may take several hours.
People should be informed about cancer being ruled out, or diagnosed at the earliest face-to-face opportunity by default, unless the person has expressed an alternative method of communication to speed up communication. In this timed pathway, this can be done at a one-stop clinic, a follow-up testing or results outpatient appointment, or at a treatment planning outpatient clinic. The diagnosis should include the tumour receptor status, ie the oestrogen receptor (ER), the human epidermal receptor 2 (HER2) receptor status, and +/- the progesterone receptor (PR). It is recognised that if HER2 fluorescent in-situ hybridisation (FISH)/in-situ hybridisation (ISH) is required, this may not be available for a further 7 days. In this case, a diagnosis should still be provided within 28 days without FISH/ISH if it is not available in time. Early consideration of a person’s fitness for definite therapy and requirements for pre-habilitation should be addressed as soon as possible in the pathway to minimise delays in expediting treatment. Patients that require a frailty assessment can be assessed using the National Audit of Breast Cancer in Older Patients frailty assessment or electronic frailty index in advance of multidisciplinary team (MDT) discussion.
Communication to primary care of the urgent referral outcome must occur as quickly as possible.
9. MDT discussion with ER, PR and HER2 further information (where relevant). The core roles at the full MDT (to be carried out following cancer diagnosis) are lead clinician, radiologist, surgeon, pathologist, oncologist, CNS and relevant AHP to review investigation results with an MDT coordinator/pathway navigator. An oncologist with experience in breast cancer and a radiologist with an established breast cancer interest should be present at the full MDT. The capacity required to deliver these core roles should be reflected in job plans. National guidance on how to maximise effectiveness of MDT meetings and the use of standards of care is available. Locally agreed, clear criteria for referral to MDT can also support with efficient pathway management. More than one MDT may be required to make all necessary management decisions.
If patients require genetic testing, providing results would not form part of the FDS diagnosis. Follow-up genetic testing should be considered for people with confirmed breast cancer.
10. Personalised care and support planning should be based on the person and clinician(s) completing a holistic needs assessment (HNA) and personalised care and support plan, usually soon after diagnosis. The HNA ensures conversations focus on what matters to the person, considering wider health, wellbeing, practical issues and support in addition to clinical needs and fitness. The personalised care and support plan also enables shared decision-making regarding treatment and care options to be documented.
11. Breast pain pathway management – new pilots seeking to address breast pain pathways should maximise patient satisfaction, safety, and cost-effectiveness. They must also be able to provide an outcome for patients within the FDS 28-day timeframe for urgent referrals. There should be named clinical leadership and clear lines of accountability for ensuring these pathways are implemented safely, and for their performance against the FDS.
All new breast pain pathways require evaluation. If you are developing a breast pain pathway, please contact the Association of Breast Surgery and Breast Cancer Now to ensure you implement a pathway that is already being evaluated or join their service evaluation.
Additional information
Cancer alliance workspace
Cancer alliances access this workspace for national guidance, resources, and to share learning. Please use this workspace to upload materials you have developed locally and that you think would be useful for colleagues implementing this pathway across the country.
Audit tool
This tool can be used to undertake a baseline audit of services being delivered and whether sufficient capacity is in place to routinely deliver, identify areas for improvement, select measurements for improvement, and conduct re-audits as part of continuous improvement.
Acknowledgements
This guidance was developed by the NHS Cancer Programme and builds on experience and expertise provided by the Breast Task and Finish Group membership outlined below which includes clinical representatives, operational representatives, patient and charity representatives, and the NHS Cancer Programme.
Catherine Harper-Wynne, Consultant Medical Oncologist (Chair), NHS Maidstone and Tunbridge Wells NHS Trust
Alison Hall, Consultant Clinical Oncologist, The Clatterbridge Cancer Centre NHS Foundation Trust
Ashu Gandhi, Consultant Breast Surgeon, Manchester University Hospital NHS Trust
Ayesha Dave, Project Manager, (Faster Diagnosis), NHS England
Chris Holcombe, Past-President; Consultant Breast Surgeon, Association of Breast Surgery; Liverpool University Hospitals NHS Foundation Trust
Leena Chagla, President; Consultant Surgeon, Breast Lead Clinician, Association of Breast Surgery
Claire Herlihy, Lead Clinical Nurse Specialist in Breast Care, NHS Northern Devon Healthcare NHS Trust
Hannah Raphael, Project Manager (Living With and Beyond Cancer Team), NHS England
Inder Kumar, Consultant Breast Surgeon (Provider Operational/Clinical Lead), East Lancashire Hospitals NHS Trust
Jacquie Jenkins, Programme Manager (Screening Representative), NHS England
Jo Chambers, Public Patient Voice Forum Representative
Jo Taylor, Cancer Alliance Patient Representative
Madeleine Webb, Policy Lead (Charity Representative), Breast Cancer Now
Matt Keeling, Transformation Lead (Faster Diagnosis), NHS England
Peter Hawkins, Programme Manager, (Faster Diagnosis), NHS England
Rahul Deb, Consultant Pathologist, Royal Derby Hospital, University Hospitals of Derby and Burton
Sarah Vinnicombe, Consultant Radiologist, Breast Imaging, Gloucestershire Hospitals NHS Foundation Trust
Tracey Irvine, Consultant Oncoplastic Breast Surgeon and Senior Clinical Advisor for Getting It Right First Time (GIRFT)
Vijay Patel, Clinical Fellow, (Primary Care GP representative), NHS England
Publication reference: PRN00536

