Foreword: best practice timed diagnostic pathways
Best practice timed pathways support the ongoing improvement effort to shorten diagnostic pathways, reduce variation, improve experience of care, and meet the Faster Diagnosis Standard (FDS).
The bladder, upper-tract urothelial carcinoma, penile, renal, and testicular cancer pathways are part of a series, published since April 2018.
The 28 Day Faster Diagnosis Standard remains the single standard by which the speed of diagnosis of cancer will be measured under Cancer Waiting Times. However, some types of cancer are particularly aggressive, and particular effort should be made to diagnose them even faster than the headline target. Some testicular cancers and large and invasive kidney cancers fall into this category. This document outlines the testicular pathway setting out the sequence of events that would be required to complete the diagnostic process wherever possible within 21 days are reflective of that.
This guidance complements existing resources such as NICE Guidelines (including NG12), clinical Audits, Getting It Right First Time’s Urology: Towards better care for patients with bladder cancer and Getting It Right First Time’s Urology: Towards better care for patients with kidney cancer and should therefore be read alongside such guidance.
While the guidance recommends clinical actions and timings to support pathway design, we recognise that responsibility for clinical decision making remains with local clinical teams with the knowledge and expertise to make appropriate decisions for patients.
The pathways in this document were developed by a multi-disciplinary consensus group with clinical leaders from local and specialist services across England, expert advice from Cancer Alliances, and people with lived experience.
For any questions about this document please email england.cancerpolicy@nhs.net.
Dame Cally Palmer, National Cancer Director, NHS England
Professor Peter Johnson, National Clinical Director for Cancer, NHS England
Matthew Hayes, Chair of the Urology Task and Finish Group
The Faster Diagnosis Standard
We committed in the NHS Long Term Plan to provide a faster diagnosis for people through the introduction of the Faster Diagnosis Standard (FDS). This standard will ensure people are told they have cancer, or that cancer is excluded, within a maximum of 28 days from referral. The standard is intended to:
- reduce the time between referral and diagnosis of cancer
- reduce anxiety for the cohort of people who will be diagnosed with cancer or receive an ‘all clear’
- reduce unwarranted variation in England by understanding how long it is taking people to receive a diagnosis or ‘all clear’ for cancer
- represent a significant improvement on the current two-week wait to first appointment target, and a more person-centred performance standard
As the key system-wide organisations for cancer services, Cancer Alliances will need to work across the local system to ensure that implementation is prioritised by senior stakeholders, clinical leaders, and operational colleagues, and that capacity is prioritised to enable the standard to be delivered.
The FDS has been formally performance managed since October 2021 activity, in line with cancer services recovery, with an initial threshold of 75%, rising to 80% by the end of 2025/26. Cancer Alliances will need to ensure that they have plans to meet the threshold, which will need to be increased in subsequent years if we are to contribute to achieving the early diagnosis ambitions in the NHS Long-Term Plan.
The case for change
- bladder, upper-tract urothelial carcinoma, penile, renal and testicular cancer account for more than 18,000 cancer diagnoses each year
- suspicion of urological cancer, which includes prostate cancer in addition to bladder, renal, testicular, and penile represents 10% of all urgent suspected cancer referrals from primary care in 2019/20 (more than 235,000 referrals)
- on 2018, (latest published data) patients with renal cancer in England had intervals between referral and commencement of treatment of 98 days (median values) for women and 102 days (median values) for men. This varied by Cancer Alliance with a range of 70 to 115 days (median values) for women and 88 to 119 days (median values) for men. Patients with bladder cancer had intervals between referral and commencement of treatment of 63 days (median values). This varied by Cancer Alliance with a range of 50 to 75 days (median values) for women and 55 to 75 days (median values) for men
- for renal cancer patients in England diagnosed between 2016 and 2020, five-year age-standardised net survival was 66.6%. For bladder cancer patients in England diagnosed between 2016 and 2020, five-year age-standardised net survival was 52%
The best practice timed pathway, when implemented in conjunction with improvement work in prevention and public health, will enable a reduction in overall waiting times and improvement in early diagnosis and survival. A streamlined and more efficient pathway will improve avoidable delays and should reduce the unwanted variation currently seen.
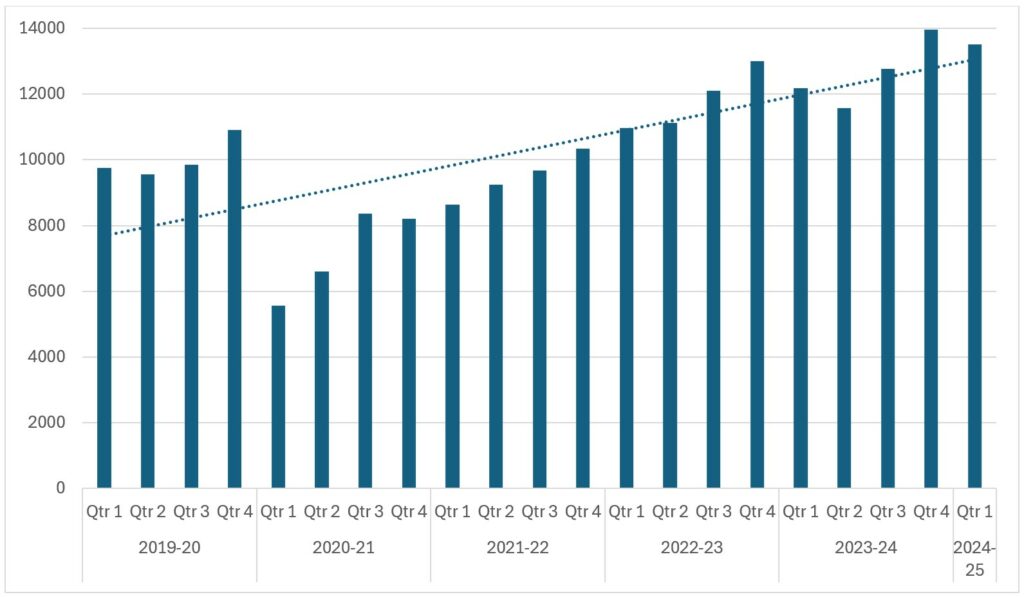
Figure 1: Urology excluding testicular 62-day referral to treatment cancer waiting times volumes for England, 2019-20 to 2024-25 Q1
“The patient pathways must consider a combination of diagnostic modalities and enable onward electronic referral. Initial diagnostics can be undertaken at local units, but more complex staging investigations may require specialist decision making. Where previously this was often done in series, FDS performance will require more steps in parallel.
The key features of these pathways are to implement rapid triaging of patients so they can access the right tests, first time, through use of an appropriately staffed one-stop clinic. This will assist the streamlining of transfer of appropriate cases between local unit and specialist centre. This should be integrated with timely booking and reporting of the diagnostic and staging investigations.
The proposed changes are simple but do require better administration. Their implementation is anticipated to reduce waiting times for critical investigations and decision making and enable prompt commencement of treatment for those diagnosed with cancer.”
Matthew Hayes, on behalf of the Urology Task and Finish Group, NHS Cancer Programme
Benefits of pathway change
For patients and carers
- reduced anxiety and uncertainty of a possible cancer diagnosis, with less time between referral and receiving the outcome of diagnostic tests
- improved patient experience from fewer visits to the hospital, particularly to specialist centres if possible, and avoiding emergency admission
- potential for earlier recognition and initiation of pre-optimisation for treatment that could reduce complications and adverse outcomes
For systems
- reduced demand in outpatient clinics with increased straight-to-test provision and use of pathway navigators to manage and track the patients on the pathway which will avoid delays in care
- allow resources to be targeted at patients with cancer by removing non-cancer patients earlier in the pathway and provide assurance earlier
- improved quality, safety, and effectiveness of care with reduced variation and improvement in outcomes
Experience of care
- Ppatients know they are urgently referred for investigation of suspected cancer and should expect diagnosis within 28 days
- ensure that patients and carers’ ability to attend appointments is taken into account and additional support is offered, where necessary
- Ppatients are communicated with clearly (in non-technical terms and in writing if required), understand the information provided, and are given additional support, such as access to a clinical nurse specialist (CNS) or navigator, psychological support, buddy system, where necessary
For clinicians
- using a nationally agreed and clinically endorsed pathway to support quality improvement and reconfiguration of urological cancer diagnostic services
- the use of predetermined diagnostic algorithms and standards of care to streamline clinical decision-making and reduce delays for MDT discussion
Improved ability to meet increasing demand and ensure best use of the highly skilled workforce
Bladder best practice timed pathway
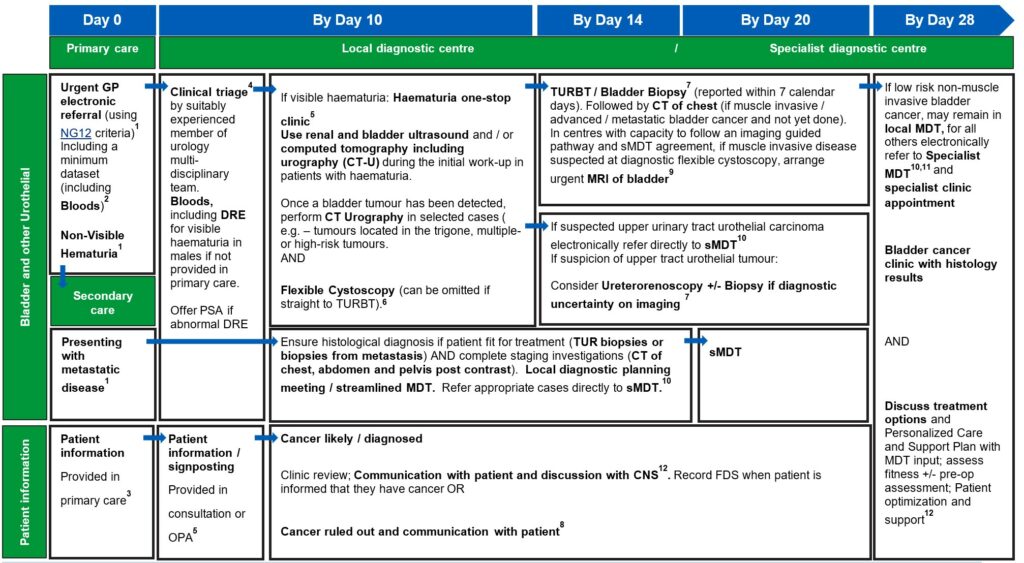
View and download a PDF version of the 4 best practice timed pathways. More detailed information can be found below.
Penile best practice timed pathway
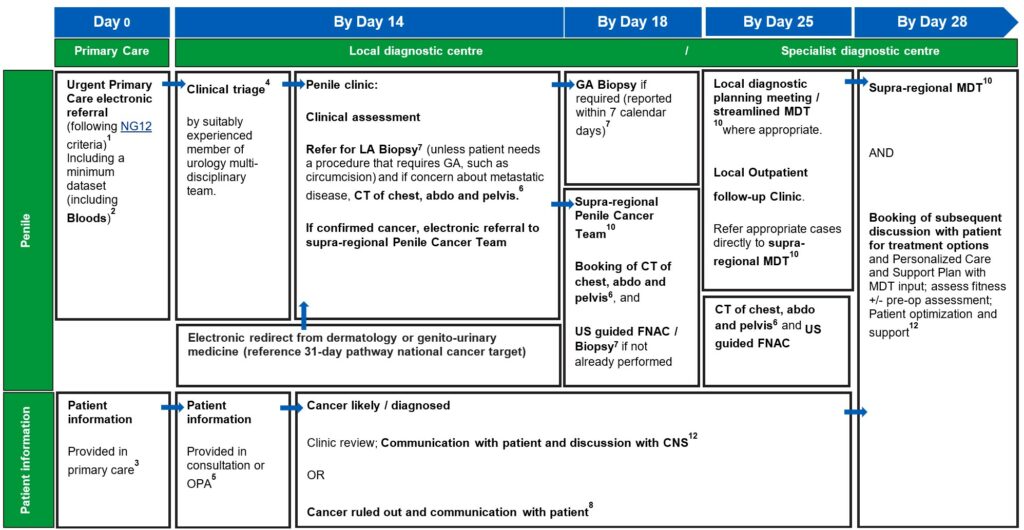
View and download a PDF version of the 4 best practice timed pathways. More detailed information can be found below.
Renal best practice timed pathway
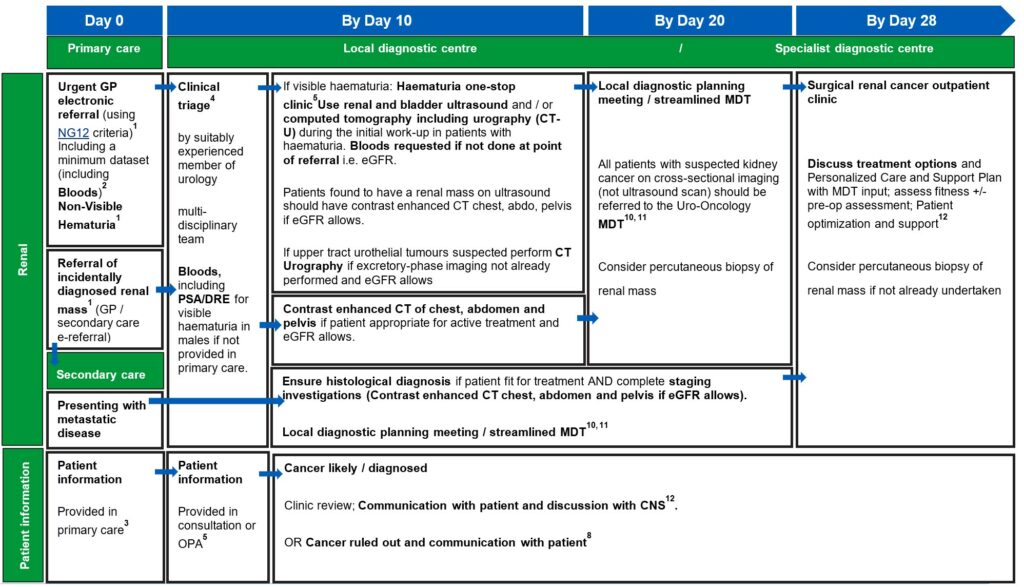
View and download a PDF version of the 4 best practice timed pathways. More detailed information can be found below.
Testicular best practice timed pathway
View and download a PDF version of the 4 best practice timed pathways. More detailed information can be found below.
Detailed information
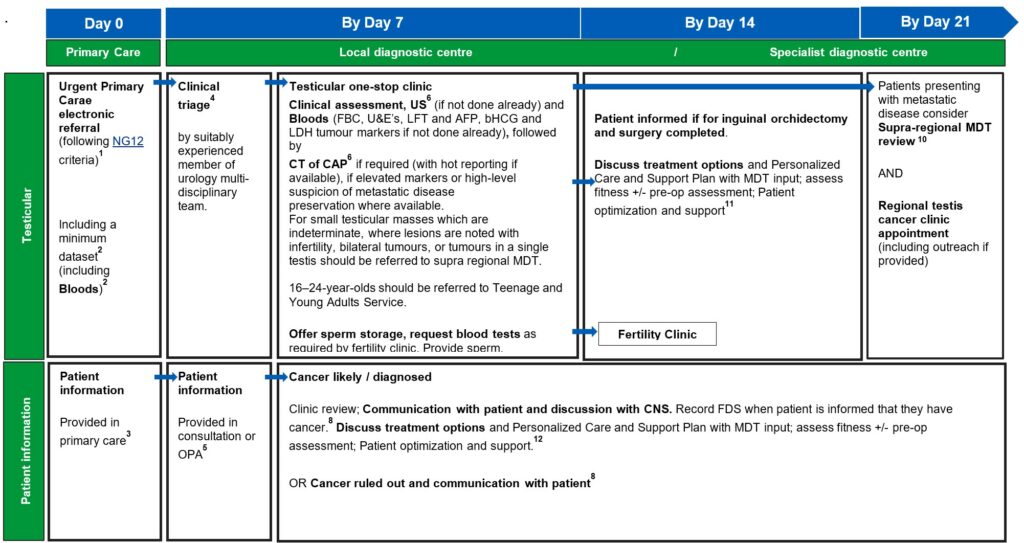
1. An urgent electronic referral pathway should be used for patients who meet NG12 criteria for suspected cancer pathway referrals. In a scenario where primary care refers the patient for a direct access test as cancer is not initially suspected, and the ultrasound, CT, or bloods are abnormal and suspicious of cancer, patients should be followed up directly by secondary care therefore referred on internally without the need for an additional referral from the GP, without the need for an additional referral from their GP.
Patients presenting with possible pain, fatigue and weight loss and there is suspicion of metastatic disease either in primary care or secondary care patients should be considered for further diagnostics.
Bladder and renal cancers: Non-visible haematuria-non-visible haematuria (NVH) is a common finding and may (uncommonly) indicate undiagnosed urological cancer. The optimal investigation of NVH is unclear, given the low incidence of cancer and the implications of testing all individuals with this finding. Further study is required to reach consensus on optimal investigation schedules, and in the meantime a risk-benefit discussion should be had with those affected to agree an individualised approach.
Evidence supports referral via a suspected cancer pathway for individuals aged 60 and over who have unexplained non‑visible haematuria and either dysuria or a raised white cell count on a full blood count (Recommendations organised by site of cancer | Suspected cancer: recognition and referral | Guidance | NICE)
The patient would then join the pathway after the first diagnostic test (labelled on this pathway diagram as ‘straight to one-stop clinic’). The National Cancer Waiting Times Monitoring Dataset Guidance v12.0 sets out consultant upgrade rules. A consultant upgrade would also apply to other scenarios where the patient may join the pathway, such as an abnormal CT result following attendance at A&E, or an incidental finding on imaging undertaken for a different indication.
Consultant upgrade patients are reported alongside urgent suspected cancer referrals from primary care within the single 62-day referral to treatment standard. Cancer Alliances may set out local arrangements to facilitate patient self-referral access to this pathway.
2. A minimum dataset to accompany the referral and facilitate straight to clinic and immediate diagnostics, to be agreed locally, may include:
- description of referral reason in line with NG12 guidelines
- patient demographics
- estimated glomerular filtration rate (eGFR)
- full blood count
- urea and electrolytes
- renal function including creatinine.
- calcium
- LFTs
- anticoagulant status
- co-morbidities, including diabetes status.
- dementia
- mental health conditions, such as claustrophobia
- Body Mass Index
- prescribed medication
- allergies
- family history of cancer
- World Health Organization performance status
- presence of metal implants or pacemakers
- need for an interpreter.
- mental capacity to consent.
The referral should not be delayed while obtaining dataset items, such as waiting for blood test results.
3. Primary care should provide information to the patient, including information about FDS and the urgent suspected cancer pathway, expected timelines, including that the patient should be available within the next 10 days initially for appointments and tests, may be required for 28 days. This is also a good point to discuss the importance of stopping smoking and provided information cessation services.
Cancer Alliances are encouraged to use this guidance and other policies to support work locally, with commissioners, to provide educational opportunities for Primary Care on cancer symptoms, urgent referral processes and the locally agreed minimum dataset. This should include hard to reach and outlier GPs.
4. Clinical triage can be undertaken by a suitably experienced clinician. This may be a supervised CNS, who has the training and authority to triage to one-stop clinics and book imaging tests. Preparation for any tests should be communicated to patients.
Suitability for treatment and any requirements for pre-habilitation (e.g. Nutrition and exercise advice) should be considered at this stage in the pathway. Patients should be triaged in accordance with NG12 Guidelines for urological cancers and any locally agreed clinical criteria for risk stratification.
Throughout the pathway, tests will need to be pre-booked at the earliest opportunity to ensure sufficient capacity and patient flow enable all relevant test to be carried to ensure diagnosis of 28 days. Therefore, a consideration should be made to ring-fence urgent cancer slots in advance and releasing them if no longer required.
5. An outpatient appointment (OPA) should be provided for any cohort of patients who are medically unfit for straight to one-stop clinic or may not need a one-stop clinic appointment. Patients who attend an OPA should have same day tests to reduce repeat visits and improve patient experience. If this is not possible, tests should be on the next day (i.e. within 24 hours).
The recommended first line investigations should be performed as a one-stop clinic where possible so that this cohort can progress on the pathway in the same timeframe. Patients and Carers should receive information about any tests and any preparations they need to make for their appointments. A clear walk through of what the patient will experience, plus leaflets and investigation information should be provided including details of whether they need to have more than one test on different days. Patients should also be asked if they require any accessibility support.
Preferences for the amount of information and when it is provided will vary, and therefore it will help to provide the pathway navigator or CNS with telephone contact details so they can provide support throughout the pathway and outside of clinic times, provide signposting to charities and support services, provide information about carer attending appointments, and offer follow-up if patients do not receive confirmation of an appointment in expected timescales.
Where possible, continuity of pathway navigator or CNS should be provided to enable familiar contact and to build trust. Patients should also be informed if they are likely to receive a procedure and/or diagnostic test on the same day at the first face-to-face appointment.
The clinical triage consultation or first OPA is also an opportunity to collect minimum dataset items from the patient, if not provided in the primary care referral. The haematuria clinic set out in both the renal and bladder pathways can be a combined clinic or separate renal and bladder clinics, as determined by pathway, resourcing and planning requirements locally. The order of diagnostic tests in the haematuria clinic may vary based on local clinical criteria and operational requirements.
6. Standard imaging protocols should be applied for all CT, MRI, and ultrasound. These should comply with Royal College of Radiologists’ recommendations or equivalent. Further information is available on the Royal College of Radiologists’ iRefer page. Ring-fenced imaging slots should be considered to ensure that capacity is available to meet demand in a timely fashion.
7. Histopathology reports for tissue sampling should usually be available in seven calendar days. This may be longer if ancillary tests are required to establish a diagnosis or if the pathway for a sample reaching the reporting laboratory is delayed. All histopathology should have a designated point of receipt, sign-off and management responsibility to ensure that reporting is not lost between different clinicians.
Perioperative Care of Older people undergoing Surgery (POPS) assessment should be carried out, at or immediately following one stop clinic, or decision to book, to assess suitability for GA biopsy.
Following tissue sampling results, confirmed cancer tumours should be tested for all clinically relevant molecular markers required to determine onward management. Given the possibility of benign disease, all patients with solid small renal masses should be considered for biopsy where technically feasible, it will impact patients’ choice or clinicians recommendation on treatment.
8. Patients should be informed about cancer being ruled out or diagnosed at the earliest face-to-face opportunity following diagnostic test being completed unless the patient has expressed an alternative method of communication to speed up communication.
When cancer is ruled out, the patient may be informed by telephone or written communication if waiting for diagnostic reporting. Patients may still require further testing in secondary care before an alternative non-cancer diagnosis can be identified.
Best practice would be to refer the patient to an alternative secondary care service within the same provider if one is identified, rather than being discharged back to primary care. Running parallel general clinics alongside one-stop clinics using ‘hot slots’ can allow patients to undergo imaging and be followed up by the general clinic on the same day.
When bladder, renal, penile and testicular cancer is ruled out, and other cancers are not ruled out, it may be appropriate to refer the patient onto an alternative tumour site specific pathway, or to a non-specific pathway, where non-specific or vague symptoms can be considered. When cancer is ruled out patient should be referred to the relevant secondary care speciality.
For continuity of care, it is best practice for the CNS from triage and diagnostics to be available in the clinic.
If a suspicious lesion is identified, patients should have access to a CNS with expertise in bladder, penile and renal, and testicular cancer for cancer for support (from the point of diagnosis onwards).
Cancer waiting time rules (including ‘clock start’ and ‘clock stop’) are set out in the National Cancer Waiting Times Monitoring Dataset Guidance v12.0.
9. MRI can be used for further characterising small renal masses if CT is uncertain but should likely be reserved for specialist MDT actioning.
Further information is available on the Royal College of Radiologists’ iRefer page. Ring-fenced imaging slots should be considered to ensure that capacity is available to meet demand in a timely fashion.
10. National guidance on how to maximise effectiveness of MDT meetings is available. This aims to improve patient experience, improve communication, and prevent delays in starting treatment. Locally agreed, clear criteria for referral to sMDT can also support with efficient pathway management.
11. In exceptional circumstances where required addition imaging should be completed as below and reviewed as part of MDT
+/- chest CT if indicated and not yet done.
+/- Imaging (e.g. MRI)
+/- Renal Tumour Biopsy / Image Guided Biopsy7 (if not already performed)
12. Outpatient clinic should include the CNS assigned to the patient earlier in the pathway, from clinical triage or when a likely cancer diagnosis was discussed. Personalised care and support planning should be based upon the patient and MDT clinician(s) completing a holistic needs assessment (HNA), usually within/days of diagnosis.
The HNA ensures conversations focus on what matters to the patient, considering wider health, wellbeing, practical issues, and support in addition to clinical needs and fitness. This enables shared decision-making regarding treatment and care options.
Early consideration of the patient’s fitness for radical therapy should be addressed as soon as possible in the pathway to minimise delays in expediting treatment. Local protocols and initiatives should be developed in collaboration with perioperative medicine, elderly care, and specialist dietitians. Anaesthetic assessments for patients with comorbidities should also be undertaken.
Audit tool
This tool can be used to undertake a baseline audit of services being delivered and whether sufficient capacity is in place to routinely deliver, identify areas for improvement, select measurements for improvement, and conduct re-audits as part of continuous improvement.
Download a word version of the audit tool
Cancer Alliance workspace
Cancer Alliances access this workspace for national guidance, resources, and to share learning. Please use this space to upload materials you have developed locally and that you think would be useful for colleagues implementing this pathway across the country.
Acknowledgements
This guidance was developed by the NHS Cancer Programme and builds on experience and expertise provided by the Bladder Task and Finish Group membership, including:
- Matt Hayes, Clinical Director, Wessex Cancer Alliance (Chair)
- Maria Physicos, Project Manager, West London Cancer Alliances (Cancer Alliance Representative
- Stefan Anastascu, Clinical Nurse Specialist, Kingston Hospital NHS Foundation Trust (Provider Operational/Clinical Representative)
- Simon Williams, Consultant Urologist, University Hospitals of Derby and Burton NHS Foundation Trust (via The British Association of Urological Surgeons) (Renal specialist)
- Vishwanath Hanchanale, Consultant Robotic and Urological Surgeon, Liverpool University Hospitals NHS Foundation Trust (via The British Association of Urological Surgeons) (Bladder specialist)
- James Armitage, Consultant Urologist, Cambridge University Hospitals NHS Foundation Trust (via The British Association of Urological Surgeons) (Renal and Testicular specialist)
- Helen Turner, Consultant Histopathologist, Newcastle Upon Tyne Hospitals Foundation Trust (Primary Care GP representative)
- Alex King, Consultant Radiologist, University Hospital Southampton NHS Foundation Trust
- Dr Caitlin Bowden, Clinical Oncologist, Gloucestershire Hospitals NHS Foundation Trust
- Simon Crabb Medical Oncologist, University Hospital Southampton NHS Foundation Trust
- Stephen Scott Policy Led, Operational Performance Team, NHS England
- Melanie Costin, Fight Bladder Cancer (charity representative)
- Paula Naraine, RM Partners Patient Advisory Group Member and Merck KGaA Representative (via West London Cancer Alliances) (patient representative for bladder cancer)
- Vijay Patel, Clinical Fellow, NHS England (Primary Care GP representative)
- Kelly Kusinski, Urology Advanced Nurse Practitioner, The Royal Wolverhampton NHS Trust (via British Association of Urological Nurses)
- Jonathan Aning, Consultant Urological Surgeon at the Bristol Urological Institute; BAUS Oncology Chair, North Bristol NHS Trust; British Association of Urological Surgeons
- Grant Stewart, Professor of Surgical Oncology, Interim Head of Department, Department of Surgery, University of Cambridge
Publication reference: PRN00661_ii

