1. Introduction
This guidance provides consolidated advice and recommendations for healthcare providers on identifying and managing patients with suspected and confirmed measles, as well as broader public health considerations relevant to NHS services.
Commissioners should use this guidance to inform clinical pathways for effective management of measles cases and outbreaks.
This guidance should be used alongside the following resources:
- NHS guidance for risk assessment and infection prevention and control (IPC) measures in healthcare settings
- UK Health Security Agency (UKHSA) national measles guidelines
- The national IPC manual (NIPCM) for England
- The Green Book of immunisation, chapter 21: measles
- National Institute for Health and Care Excellence (NICE) guidance on diagnosis and management of measles
2. Assessment of patients with suspected measles
Measles is highly infectious and has multiple differential diagnoses, so a high index of suspicion is required when assessing patients with compatible symptoms.
- Correct triage is essential. Ensure receptionists and triage teams ask patients, parents and carers about fever and rash.
- Any unvaccinated patient presenting with a fever and rash is potentially infectious and should wait in a separate room, away from other patients.
- Measles is a clinical diagnosis and alternative diagnoses including streptococcal and meningococcal infections and Kawasaki disease should be considered and excluded.
- Consider a measles diagnosis in children or unvaccinated adults, including pregnant women, presenting with a measles-like rash, fever, and other symptoms suggestive of measles, including:
- high temperature
- runny or blocked nose
- sneezing
- cough
- red, sore, and watery eyes
2.1. Clinical presentation
The following signs and symptoms constitute a classical presentation of measles:
1. A prodrome of fever (often ≥39ºC) associated with coryza, cough and/or non-purulent conjunctivitis, followed by:
2. A blanching, blotchy, maculopapular rash which typically appears 2-3 days after the onset of fever, often started on the face and spreading to the trunk over the next 3-4 days.
- on white skin, the rash is usually red or brown
- on black or brown skin, the rash may be more difficult to detect and may appear purple or darker than the surrounding skin
For more information on the clinical diagnosis and differential diagnoses for measles, please see the relevant NICE clinical knowledge summary and UKHSA quick reference poster.
The following considerations may assist in assessing the likelihood that a given clinical presentation may be due to measles:
2.2. Immunisation history
- 1 dose of MMR (measles, mumps and rubella) vaccine is effective at protecting against measles in 95% of people, increasing to 99% effectiveness with the second dose.
- In people who are fully vaccinated against measles, or who have previously had measles infection, presentation with signs and symptoms of measles is more likely to be due to another differential diagnosis.
- Breakthrough measles infections in previously immune individuals are rare but can occur, particularly in those exposed to high viral loads. Symptoms tend to be milder and may lack the characteristic rash.
2.3. Exposure history
- Contact with a suspected measles case is a significant risk factor. The infectious period of cases generally starts from 4 days before the rash and lasts up to 4 full days after the onset of rash.
- Transmission is most likely with face-to-face contact or prolonged exposure (>15 minutes), but can occur from casual contact over much shorter durations.
- Patients may have been notified that they have been exposed to measles, for example in a letter from school, nursery or a healthcare setting.
- Individuals who have travelled to measles endemic countries or countries with outbreaks are more likely to have been exposed to infection.
- Risk is higher in groups with lower vaccine coverage, for example, Charedi Orthodox Jewish, Traveller and Anthroposophic (Steiner) communities, and migrants.
- Have a low threshold for suspecting measles in any patient who reports having been exposed and consider providing exclusion advice during the prodromal period as well as after the rash appears.
- Post-exposure prophylaxis may be advised for vulnerable patients including those who are immunosuppressed, pregnant women, and infants under 1 year of age.
2.4. Other medical history and vulnerable groups
- Immunosuppressed patients may not develop typical symptoms.
- Consider measles as a differential diagnosis in immunosuppressed patients with evidence of infection, especially in areas with known outbreaks, and consider isolating and testing these patients for measles early in the clinical course.
3. Investigation and notification
Measles is a clinical diagnosis, and isolation and exclusion advice should not be delayed pending testing results where there is clinical suspicion. However, the reliability of clinical diagnosis for measles is low because of generally low prevalence of the disease in England and the broad range of differential diagnoses, particularly in young children. It is therefore important that suspected cases are also tested using appropriate laboratory methods to confirm the diagnosis, particularly if measles is not known to have been circulating recently in the local community.
3.1. Diagnostic testing
- Diagnostic testing for active measles infection is by viral Polymerase Chain Reaction (PCR), with detection being most successful in samples taken as close as possible to the first day of onset of rash.
- Samples are most easily obtained using a mouth swab. These should ideally be taken using a viral swab and viral transport medium, but any dry swab can be used provided it is placed in a sterile universal container.
- Bacterial transport media such as charcoal cannot be used, as these contain PCR inhibitors.
3.2. Surveillance testing
- In addition to any diagnostic samples, UKHSA will send an oral fluid kit to all suspected measles cases for them to self-administer.
- This is for surveillance purposes and does not replace diagnostic testing.
- Patients should be advised to complete the oral fluid test in addition to the diagnostic swab.
- Surveillance testing is important to enable UKHSA to monitor importations of measles strains, spread and chains of transmission, to meet reporting requirements to the World Health Organization (WHO), documenting progress towards achieving and maintaining measles elimination status.
3.3. Notification process
- Measles is a notifiable disease. Clinicians should notify all suspected cases to the regional health protection team by telephone call as soon as reasonably practical and within 24 hours.
- Measles virus is also a notifiable causative agent. Laboratories should notify all positive samples to the relevant health protection team as soon as practically possible and within 24 hours.
4. Management of suspected and confirmed measles cases
4.1. Suspected measles in primary and community care
- Place the patient in a room, away from other patients on arrival.
- If the patient with suspected measles is assessed in a face-to-face setting, the assessment should be done in an isolated room.
- Wear appropriate personal protective equipment (PPE). Refer to the National infection prevention and control manual (NIPCM) sections 1.4, 2.4 and appendices 5b and 6 for further information of the use of PPE. NHS England’s Infection Prevention and Control guidance on PPE is available online.
- Measles is a clinical diagnosis, and isolation should not be delayed awaiting PCR results where there is clinical suspicion.
- Notify the local Health Protection team (HPT) promptly. Contact details for HPTs are online on the gov.uk website. They will advise on public health measures including contact tracing to identify vulnerable individuals and arrange surveillance testing.
- Most children and young people will not need admission and should not be referred to secondary care for confirmatory testing or review unless clinically indicated.
- Family members should be vaccinated promptly against measles as per national guidance.
- Seek immediate advice on management from local paediatric services through locally agreed rapid access routes if the patient has a measles-like rash and is in 1 of the following high-risk groups:
- younger than 1 year of age
- immunocompromised (regardless of immunisation status)
- pregnant
And/or if the patient presents with or reports symptoms of a serious complication:
-
- pneumonia
- neurological problems
- fever and immunocompromised
- Where admission is planned, contact the hospital regarding isolation before admission.
- Advise on rest, fluids, and paracetamol and ibuprofen for symptomatic relief. Children younger than 16 years old should not take aspirin.
- Vitamin A should not be prescribed in suspected or confirmed cases of measles when the patient has not been hospitalised. Vitamin can cause harm if prescribed inappropriately.
- Patients with measles should be advised to stay away from nursery, school, or work for at least 4 days after the initial development of the rash.
- For individuals with immunosuppression, discuss management with clinicians managing their immunosuppression as they are at more risk of severe disease and complications, and may be infectious for longer.
- Provide written advice about measles to parents and carers. The QR code and link to the NHS measles page is in appendix 4.
- Individuals who received measles vaccination outside the UK are generally considered protected. However, in the absence of documented evidence of vaccination, they should be offered the MMR vaccine to ensure full immunisation.
4.2. Individuals with confirmed measles in hospital
- Ensure triage in place and that suspected cases are isolated on arrival.
- Clinicians should work with their local HPT to risk assess close contacts.
- Measles is diagnosed clinically, and PCR testing should be used for confirmation of diagnosis.
- The management of unwell children, particularly those under 2 years of age, malnourished or with underlying health conditions should be discussed with the regional paediatric infectious diseases team.
- For a child likely to be in hospital for 2 or more days, aged under 2 years, malnourished and diarrheal, the use of high-dose unlicensed vitamin A can be considered. These cases should be discussed with the regional paediatric infectious diseases team. Vitamin A (Provepharm) 100,000 international units per 2ml ampoules is an unlicensed medicine imported from France. It is available to import via the usual UK unlicensed medicines importers. Please contact your hospital pharmacy procurement team. There is no dosage currently listed in the British National Formulary for Children (BNFC) or other commonly used reference sources.
- There is limited evidence of benefit of anti-viral treatments in children and young people with severe measles infection and they are not routinely recommended. There may be some benefit for a subset of children with significant immunocompromise and overwhelming infection, but this should be agreed on a case-by-case basis at a multidisciplinary team (MDT) meeting including paediatric infectious diseases specialists.
5. Management of patients exposed to measles
5.1. Individuals who have been in contact with suspected measles
- Determine immunisation status and whether there has been significant contact with a suspected case, or is immunocompromised.
- Contact the local HPT immediately if the individual is:
- immunocompromised
- younger than 1 year of age
- pregnant
- susceptible to measles infection but the MMR vaccine is contraindicated
The risk assessment for the post exposure prophylaxis of susceptible vulnerable close contacts is detailed in the national measles guidance.
- For immunocompromised patients, intravenous immunoglobulin is recommended.
- For pregnant women and infants, human normal immunoglobulin (HNIG) is recommended.
Please refer to national guidance for further information and work with your local HPT who will support risk assessment.
|
Table 1. Assessment and treatment of infants in contact with measles (UKHSA) | ||
|
Infants under 6 months |
Assume susceptible and administer intramuscular human normal immunoglobulin (HNIG), ideally within 72 hours but up to 6 days, regardless of maternal status. | |
|
Infants aged 6 to 8 months |
For household exposure, administer HNIG, ideally within 72 hours but up to 6 days if necessary. |
For exposures outside of the household, administer MMR, ideally within 72 hours. |
|
Infants 9 months and older |
Administer MMR vaccine, ideally within 72 hours of exposure. | |
For children more than 1 year of age, not immunocompromised, and with no other contraindications to the MMR vaccine:
- offer prompt vaccination
- the MMR vaccine should ideally be given within 3 days of contact with a possible case and repeated after an interval of at least 1 month
- for children younger than 15 months old when they receive their second dose, another routine (third) dose should be given after 18 months for full protection
- for children younger than 12 months old when they receive their first dose, 2 further doses will be required at the normal ages in accordance with the Childhood Immunisation Programme
- provide written advice about measles to parents and/or carers. If the information is required in a language other than English, advice is available on the NHS website
5.2. Clinically vulnerable groups
Anyone who has not had measles before and has not had 2 doses of a measles-containing vaccine is susceptible to the infection. The following groups are at highest risk of developing severe complications:
- immunosuppressed individuals are at particular risk of developing severe and prolonged measles and associated complications
- infants under 1 year are unlikely to have received any MMR vaccines and have immature immune systems
- pregnant women are at higher risk of hospitalisation and severe complications compared to non-pregnant adults, with associated pregnancy complications including preterm birth and pregnancy loss
Complications of measles predominantly affect the respiratory, gastrointestinal and central nervous systems and can be fatal. For more details on the complications associated with measles, please see the NICE clinical knowledge summary (CKS).
Further information on immunosuppression and pregnancy in relation to vaccination is available in chapter 6 of the Green Book.
5.3. Post-exposure prophylaxis (PEP) and immunoglobulin pathways
Urgent post-exposure prophylaxis should be considered in any vulnerable individual who is exposed to measles. The treating clinician (in primary care or hospital) should contact the patient’s local health protection team and/or the hospital duty virologist and undertake a risk assessment. Guidance on exposure risk assessment and post-exposure management is available in the UKHSA national measles guidelines.
Integrated care boards (ICBs) must ensure that clinical pathways for the provision of immunoglobulin and post-exposure vaccination are available and clearly defined within their system, as described in section 3 of the of the 2025 infectious disease incident commissioning guidance. Recommendations for immunoglobulin treatment will be given by a consultant in health protection or virologist, based on an individual risk assessment.
Infection prevention and control teams will work with clinicians to identify exposed, susceptible contacts within the healthcare setting. Identification of contacts should be in the following order of priority:
- immunosuppressed contacts
- pregnant women and infants less than 12 months
- health care workers
- healthy contacts
Measles post exposure treatment may include the following:
- intravenous immunoglobulin (IVIg) is indicated for immunosuppressed patients without adequate current immunity to measles. Any level of contact should trigger an assessment, even if the index case is presumed to have breakthrough measles. IVIG should be administered in a hospital setting, as soon as possible and ideally within 72 hours of exposure
- human normal immunoglobulin (HNIg) may be administered by the intramuscular route to immunocompetent but clinically vulnerable contacts (pregnant women and infants) without evidence of measles immunity. This may be given in community healthcare settings, but it may be necessary to administer this in the local hospital outside of GP surgery hours to ensure administration within post-exposure prophylaxis windows. HNIG should be administered as soon as possible and within 6 days of exposure to measles
- MMR vaccine may be suitable as post-exposure prophylaxis for susceptible individuals aged 6 months and over, and should be given within 72 hours of exposure
Details regarding suitable immunoglobulin products, dosing and administration can be found in the UKHSA national measles guidance, including annex 5.3.
5.4. Prescribing and supply of immunoglobulin for measles PEP following risk assessment
The NHS England commissioning criteria policy for the use of therapeutic Ig includes the indication for use following exposure to measles available in the Commissioning criteria policy for the use of therapeutic immunoglobulin (Ig) England, 2021.
Use for PEP of pregnant women and infants should follow a risk assessment supported by the local health protection team, sharing patient details and agreeing the indication for Ig. If eligibility criteria are fulfilled, approval from the Sub-Regional Immunoglobulin Assessment Panel (SRIAP) is not required on a case-by-case basis.
Trusts are requested to use local stocks of subcutaneous Ig (SCIg) for PEP of infants and pregnant women where possible, which will also support timely administration within PEP windows. This is most effective if given within 72 hours of exposure but may still be effective if given within 6 days of exposure.
|
Indication |
Eligibility criteria |
Position of immunoglobulin, taking into account alternative therapies |
Recommended dose |
Prior panel approval required |
|
Immune-suppressed individuals |
Immunosuppressed individuals (group A and group B based on level of immunosuppression) who have had a significant exposure to measles and are known to be susceptible (based on vaccine history and /or IgG testing). |
For immunosuppressed contacts IVIg is mainstay management |
0.15g/kg of IVIg recommended ideally within 72 hours of exposure although can be given up to 6 days. Where exposure recognised late or found to be antibody negative between 6 and 18 days after exposure, IVIg may be considered following discussion with specialist clinician. |
Prior approval is via discussion with UKHSA health protection team. Find your local protection team. |
|
Pregnant women and infants |
Pregnant women who have identified as susceptible based on vaccine history and /or antibody testing who have had a significant exposure to measles. Infants under 9 months of age with a significant exposure to measles. Advice is available on the gov.uk website. |
For pregnant contacts, immunoglobulin is mainstay management for PEP For infants below 6 months immunoglobulin is mainstay treatment; For infants aged between 6-8 months, MMR vaccine can be offered if exposure occurred outside household setting and ideally should be given within 72 hours. |
For pregnant contacts, approximately 3000mg of human normal immunoglobulin (HNIG) |
Prior approval is via discussion with UKHSA health protection team. Find your local protection team |
All close contacts of measles should be given warn and inform information so they are aware of the signs and symptoms of measles and understand the action to take should they become unwell.
5.5. Pre-exposure assessment of immunosuppressed patients
During periods of increased measles transmission, clinicians caring for patients who are immunosuppressed or soon to become immunosuppressed should assess the patient’s immune status. Clinicians should:
- assess each immunosuppressed patient at their next scheduled face-to-face appointment. Additional appointments exclusively to enable pre-exposure measles risk assessment are not recommended
- document measles vaccination status and arrange antibody testing for measles immunity to facilitate appropriate management in the event of an exposure
- offer re-vaccination or immunity testing following treatment completion where appropriate, for example, after bone marrow transplantation
Clinicians caring for immunosuppressed patients should provide tailored information and advice on protection against measles, including:
- information about current measles transmission, routes of exposure and the potential risks for their health
- advice that all family members and close contacts should either be fully immunised with 2 MMR doses or have a reliable history of previous measles infection
- encouraging patients or parents to inform workplaces, schools, nurseries and social groups that they or their child are immunosuppressed so that they can be alerted should an exposure occur
- advice on seeking immediate medical advice in the event of a possible exposure to measles, for example from the patient’s supervising specialist, specialist nurse or GP, for consideration of post-exposure treatment
6. Preventing measles transmission in healthcare settings
Healthcare settings are high risk for measles transmission during measles outbreaks due to a combination of infectious patients presenting for assessment and clinically vulnerable patients being managed in the vicinity. Settings at greatest risk are those with unselected admissions such as walk-in centres, GP practices and emergency departments, as well as those managing clinically vulnerable patients such as neonatal and paediatric units, antenatal and maternity units and haematology and oncology units. Even single measles exposures, and outbreaks, require significant staff resource to contact trace and provide advice and care for vulnerable contacts. Cases of measles in healthcare staff also place patients at risk and stretch services through exclusion of unwell or susceptible exposed staff contacts from work.
All healthcare providers should ensure that:
- appropriate triage, isolation and IPC procedures are in place to prevent transmission of measles. Please see the NHS England guidance on risk assessment and IPC measures for measles in healthcare settings for more information on patient placement, PPE, and completing a measles risk assessment
- a respiratory season or winter plan is in use to ensure appropriate segregation of patient cases and management of increasing case numbers as required
- all staff are up to date with their immunisations as per the national routine immunisation schedule, including 2 documented doses of MMR vaccine (or to have documented evidence of measles immunity)
- Filtering Face Piece (FFP) 3 respirator fit testing is completed for staff who may be required to assess or provide clinical care for suspected or confirmed measles cases
- IPC training is provided to all staff, including the correct use of PPE and the correct techniques for putting on and removing PPE safely. All staff should be familiar with standard infection control precautions (SICPs) and transmission-based precautions (TBPs) as set out in chapters 1 and 2 of the NIPCM
- risk assessments are undertaken for staff identified as high risk for infection with and/or complications of measles. Please see ‘occupational health assessment’ below for further information
- clinicians report suspected cases early to the local health protection team (HPT). The HPT will in turn alert acute trusts and primary care should measles be transmitting in the local area. Community and acute care partners will support incident management team meetings as required during outbreaks
- pathways are in place for the timely administration of Ig products to vulnerable contacts as post exposure prophylaxis in both the community and secondary care in and out of working hours
- all opportunities are being taken to encourage good uptake of MMR vaccine by staff and patients.
6.1. Occupational health assessment
All NHS trusts are required to carry out health assessment screening on their staff to determine suitability and fitness to undertake their job roles. Similarly, healthcare workers have a duty to take reasonable precautions to protect patients from communicable diseases, including ensuring that they are appropriately vaccinated.
MMR vaccination is very effective at preventing measles infection and transmission to clinically vulnerable patients and staff. All staff working in proximity to patients should have 2 documented MMR vaccinations or be IgG positive for measles. Staff who do not have immunity should not be involved in the assessment or management of patients with suspected measles.
Healthcare employers have a responsibility to ensure that staff receive vaccinations appropriate to their role and should be able to show that their organisation has an effective employee immunisation programme in place. As standard practice, employers and occupational health services should:
- check the measles immunity status of all staff during pre-employment screening, with evidence of immunity provided by documentation of 2 MMR doses or a positive measles antibody test
- unless contraindicated, offer MMR vaccination to any eligible staff without evidence of immunity, even if they think they may have been vaccinated or had measles in the past
- where vaccination is declined or contraindicated, advise the staff member’s manager to conduct a personal risk assessment to inform their placement and responsibilities as per local trust policy
During periods of increased measles transmission locally or nationally, employers and occupational health services should also:
- review the measles immunity status of existing staff via occupational health records
- recall any staff without evidence of measles immunity for vaccination unless contraindicated
- if vaccination is contraindicated or declined, advise the staff member’s manager to review their personal risk assessment to inform their placement and responsibilities
6.2. Staff vaccination in general practice
Owing to the ongoing sustained transmission of measles in England, GP practices are permitted to administer MMR vaccines to their eligible staff who are not registered at the administering practice
- this is a temporary arrangement aimed at boosting staff MMR coverage between 1 August 2025 and 31 March 2026
- indemnity cover will be provided through the Clinical Negligence Scheme for General Practice
- nationally supplied MMR stock can be used
- an item of service fee cannot be claimed for MMR vaccines administered to staff registered with another practice
- staff must be strongly encouraged to inform their registered practice that they have received an MMR vaccine and request that it be included in their medical record
6.3. Personal protective equipment (PPE) and measles exposure
Vaccination is very effective at reducing the risk of measles infection following exposure, with 2 MMR doses conferring immunity in 99% of people. However, breakthrough measles cases can occur, especially in healthcare workers exposed to high viral loads for prolonged periods. All staff members should therefore wear appropriate PPE when attending patients with suspected measles. For further information on recommended PPE, please see the NHS England measles IPC guidance and chapter 2 of the national IPC manual.
In a healthcare setting, exposure to a confirmed case of measles is defined as:
- face-to-face contact of any duration, or
- more than 15 minutes in a small, confined area (for example, 4 bed bay) without wearing appropriate PPE
Following exposure to a confirmed case of measles, exclusion from patient-facing work may be appropriate depending on the healthcare worker’s immunity status. Please see section 2.6.2. of the NHS England measles IPC guidance and section 3.2.3 of the UKHSA national measles guidelines for more information on risk assessment and exclusion advice in healthcare workers.
7. Pre-exposure vaccination
7.1. Routine childhood vaccination
The most effective way to prevent measles infection and transmission is through high uptake of 2 doses of the MMR vaccine. At present, the first dose (MMR1) is offered to children at 12 months, and the second dose (MMR2) at 3 years and 4 months.
- The timing of MMR2 is due to be brought forward as per the system communication published 30 April 2025. From 1 January 2026, children born on or after 1 July 2024 will be eligible to receive MMR2 at their 18-month vaccine appointment.
- In areas with current or anticipated measles outbreak, providers may choose to adopt this offer earlier. However, the focus of local vaccination efforts should be on achieving high uptake of MMR1, which achieves satisfactory immunity in 90-95% of people.
- The greatest opportunity for reducing transmission and severe disease therefore comes from ensuring first dose uptake is as high as possible, both within the general population as well as specific under-vaccinated groups.
- Providers should refer to the quality criteria for an effective immunisation programme and NICE Quality Standard 145 for additional information about high-quality care in vaccine uptake and priority areas for improvement.
7.2. Reactive and catch-up vaccination
- Variation in uptake by ethnicity, deprivation, and geography results in health inequalities, with the burden of measles falling disproportionately on under vaccinated populations.
- Alongside the core offer of universal vaccination for all children, providers should recognise the potential of targeted campaigns for increasing uptake among under vaccinated communities. Following a catch up campaign in 2023/24, the largest increases in MMR1 coverage were consistently seen in under-vaccinated groups, indicating the effectiveness of targeted catch-up activities.
- There is no upper age limit to offering MMR vaccine, and patients who have missed MMR doses should be caught up opportunistically or in local catch-up campaigns.
- New entrants from abroad and new GP registrants should have their immunisation history checked and missing doses administered.
- An extra dose of MMR vaccine can be administered to children between 6 and 12 months if travelling to an endemic country or exposed to a case or outbreak of measles. These children still require the full 2 doses after their first birthday.
For further information on eligibility for MMR vaccine and the vaccination schedule, please see chapter 21 of the Green Book.
Appendix 1: Practical steps towards completing a local risk assessment for measles in healthcare settings
To support organisations, practices and employers to undertake a local risk assessment in the context of managing cases of suspected or diagnosed measles based on the measures as prioritised in the hierarchy of controls.
Download a word copy of this template: Practical steps towards completing a local risk assessment for measles in healthcare settings.
Appendix 2: Think Measles – primary care actions for screening, triage and management
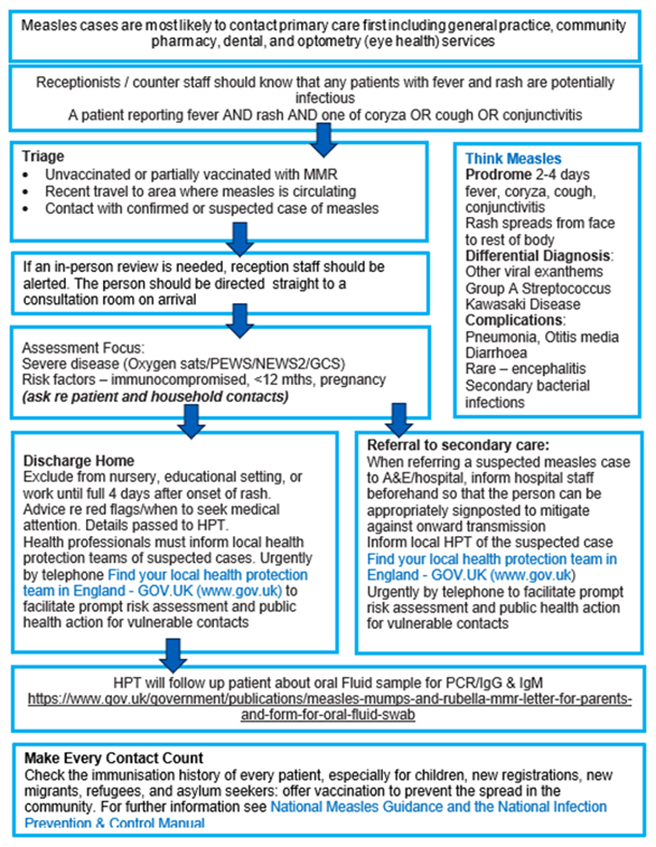
The above flowchart, and accessible text explaining what this flowchart is showing, is available in Guidance for risk assessment and infection prevention and control measures for measles in healthcare settings.
Appendix 3: Think Measles – urgent and emergency care actions
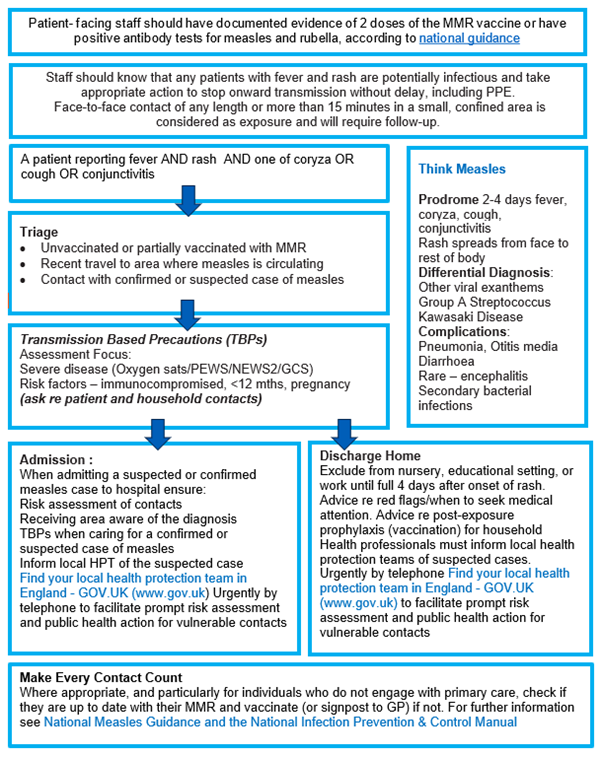
The above flowchart, and accessible text explaining what this flowchart is showing, is available in Guidance for risk assessment and infection prevention and control measures for measles in healthcare settings.
Appendix 4: QR code for NHS.UK measles webpage

QR code web address: https://www.nhs.uk/conditions/measles/
Publications reference: PRN01256

