Applicability
This NETB applies to all healthcare setting who have centralised or large scale Sterile Services Departments.
Objective
The objective of this NETB is to:
1. Outline the knowledge, skills, abilities and behaviours required by staff working in decontamination units to ensure the highest standards of decontamination are achieved; and
2. To give general recommendations for improving board-level commitment and oversight of decontamination quality.
Status
The information contained within this bulletin is a supplement to the current HTM. It should be read in conjunction with HTM01-01 Decontamination of surgical instruments and its contents should be implemented in the same way. This is version 2.0 of the document in which appendix 1 and associated references have been removed and minor amendments made to table 1.
Content
The bulletin sets out a national competency framework outlining the skills, qualifications, experience and registration requirements for sterile services staff at all levels. It provides detailed job specifications and career development pathways.
NHS Estates Technical Bulletin (NETB)
With responsibility for producing Standards and Guidance for the NHS Estate, we are responsible for ensuring that the information and guidance they contain remains up-to-date and relevant for users. This can involve revising and updating the documents themselves, but this is not the optimum approach in all cases and therefore an alternative approach is needed where such full revision is not appropriate. Where appropriate, NHS Estates Technical Bulletins (NETB) will be issued instead.
NETB need to be considered by all applicable organisations, as noted above, and implemented as required. Boards are responsible for its assessment and application to their organisations.
Background
In May 2022 the Health Services Safety Investigations Body (HSSIB) published a report into their concerns around the decontamination of surgical instruments. A copy of the report can be found here Decontamination of surgical instruments (hssib.org.uk)
Within the report there were specific safety recommendations relating to the Estate. They were:
1. HSIB recommends that NHS England and NHS Improvement amends Health Technical Memorandum 01-01 to define ‘top management’ and its commitment to quality, and that external independent audits are reported directly to the responsible executive director in a trust who is accountable for the service, not just the certified department; and
2. HSIB recommends that NHS England and NHS Improvement develops a competency framework, stating skills, qualifications and professional registration as required, for all sterile services staff and includes it in Health Technical Memorandum 01-01.
This technical bulletin aims to address the above items.
Monitoring of implementation
The implementation of this NETB will be monitored in line with overall compliance to the HTM’s and HBN’s through the NHS Premises Assurance Model.
Point of contact/feedback
For any queries please contact the estates and facilities mailbox england.estatesandfacilities@nhs.net
Executive summary
This technical bulletin was written in response to recommendations from a 2022 Health Service Safety Investigations Body (HSSIB) report on an incident involving contaminated medical devices being identified during a surgical procedure. The HSSIB investigation found several areas of concern in decontamination practices, including a lack of standardised training and competency frameworks for sterile services staff across facilities.
The key problem identified is inconsistent and variable training standards for decontamination staff. This creates risks that some staff may not have the requisite skills, knowledge and competence to ensure instruments are correctly decontaminated, which includes cleaning and sterilisation. Improper decontamination heightens infection risks for patients.
This technical bulletin seeks to address this, setting out a national competency framework outlining the skills, qualifications, experience and registration requirements for sterile services staff at all levels. It provides detailed job specifications and career development pathways.
One of the aims of the competency framework is to provide clear progression pathways for all staff through relevant qualifications. There are no formal restrictions regarding age or experience that would preclude any staff from pursuing requisite qualifications or apprenticeships to advance their career development per the framework. The competency framework aims to benefit experienced and new staff alike by aligning career advancement to attainment of recognised qualifications.
Other key recommendations include:
- implementing standardised job profiles and descriptions for consistency across facilities
- requiring certain minimum qualifications and Institute of Decontamination Sciences (IDSc) membership for senior roles
- expanding apprenticeship training routes through the existing training programmes available and being developed through the apprenticeship scheme and IDSc working towards accredited professional registration for staff via the Academy for Healthcare Science (AHCS)
- enhancing continuing professional development programmes
- increasing board-level oversight, governance and accountability regarding decontamination quality management
- board level involvement in external certification audits by notified bodies and a robust assurance process for incidents relating to decontamination
- clarity of the Decontamination Lead and responsibilities.
Implementing these recommendations will strengthen skills, uphold consistent high standards in decontamination practices and crucially protect patient safety.
Note: Decontamination requirements have minor regional variations between the devolved nations. Therefore organisations providing decontamination services should acquaint themselves with any region-specific regulations and guidance that are applicable:
Health Technical Memoranda (HTMs), Welsh Health Technical Memoranda (WHTMs), Scottish Health Technical Memorandums (SHTMs), Northern Ireland Health Technical Memoranda and Health Building Notes (HBNs). The devolved nations will need to assess and consider whether to implement the recommendations in this competency framework.
Definitions
1. Competence: the combination of training, skills, experience and knowledge that a person has and their ability to apply them to perform a task safely. Other factors, such as attitude and physical ability, can also affect someone’s competence.
2. Decontamination: a combination of processes that removes or destroys contamination so that infectious agents or other contaminants cannot reach a susceptible site in sufficient quantities to initiate infection, or other harmful response (definition from the Health and Safety Executive (HSE)).
3. Decontamination unit: for the purpose of this document, the decontamination unit has been used for the various names that are used in different organisations e.g., central sterile services department (CSSD), sterile services department (SSD), hospital sterilisation and disinfection unit (HSDU), central decontamination unit (CDU), theatre sterile services unit (TSSU).
4. Medical device: any apparatus, appliance, software, material or other article, whether used alone or in combination, intended by the manufacturer to be used by human beings for a medical purpose. For example, walking sticks, contact lenses and breast implants (definition from the Medicines and Healthcare products Regulatory Agency (MHRA)). Decontamination units typically process surgical instruments, endoscopes, ENT probes, and dental and podiatry instruments.
5. Operational experience: skill or knowledge obtained through employment.
1. Introduction
1.1 Decontamination services deliver appropriately decontaminated reusable medical devices for surgery, clinical investigations, diagnoses and treatment of patients. The range of services delivered by decontamination units varies considerably and are:
- either provided by NHS personnel based on a hospital site or off-site
- or provided by private contractors under contracts to the NHS on site or at off-site central hubs.
1.2 Healthcare providers may provide decontamination services only to their own organisation or supply other acute hospitals, community dental units, diagnostic centres, primary care and GP surgeries. Typically, private hospitals provide their own decontamination services either on site or off site, or use a contracted decontamination services provider.
1.3 Correct and effective decontamination is essential in ensuring patient safety as any device that has not been decontaminated correctly could lead to a patient acquiring a healthcare-associated infection (HCAI). These infections may necessitate additional treatment and endanger patient health, potentially resulting in poorer health outcomes or the death of the patient. Therefore, this is an essential service that should be supported financially and resourced correctly to meet the latest standards.
1.4 If the decontamination unit provides services to a third party, they must register with the MHRA and have a certified quality management system (QMS) that complies with an appropriate conformity assessment route under UK or EU Regulations as issued by a UK approved body or EU notified body.
1.5 If the decontamination unit only supplies to their own organisation, they are expected to have a QMS but do not have to register with the MHRA. Therefore, there is no method to identify where these units are sited.
1.6 Organisations have different management structures and responsibilities for decontaminating medical devices. Some organisations have centralised all decontamination under one management team, while others have kept separate areas and management responsibilities for decontamination.
1.7 A centralised decontamination unit processes all or most of the following:
- surgical instruments
- endoscopes
- nasendoscopes
- transoesophageal echocardiogram (TOE) probes
- transrectal (TR) and transvaginal (TV) probes
- dental instruments
- cutting-edge medical devices that utilise recent innovations (such as robotic surgical instruments and minimally invasive devices).
1.8 These units will be managed by a decontamination manager. Technicians that have the specialist training, knowledge, skills and competencies required will carry out the day-to-day processing tasks. This approach is considered best practice as the units will work to standard operating procedures (SOPs) and Health Technical Memorandum (HTM) guidance, which form part of a quality management system.
1.9 The level of decontamination will vary depending on the device and the information in the manufacturer’s instructions for use. In all cases, decontamination staff need to be trained and competency-assessed on the decontamination tasks they will carry out, and documented evidence of all training and competency assessments should be maintained and retained.
1.10 This was highlighted in the Department of Health and Social Care’s (DHSC) ‘National Decontamination Survey Report 2008-2010’, which found that there is scope for broader training and professional development among decontamination staff. It said that staff training, education and qualifications in relevant medical device decontamination sciences is of significant relevance in terms of prion removal and decontamination.
1.11 The survey found that 82.5% of operator staff were practically trained and skilled in the tasks they were expected to perform but did not necessarily have the underpinning knowledge that theoretically supports the training. However, it also highlighted that this did not always bridge the theory-practice gap, with some aspects of training provided being better quality than others. For example, while 97% of staff who carried out manual washing were trained in the activity, very few had taken part in a training programme leading to a formal qualification.
1.12 Up to 76% of decontamination units reported quality issues which they considered to be closely related to a requirement that staff be trained adequately in the application of quality systems based on international standards, and it was found that staff lacked formal qualifications in much of this area:
There is an identifiable absence of professional training and recognition amongst many grades of staff; this includes the trainers and department leads. [Department of Health and Social Care’s ‘National Decontamination Survey Report 2008-2010’]
1.13 It identified that the introduction of formal education and qualification frameworks was essential to reduce the risk of infection transmission during invasive procedures.
1.14 Since 2013, UNISON, NHS Employers, the Institute of Decontamination Sciences (IDSc) and Health Education England have been working collaboratively to raise national standards and training for staff working in sterile services roles.
1.15 It was recognised in 2015 that because of the complexities and science knowledge required to provide effective decontamination of medical devices, all medical device decontamination staff were aligned to the national profiles for “healthcare science”. This was part of the Modernising Scientific Careers (MSC) project which was signed by the Chief Scientific Officers from the four devolved nations.
1.16 However, despite the progress made, there are still urgent concerns throughout the decontamination community about the lack of training and formal qualifications in medical device decontamination. The Health Service Safety Investigations Body (HSSIB) (formerly known as the Healthcare Safety Investigation Branch (HSIB)) was notified in 2022 of a patient safety concern relating to contaminated surgical equipment being used during a kidney stone removal procedure. HSIB conducted an initial scoping investigation which determined that the patient safety concern met the criteria for investigation. The investigation found several areas of concern, one of which was the following:
Staff training and competence was another key area that the investigation considered. It was discovered that all training and training standards for decontamination are set locally by individual SSDs rather than set out in a national framework. This creates challenges for regulators, SSDs and NHS trusts as currently the system of safety relies on trained and experienced staff carrying out decontamination tasks to a set standard and identifying problems as they arise. If the standard of staff training varies across the country, then the standard of decontamination may also vary. (HSSIB (2022)
As a result, the report identified that there was no national competency framework for staff working in decontamination units to ensure consistency and standardisation. It therefore made the following recommendation:
Safety recommendation R/2022/195: HSIB recommends that NHS England and NHS Improvement develops a competency framework, stating skills, qualifications and professional registration as required, for all sterile services staff and includes it in Health Technical Memorandum 01-01. (HSSIB (2022)
1.17 The HSSIB report (2022) also identified that “top management” currently lacks involvement, oversight and accountability regarding the quality management systems and risks in decontamination units. It recommended that changes be made to better define, involve and inform trust leadership regarding quality and risks in decontamination units.
1.18 This technical bulletin aims to address the HSSIB’s recommendations by:
- outlining the knowledge, skills, abilities and behaviours required by staff working in decontamination units to ensure the highest standards of decontamination are achieved (see table 1), and
- giving general recommendations for improving board-level commitment and oversight of decontamination quality.
Note: Most healthcare professionals will decontaminate medical devices such as infusion pumps, ultrasound probes, stethoscopes, respiratory equipment and incubators as these devices may not be decontaminated in a decontamination unit. Therefore, it is important that the following points have been considered and risk-assessed:
- The correct and latest decontamination procedures and guidelines applicable for the devices they are decontaminating should be followed (the approach and method of decontamination of devices can be found in the manufacturer’s instructions for use, which is supplied with the device when purchased).
- Adequate training on the specific devices to be decontaminated has been given and documented.
- Healthcare professionals should understand the risks of improper decontamination when it is done in a decentralised way by many different people.
- There is a need for oversight and auditing of all decontamination practices by decontamination experts and IPC teams on an approved audit schedule.
- Appropriate levels of staffing and dedicated facilities and equipment should be allocated to meet decontamination and health and safety standards.
- There should be a documented protocol to escalate all incidents and non-conformities, aligned with organisational policy, to ensure the Decontamination Lead is notified as part of the escalation process.
2. Current legislation, standards and guidance
Acts and regulations
2.1 Regulation 19 of the Health and Social Care Act 2008 (Regulated Activities) Regulations 2014 states:
Persons employed for the purposes of carrying on a regulated activity must be of good character, have the qualifications, competence, skills and experience which are necessary for the work to be performed by them.
Code of practice
2.2 The ‘Health and Social Care Act 2008: Code of Practice on the prevention and control of infections and related guidance’ is directly relevant to decontamination of reusable medical devices and is part of the measures required to prevent the risk of HCAIs and cross-contamination between patients. It says reusable medical devices and equipment decontamination policy should demonstrate that:
Staff are trained in cleaning and decontamination processes and the safe use of decontamination equipment and hold appropriate competences for their roles.
2.3 Clause 2.4 in the Code of Practice also outlines the roles and responsibilities of the Decontamination Lead.
Quality management systems and compliance audits
2.4 Although decontamination units are presently expected to implement a QMS, upcoming reforms to medical device regulations in the UK will likely make QMS adoption mandatory across all units.
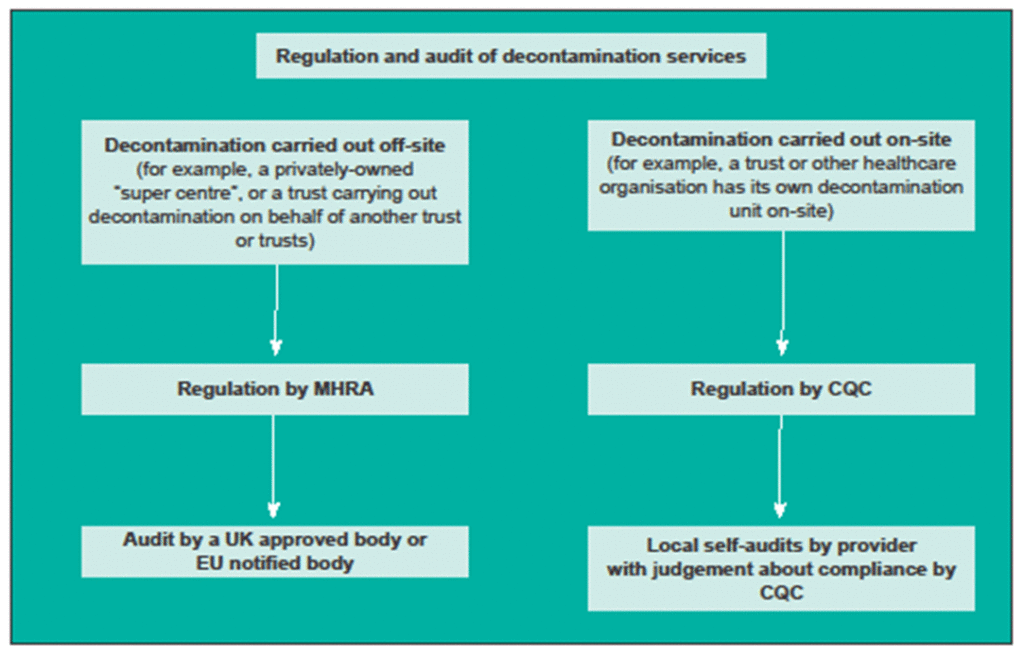
2.5 Decontamination units that supply services to external organisations are required to have a certified QMS system that complies with an appropriate conformity assessment route under UK or EU Regulations as issued by a UK approved body or EU notified body. Compliance with the Medical Devices Regulations is also required. (Note: at the time of writing this is currently under review as part of the UK’s withdrawal from the European Union.)
2.6 Figure 1 illustrates the regulatory framework and the compliance routes for reusable medical devices transferred between legal entities and for reusable medical devices remaining within one legal entity.
2.7 Decontamination units subject to UK approved body or EU notified body certification are generally audited on an annual basis.
Figure 1 Table of regulation and audit of decontamination services
2.8 ISO 13485 section 6.2 states that “there must be a departmental training programme with associated evidence of competencies for each staff member”. As there is no national training programme, decontamination units have developed their own programme and methods of evidencing competency. There is no oversight of the programmes in place and therefore authorised bodies will audit against that stated in the decontamination unit’s QMS.
Healthcare guidance
2.9 Health Technical Memorandum (HTM) 01-01: ‘Management and decontamination of surgical instruments (medical devices) used in acute care’ offers best practice guidance on the whole decontamination cycle including the management and decontamination of surgical instruments used in acute care. Health Building Note 13 (HBN) – ‘Sterile services department’ provides guidance on the infrastructure and fabric of a decontamination unit.
2.10 HTM 01-01 Part A gives guidance on the education, training registration and professional membership advice for decontamination personnel:
The ACDP-TSE Subgroup therefore recommends that decontamination staff should undertake appropriate formal training…… the training package offered by the Institute of Decontamination Sciences (IDSc) or other equivalents initiative apprenticeship programme. It also states that it would be best practice for senior SSD staff (for example the User) to be members of a relevant professional body such as the IDSc and implementing the generic job descriptions will improve patient safety and staffing structures.
2.11 Paragraph 4.29 in HTM 01-01 states that the decontamination policy should demonstrate that staff are trained in cleaning and decontamination processes and hold appropriate competencies for their role.
2.12 In addition, Health Education England’s (2017) ‘Commission on education and training for patient safety’ progress report stated that “the NHS cannot expect to achieve improvements in patient safety if it is not embedded within education and training and if we cannot safely allow staff the time away from the workplace to undergo training”.
3. Training and competency
3.1 National profiles for the “healthcare science” workforce is intended to cover a wide range of professional groupings. These include medical device decontamination sciences.
3.2 BTEC Level 2 Diploma in Healthcare Science (Healthcare Science Assistant) and BTEC Level 4 Diploma in Healthcare Science (Healthcare Science Associate) are both competency-based qualifications and can be accessed through the apprenticeship levy funding in the NHS and private organisations that contribute to the fund. The apprenticeship contains knowledge, skills and behaviour requirements and combines practical and theoretical knowledge.
3.3 These courses take between 12 and 24 months to complete, cover generic modules that are applicable to all healthcare science personnel and then specialist modules in decontamination of both surgical instruments and flexible endoscopy. There is an on-site end point assessment on completion of the course that involves competency and knowledge of the apprentice(s).
3.4 Commercial training providers can deliver the content or the organisations can deliver it in house; however, they must be approved by the qualification provider.
3.5 The IDSc provides a Technical Certificate training programme level 3. This programme has been accredited since 2021 having previously been non-accredited since its introduction in 2011. This is aimed at supervisor and team leader level, and at the time of writing over 900 people have completed the course with 250 people currently on the course.
3.6 There is currently no provider or apprenticeship programme for the practitioner and clinical scientist levels. Anglia Ruskin University used to offer a foundation degree course (2012–2018) but uptake was low as it was not a requirement for the role and organisations were reluctant to fund members.
3.7 The apprenticeship route is the only formal framework for training staff in decontamination. Requirements for higher level staff (for example, production managers and departmental managers) are not addressed and there has never been a requirement to have a framework. Yet, all departments that are accredited to ISO 13485 are required to have a training and competency assessment programme for production and quality staff levels. These are developed by the departments and audited by the authorised body. If the department is not accredited, then local programmes may be in place and may vary in quality.
3.8 One provider provides City & Guilds-accredited training programmes for higher level staff and they include a “User” course for decontamination unit supervisors and managers. They also offer a Decontamination Lead course.
3.9 It is important to recognise that engineering staff that work on decontamination equipment should also have appropriate training and competencies as this is integral to the same decontamination of medical devices (see HTM 01-01).
Professional membership
3.10 HTM 01-01 Part A states that it would be best practice for senior decontamination staff (for example, the User) to be members of a relevant professional body such as the IDSc. This should be chartered member.
3.11 The two-level membership of the IDSc was introduced after HTM 01-01 was published and therefore the guidance should relate to Chartered Member status as this requires specified qualifications. A member does not require any qualifications.
Trainer qualifications
3.12 It is recognised that for effective training of decontamination personnel, those delivering the training should be highly skilled and qualified at providing instruction and mentoring, ideally supported by an appropriate qualification. These trainers should have a level of competency higher than that of the staff they are training.
3.13 Further details on the recommended training, competency, membership qualification requirements for each job type are set out in Table 1.
4. Organisational structure, job descriptions and job profiles
It is important to note that there is an aging profile of existing decontamination management and a significant number will be retiring in the next ten years. This is reflected in NHS England’s (2022) ‘Estates and Facilities Workforce Action Plan’. This will lead to a knowledge gap at a time when the complexity and scientific knowledge of decontamination personnel is at its highest.
The proliferation of innovative medical devices in recent years (including minimally invasive devices and robotics) has reinforced the need to consider all clinical and decontamination aspects of the medical devices under consideration before purchase. Decontamination of these modern medical devices often requires specialist facilities, equipment and staff with specialised knowledge, and the costs associated with these should be considered along with the clinical benefits as part of the comprehensive decision-making process.
Organisational structure for decontamination staffing, reporting and safety oversight
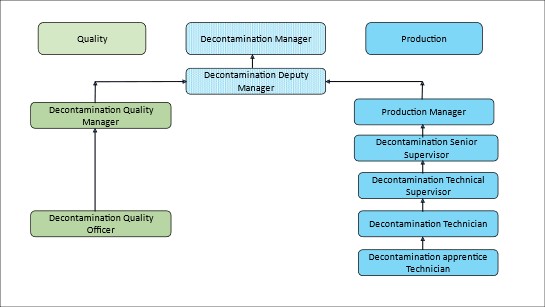
4.1 Figure 2 shows an example of a decontamination unit staff structure incorporating the job roles and profiles. Dependent on the size and structure of the department not all roles will be required; however, the basic structure should be used.
Figure 2 An example of a decontamination unit staff structure
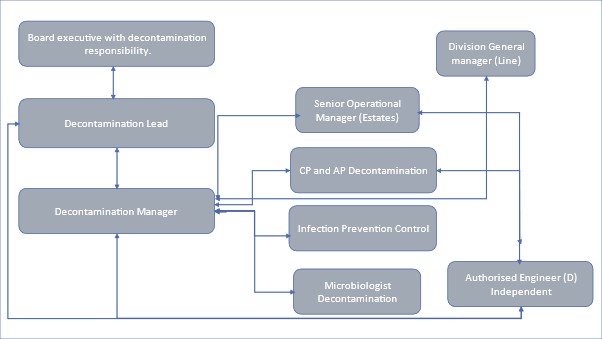
4.2 Figure 3 shows an organisational decontamination reporting structure from a decontamination manager to CEO (adapted from HTM 01-01 Part A) and includes the managerial reporting structure for the decontamination manager.
4.3 Decontamination units provide services to all clinical divisions and therefore consideration should be given to the service sitting in a clinical support division as do pathology, radiology and pharmacy.
Figure 3 Organisational decontamination reporting structure
4.4 The recommended membership of a decontamination safety group (DSG), which should be implemented within each organisation to ensure there is full coverage of the decontamination of medical devices as covered in the Health and Social Care Act, should include:
- decontamination lead (chair)
- decontamination manager
- endoscopy lead
- external provider
- management from each clinical division
- infection, prevention and control lead
- head of estates
- radiology lead
- pathology lead
- divisional lead nurses
- risk/governance/medical devices safety officer
- clinical leads
- microbiologist (decontamination)
- authorising engineer (decontamination)
- authorised person (decontamination)
- senior operational manager.
4.5 The HSSIB investigation (2022) found that “top management” are not involved in the quality management systems used by their decontamination units. Moreover, external independent audits are only reported to the decontamination unit and not to the organisation leadership:
“HSIB recommends that NHS England and NHS Improvement amends Health Technical Memorandum 01-01 to define ‘top management’ and its commitment to quality, and that external independent audits are reported directly to the responsible executive director in a trust who is accountable for the service, not just the certified department”. (HSSIB, 2022)
4.6 It concluded that risks are not escalated or considered at the Trust board level and thereby recommended that changes are needed to better define, involve and inform organisation leadership.
4.7 HTM 01-01 Part A (Management and provision) defines the following roles in relation to ‘top management’:
Management
Management of a healthcare organisation performing decontamination is defined as the owner, chief executive or other person of similar authority who is ultimately accountable for the safe operation of the premises, including decontamination.
Executive manager
The Executive manager is defined as the person with ultimate management responsibility, including allocation of resources and the appointment of personnel, for the organisation in which the decontamination equipment is installed.
Depending on the nature of the organisation, this role may be filled by the general manager, chief executive or other person of similar authority.
Decontamination lead
Every healthcare provider should have a nominated decontamination lead with responsibility for decontamination, either at board level or who has line management responsibility to a senior responsible person at that level.
The decontamination lead should report directly to the executive manager.
The decontamination lead is organisationally responsible for the effective, and technically compliant, provision of decontamination services.
The decontamination lead is responsible for the implementation of operational policies for decontamination and should ensure specific operational policies are in place for the decontamination of all medical devices. He/she should ensure that the operational policy clearly defines the roles and responsibilities of all personnel who may be involved in the use, installation and maintenance of decontamination equipment. The Decontamination Lead is also responsible for monitoring the implementation of the policy and should have a competent understanding of the decontamination of medical devices, guidance, legislation and standards.
4.8 It is imperative that all organisations have a commitment from the executive responsible for decontamination as described above and that there is a formal process in place that they are notified by the department manager of impending audits and that the reports are shared with them immediately post visit.
4.9 It is further recommended that the executive should attend the department during the audit visit and meet with the auditor. This will demonstrate to the auditor that there is commitment to quality within the department by the “top management” of the organisation.
Requirements for decontamination staff: experience, skills, qualifications and registration
- In conjunction with NHS Employers, the IDSc has developed a suite of job profiles and job descriptions that should be used for ensuring consistency of job roles in decontamination departments. The job profiles are available on Education » IDSC (idsc-uk.co.uk)
- The profiles state the qualification that should be achieved for each role and describes the limits of authority.
Note: At time of writing the job profiles are being reviewed with NHS Employers and the IDSc with the aim of having these available on the NHS Employers website in 2024.
- The appropriate national job profiles should be used by the job matching evaluation panel for all vacancies when assessing pay grades. The job profiles cannot state the pay grade, as each employer defines these locally.
- NHS England’s (2023) Long-Term Workforce Plan sets out how the NHS will address existing and future workforce challenges by recruiting and retaining thousands more staff and working in new ways to improve the experience of staff and patients. The three areas that NHS England have focused on in the plan are:
- Train: Substantially growing the number of doctors, nurses, allied health professionals and support staff. This is underpinned by a £2.4 billion funding commitment.
- Retain: A renewed focus and major drive on retention, with better opportunities for career development and improved flexible working options. This comes alongside reforms to the pension scheme, with an aim to retain 130,000 staff working in the NHS for longer.
- Reform: Working differently and delivering training in new ways. Advances in technology and treatments will be explored and implemented to help the NHS modernise and meet future requirements. The apprenticeship programme would contribute to these areas for managers of decontamination staff.
- Table 1 shows the requirements for all staff at each level within a decontamination structure in regard to registration, qualifications, experience and skills. It combines the information from various sources including job profiles (role names and skills), HTM role profiles, registration with the Academy for Healthcare Science (AHCS) and qualifications. These changes take a period of time to implement but a five-year plan should be developed and implemented by the Trust/healthcare organisation.
- Competency development should include annual refresher training packages, including reassessment and ongoing continual professional development (see Chapter 5) at each level.
Table 1: Requirements for all staff at each level within a decontamination structure
Job role: Apprentice/trainee Decontamination Technician – Band 2
Equivalent HTM role: Operator
Technical skills and competencies:
General:
- Basic skills to undertake decontamination of medical devices in a decontamination unit (DU) under supervision and assist the workforce of Decontamination Technicians
Workload:
- Work within own competencies and escalate issues where appropriate.
- Work in compliance with the quality management systems and standard operating policies
Relationship management:
- Good communication skills, able to use tact and persuasion.
Interpersonal behaviours:
- Follows team’s objectives and recommendations.
- Professional to those around them.
Experience (medical device decontamination):
- None
Professional membership/registration qualifications:
- Membership none
Qualifications:
-
Department induction programme
-
Working on Healthcare Science Assistant Level 2 qualification
Job role: Decontamination Technician – Band 3
Equivalent HTM role: Operator
Technical skills and competencies:
General:
- Competent skills to undertake effective / compliant decontamination of medical devices in all areas of the DU
- To provide supervision to apprentice Decontamination Technicians
- Plan own workload with limited supervision
Workload:
- Work within own competencies and escalate issues where appropriate
- Work in compliance with the quality management systems and standard operating policies
Relationship management:
- Good communication skills, able to use tact and persuasion
Interpersonal behaviours:
- Follows team’s objectives and recommendations
- Professional to those around them
Experience (medical device decontamination):
- Operational experience at apprentice/trainee Decontamination Technician
Professional membership/registration qualifications:
- Membership None
- Register Academy for Healthcare Science non-accredited HCS Assistant register level 2
- Recommended
Qualifications:
- Attained Healthcare Science Assistant Level 2 qualification.
- Working towards IDSc Technical Certificate Medical device decontamination
Job role: Decontamination Technician Supervisor / Team Leader / Quality Officer – Band 4
Equivalent HTM role: Operator
Technical skills and competencies:
General:
- Competent to perform a full range of decontamination duties, plan own workload and work with minimal supervision
- Manages decontamination records in own area of work
Problem solving:
- Able to recognise routine problems and provide solutions
- Work within own competencies and escalate issues where appropriate
- Undertake QA/ QC testing in compliance with QMS and National guidance
- Able to effectively manage their assigned workload
Relationship management:
- Manage a team that works to the quality standards and responds quickly and efficiently to production and customer requirements whilst maintaining standards
- Liaises effectively with clinical staff to meet demand
Analysis and research:
- Able to effectively review information and provide responses to the requester
- Able to communicate findings to team members
- Investigate incidents and provide recommendations
Interpersonal behaviours:
- Follows team’s objectives and recommendations
- Professional to those around them
Experience (medical device decontamination):
- Operational experience at Decontamination Technician
Professional membership/registration qualifications:
- Membership None
- Register Academy for Healthcare Science non-accredited IDSc Technical Certificate register.
- Essential
Qualification
- Attained Institute of Decontamination Science IDSc Technical Certificate
- Scottish Qualification Authority level 3
- Working towards Healthcare Science Associate Level 4 qualification
Job role: Senior Decontamination Supervisor – Band 5
Equivalent HTM role: User
Technical skills and competencies:
General:
- Performs a full range of decontamination duties and be accountable for work area within department
- Manages decontamination records in own area of work
- Interprets and reviews technical data and production reports
- Supervises, organises and allocates work and /or trains less experienced/qualified staff
Problem solving:
- Able to recognise routine problems and provide solutions
- Work within own competencies and escalate issues where appropriate
- Undertake QA/ QC testing in compliance with QMS and national guidance
- Able to effectively manage their assigned workload
Relationship management:
- Manage a team that works to the quality standards and provides a compliant efficient decontamination process to meet customer requirement
- Responds quickly and efficiently to production and customer requirements whilst maintaining standards
- Liaises effectively with clinical staff to meet demand
Analysis and research:
- Able to effectively review information and provide responses to the requester
- Able to communicate findings to team members
Experience (medical device decontamination): Operational experience to Decontamination Technician Supervisor/ Team Leader / Quality Officer
Professional membership/registration qualifications:
- Membership IDSc Member
- Registration Academy for Healthcare Science non-accredited HCS Associate register associate level 4.
- Essential
Qualifications:
- FdSc Post graduate degree Medical Devices decontamination
- Healthcare Science Associate Level 4 qualification
- User course
Job role: Decontamination Quality Manager – Band 6
Equivalent HTM role: User
Technical skills and competencies:
To perform a full range of decontamination duties, with senior accountability within department.
Work independently as an autonomous Practitioner, planning own workload.
Apply professional judgement and scientific knowledge when reviewing and interpreting technical data.
Supervises, organises, allocates work and trains less experienced/qualified staff.
To assist with the development of the Department’s Standard Operating Procedures and staff’s compliance.
Work with minimal managerial direction
Problem solving:
- Able to actively identify problems and develop solutions.
- Seeks information to identify the root cause of a problem.
- Outlines clear steps to learn from any mistakes.
Department management:
- Has a working knowledge of the department.
- Able to prioritise their own tasks and those of other staff members that report to them.
Relationship management:
- Works proactively to initiate and develop new relationships.
- Able to see issues from the stakeholder’s point of view.
Analysis and research:
- Able to interpret and effectively communicate quality and technical results and take appropriate action.
- Able to support and undertake analysis to provide insights into issues and solutions and provides the data in an appropriate form to appropriate colleagues.
Interpersonal behaviours:
- Demonstrates awareness of self and others.
- Reads situations and develops appropriate solutions to influence people.
Experience (medical device decontamination): Operational experience / Senior Decontamination Supervisor/ Quality Officer
Professional membership/registration qualifications:
- Membership IDSc Chartered Member
- Registration Academy for Healthcare Science Practitioner Register level 6
- Essential
Qualifications:
- Postgraduate degree medical device decontamination
- Healthcare science practitioner level 6 qualification
- User (HTM) course
Job role: Deputy/Production Decontamination Manager – Band 6/7
Equivalent HTM role: User
Technical skills and competencies:
To provide day to day operational management of the service delivery under the guidance of the Decontamination Manager.
To practice as a qualified Senior Specialist and provide a high quality service at all times.
To support the provision of the specialised decontamination service, contributing to the clinical care of the patient.
To apply professional judgement and utilise specialist skills and detailed scientific knowledge when reviewing and interpreting scientific technical data and production reports to ensure a safe service is provided.
To work independently in all areas of the specialty and be able to work as an autonomous practitioner.
To deputise for the Decontamination Manager when necessary.
To work unsupervised, plan your own workload and work with minimal managerial direction.
Problem solving:
- Able to actively identify problems and develop solutions.
- Seeks information to identify the root cause of a problem.
- Outlines clear steps to learn from any mistakes.
Department management:
- Has a working knowledge of the department.
- Able to prioritise their own tasks and those of other staff members that report to them.
Relationship management:
- Works proactively to initiate and develop new relationships.
- Able to see issues from the stakeholder’s point of view.
Analysis and research:
- Able to interpret and effectively communicate quality and technical results and take appropriate action.
- Able to support and undertake analysis to provide insights into issues and solutions and provides the data in an appropriate form to appropriate colleagues.
Interpersonal behaviours:
- Demonstrates awareness of self and others.
- Reads situations and develops appropriate solutions to influence people.
Experience (medical device decontamination): Operational experience Senior Decontamination Supervisor
Professional membership/registration qualifications:
- Membership IDSc Chartered Member
- Registration Academy for Healthcare Science Registration Practitioner Register level 6
- Essential
Qualifications:
- Postgraduate degree medical device decontamination
- Healthcare science practitioner level 6 qualification
- User (HTM) course
Job role: Decontamination Manager/Head of Decontamination Services/Lead – Band 8 A-C
Equivalent HTM role: User
Technical skills and competencies:
Operationally responsible for managing the decontamination of medical device service.
To manage all staff working within the DU.
To apply professional judgement, scientific knowledge to provide a high quality and timely service.
To plan and organise current and future plans and strategies in conjunction with the Decontamination Lead/Division/Directorate Manager and Integrated Clinical Lead.
To work independently in all areas of the service and be able to work as an autonomous practitioner.
To work unsupervised and manage workload with minimal managerial direction.
Problem solving:
- Able to apply broad thinking to own area of work.
- Thinks long-term in a multifaceted way.
- Able to make decisions and identify relevant facts.
- Able to translate complex ideas into simple messages.
Department management:
- Proactive in taking on work, yet still delegates effectively.
- Able to balance short- and long-term priorities.
- Able to establish a culture that ensures team deadlines are met.
Relationship management:
- Able to bring together groups across organisations.
- Deliberately fosters strategic internal and external relationships.
- Leads an MDT within the organisation ensuring safe medical device decontamination.
- Establishes a positive working relationship between their department and their customers.
Analysis and research:
- Able to recognise where analysis is needed and involve appropriate other professional to collect the data.
- Able to present analysis, adjusting to the audience providing solutions.
- Able to quickly judge and direct the evidence and analysis that needs to be undertaken.
- Able to provide technical leadership to the department and compliance to national and international guidance.
Interpersonal behaviours:
- Recognises underlying needs and motivations of stakeholders and responds accordingly.
Able to anticipate future needs, and what current trends will mean for these. - Communicate at all levels within own and external organisation technical and complex information.
- Able to manage own workload and recognise that they are the decontamination SME in the organisation
Experience (medical device decontamination): Operational experience Deputy / Production Decontamination Manager
Professional membership/registration qualifications:
- Membership IDSc Chartered Member
- Register Academy for Healthcare Science Registration BSC level 6 apprenticeship or apprenticeship 7/8
- Essential
Qualifications:
- Postgraduate degree medical device decontamination
- MSc Medical Device
- Professional management and scientific knowledge to master’s degree
- Healthcare science practitioner Clinical Scientist, level 7
Job role: Organisation Executive with decontamination within their portfolio
Equivalent HTM role: Decontamination Lead
Technical skills and competencies:
General:
- Basic skills to undertake decontamination of medical devices in a DU under supervision and assist the workforce of Decontamination Technicians
Workload:
- Work within own competencies and escalate issues where appropriate.
- Work in compliance with the quality management systems and standard operating policies
Relationship management:
- Good communication skills, able to use tact and persuasion.
Interpersonal behaviours:
- Follows team’s objectives and recommendations.
Professional to those around them.
Experience (medical device decontamination): Direct access to organisational Board executives, Technical competent support, e.g., Decontamination Manager
Professional membership/registration qualifications: IDSc Member
Qualifications: Decontamination Lead Course (HTM)
Engineering personnel
Job role: Competent Person (Decontamination)
Technical skills and competencies: The CP(D) is defined as a person designated to carry out maintenance, validation and periodic testing of washer-disinfectors, sterilizers and endoscope decontamination equipment.
The CP(D) should be assessed at periodic intervals by an AP(D) and should undertake refresher training when deemed appropriate (as example a minimum of 5 years and a maximum of 8-year intervals).
It is acknowledged the CP(D) may complete restricted work activities under the AP(D)’s scrutiny and at their discretion during a training period and prior to acceptance as a CP(D).
Experience (medical device decontamination): Post apprenticeship experience. Appropriately qualified in a mechanical/electrical discipline to a minimum of NVQ Level 3 qualification.
Manufacturers training.
Training in testing/validation disciplines.
Routine work activity on relevant decontamination equipment.
Professional membership/registration qualifications: Member of a recognised Institute – IHEEM/CIBSE.
Qualifications: Formal apprenticeship. NVQ, City & Guilds qualification in mechanical/electrical discipline. Accredited validation courses.
Job role: Authorised Person (Decontamination)
Technical skills and competencies:
The AP(D) should have knowledge of the specific equipment installed on site and not simply a generic overview of decontamination equipment.
The AP(D) should have received appropriate training to complete the role and be conversant with maintenance, periodic testing and governance requirements. They should have completed an accredited course to become an AP(D) and understand requirements of the CP(D)s and successfully passed the examination.
When individuals are completing the AP(D) duties with acknowledged limitations, these need to be documented and approved by the AE(D).
Experience (medical device decontamination): Supervisory/management role.
Professional membership/registration qualifications: Member of appropriate institute such as IHEEM.
Qualifications: Formal training to complete the AP(D) role. B/TEC Higher National Certificate in engineering. CP(D) Training courses.
Job role: Authorising Engineer (Decontamination)
Technical skills and competencies: The role of the AE(D) should be fully independent of the organisations structure for maintenance, testing and management of the decontamination equipment.
The AE(D) must have passed the Advanced Course in Sterilization Technology or the AE(D) Competency Framework.
Experience (medical device decontamination): Management experience
Professional membership/registration qualifications: Be accepted/registered through the peer review process and accepted as a registered AE(D). The registration process is managed by IHEEM
Qualifications: Degree and/or relative experience within healthcare engineering, microbiology and pharmaceutical technologies
Notes: Training providers of the above qualifications can be found on the IDSc website.
Level 2 = GCSE Any grade higher than a pass. Healthcare Science Assistant
Level 3 = A level
Level 4 = First year of a bachelor’s degree which is referred to as FHEQ Level 4, or a certificate of higher education (CertHE). Healthcare Science Associate
Level 6 = Bachelor’s degree or a degree apprenticeship
Level 7 = level 7 diploma Masters
Level 8 = level 8 diploma Doctorate
Training and competency matrix apprentice/trainee decontamination technician and decontamination technician
Table 1 shows the basic training required for each staff member at apprentice/trainee Decontamination Technician (under supervision only) and Decontamination Technician (competent to undertake work independently). After staff have received the training from the appropriate provider, then they must be competency-assessed before carrying out the task unsupervised. All competency assessments should be completed under the direction of a suitably qualified person either internal or external to the organisation. A log of all staff training and competency assessments should be recorded in the person’s training file and the organisation’s workforce management platform. For NHS staff, this should be recorded on ESR, the NHS’s workforce management platform.
Sources of training could be an accredited training provider, manufacturers or their certified representative of the medical device, manufacturers or their certified representative of the equipment or suitably qualified personnel. Training can be undertaken either remotely or on-site. All competency assessments should be delivered by a person certified either by the manufacturer or by qualification who has the knowledge to assess competency. Evidence should be submitted that individuals undertaking the training are competent for the tasks. Where internal training programmes are in place, periodic assessment should confirm that the information taught is in accordance with updated guidance/standards.
It is recommended that all competencies are reassessed annually (ISO 13485) and when processes are changed.
Table 2: Basic training required for each staff member at apprentice/trainee decontamination technician (under supervision only) and decontamination technician
|
Criteria |
Training delivered |
Comments |
Competency assessed |
Comments |
|
General: | ||||
|
The components and construction of a surgical instrument |
|
|
|
|
|
Manufacturer’s instructions for use compliance |
|
|
|
|
|
Identify devices that may need maintenance or repair of medical devices. |
|
|
|
|
|
Follow Standard Operating Procedures (SOPs) |
|
|
|
|
|
Infection prevention and Control processes |
|
|
|
|
|
Hand hygiene |
|
|
|
|
|
Donning and doffing PPE |
|
|
|
|
|
Steps of hand hygiene |
|
|
|
|
|
PPE for washroom |
|
|
|
|
|
PPE for Inspection, Assembly and Packing room (IAP) |
|
|
|
|
|
PPE for sterilisation area |
|
|
|
|
|
Disposal of waste streams |
|
|
|
|
|
Clinical / contaminated |
|
|
|
|
|
General/ domestic |
|
|
|
|
|
Sharps |
|
|
|
|
|
Recycling |
|
|
|
|
|
Cleaning of workspace |
|
|
|
|
|
Use of Wipes |
|
|
|
|
|
Frequency of cleaning surfaces |
|
|
|
|
|
Clearing up spills |
|
|
|
|
|
Disinfection of manual cleaning equipment. |
|
|
|
|
|
Workflow requirements |
|
|
|
|
|
Disposal of Single use items |
|
|
|
|
|
Disinfection for pre-cleaning equipment |
|
|
|
|
|
| ||||
|
Department Information system: | ||||
|
Scanning stages during the decontamination process |
|
|
|
|
|
Reason for recording the process into the system |
|
|
|
|
|
Consequence of not using system/ product recall |
|
|
|
|
|
| ||||
|
Washroom: | ||||
|
Checks made on receipt of surgical instruments |
|
|
|
|
|
Checking medical devices against master lists |
|
|
|
|
|
Preparing devices for the appropriate cleaning process (Manufacturer’s Instructions for Use) |
|
|
|
|
|
Disassembly requirements of devices |
|
|
|
|
|
Pre cleaning requirements |
|
|
|
|
|
Manual clean process procedure |
|
|
|
|
|
Knowledge of detergents |
|
|
|
|
|
Automated clean washer disinfector process cycle |
|
|
|
|
|
Automated clean ultrasonic process cycle |
|
|
|
|
|
Loading carriages configuration |
|
|
|
|
|
Reporting non conformances |
|
|
|
|
|
Operating the washer-disinfectors |
|
|
|
|
|
Daily checks requirements for process equipment |
|
|
|
|
|
Daily checks requirements for environmental conditions |
|
|
|
|
|
| ||||
|
Inspection, assembly and packing room: | ||||
|
Acceptance criteria for release from washer-disinfector. |
|
|
|
|
|
Process to follow for a “failed load” |
|
|
|
|
|
Product release procedure from washer-disinfector |
|
|
|
|
|
Checking medical devices to ensure they are fit for purpose |
|
|
|
|
|
Laying up instruments in din basket to ensure sterilisation |
|
|
|
|
|
Compliance to the master list contents |
|
|
|
|
|
Wrapping and sealing of din baskets / containers/ packs |
|
|
|
|
|
Preparing supplementary instruments for packing and sterilisation |
|
|
|
|
|
Compliance to master list content |
|
|
|
|
|
Wrapping and sealing techniques |
|
|
|
|
|
Wrapping materials requirements |
|
|
|
|
|
Labelling requirements |
|
|
|
|
|
Reporting non conformances |
|
|
|
|
|
Daily checks requirements for processing equipment |
|
|
|
|
|
Daily checks requirements for environmental conditions |
|
|
|
|
|
| ||||
|
Sterilising area: | ||||
|
Checking sets prior to loading on steriliser carriage |
|
|
|
|
|
Sterilisation carriage configuration |
|
|
|
|
|
Operation of all sterilising equipment in department. |
|
|
|
|
|
Carry out daily tests |
|
|
|
|
|
Product release procedure from sterilisation |
|
|
|
|
|
Load cooling requirements |
|
|
|
|
|
Checks prior to product release to customer/ storage |
|
|
|
|
|
Despatch process |
|
|
|
|
|
Daily checks requirements for processing equipment |
|
|
|
|
|
Despatch process |
|
|
|
|
|
Daily checks requirements for environmental conditions |
|
|
|
|
|
Daily checks environmental |
|
|
|
|
|
Collection delivery of equipment |
|
|
|
|
|
Trolley requirements |
|
|
|
|
|
Manual handling requirements |
|
|
|
|
Training and competency matrix Decontamination Technician Supervisor/Senior Decontamination Supervisor
Table 3 shows the basic training required for each staff member at Decontamination Technician Supervisor/Senior Decontamination Supervisor level. After staff have received the training from the appropriate provider then they must be competency assessed before carrying out the task unsupervised. A log of all staff training and competency assessments must be recorded in the person’s training file and the organisation’s workforce management platform. For NHS staff, this should be recorded on ESR, the NHS’s workforce management platform.
Sources of training could be an accredited training provider, manufacturers or their certified representative of the medical device, manufacturers or their certified representative of the equipment or suitably qualified personnel. Training can be undertaken either remotely or on-site. All competency assessments should be delivered by a person certified either by the manufacturer or by qualification who has the knowledge to assess competency.
Evidence should be submitted that individuals undertaking the training are competent for the tasks. Where internal training programmes are in place, periodic assessment should confirm that the information taught is in accordance with updated guidance/standards.
Table 3 Basic training required for each staff member at Decontamination Technician Supervisor/Senior Decontamination Supervisor level.
|
Criteria |
Training delivered |
Comments |
Competency assessed |
Comments |
|
General: | ||||
|
The components and construction of a surgical instrument to confirm fit for use |
|
|
|
|
|
Understand the purpose of medical device Manufacturer’s Instructions for Use (IFU) and identify areas that may not comply with departments processing parameters |
|
|
|
|
|
Managing the repair and maintenance procedure ensuring traceability is maintained |
|
|
|
|
|
Completion of the decontamination certificates for repair and loan equipment |
|
|
|
|
|
Quality assurance/control: | ||||
|
Demonstrate the ability to participate in quality processes in own area of work including the below. Able to read test results and report to an appropriate colleague if parameters are not met and sign off results within your areas of authority and competence |
|
|
|
|
|
Daily washer-disinfector checks |
|
|
|
|
|
Daily washer-disinfector tests |
|
|
|
|
|
Daily ultrasonic checks |
|
|
|
|
|
Daily ultrasonic tests |
|
|
|
|
|
Daily steriliser checks |
|
|
|
|
|
Daily steriliser tests |
|
|
|
|
|
Heat sealer checks |
|
|
|
|
|
Weekly washer-disinfector checks |
|
|
|
|
|
Weekly washer-disinfector tests |
|
|
|
|
|
Weekly ultrasonic checks |
|
|
|
|
|
Weekly ultrasonic tests |
|
|
|
|
|
Weekly steriliser checks |
|
|
|
|
|
Weekly steriliser tests |
|
|
|
|
|
Environmental monitoring and testing requirements |
|
|
|
|
|
Review prepared reports for the testing of the steriliser for referral to an appropriate senior colleague |
|
|
|
|
|
Review all housekeeping documentation and, when identified, highlight areas of concern or gaps to an appropriate senior colleague |
|
|
|
|
|
Water testing |
|
|
|
|
|
Regulation and guidance |
|
|
|
|
|
Identify areas in the department that do not meet the H&S legislation or department policy. |
|
|
|
|
|
Monitoring compliance to the department SOPs |
|
|
|
|
|
Monitoring compliance to the departmental QMS |
|
|
|
|
|
In retaining the documentation for the period defined in the departmental QMS |
|
|
|
|
|
Incident reporting: | ||||
|
Complete investigation into non-conformances and report |
|
|
|
|
|
Identify appropriate remedial actions and refer to appropriate senior staff |
|
|
|
|
|
Department information system: | ||||
|
Use of the system to manage non-conformance including repair/ maintenance, limited use devices, location of device, omissions in scanning |
|
|
|
|
|
In identifying locations of sets to assist clinical teams |
|
|
|
|
|
Use system to identify performance of staff |
|
|
|
|
|
Remove processing equipment from system if in breakdown or testing |
|
|
|
|
|
Use system to assist with contractual compliance and turnaround times |
|
|
|
|
|
Machine management |
|
|
|
|
|
Able to correctly remove machine from use to prevent failed loads |
|
|
|
|
|
Identifying when equipment needs to be tested, maintained and remove from use if beyond permitted period |
|
|
|
|
|
In interpreting cycle results using the machine information and independent monitoring system |
|
|
|
|
|
In management of fail cycles and subsequent medical device and equipment management |
|
|
|
|
|
Complete permit to work documentation |
|
|
|
|
|
Audit: | ||||
|
In participating in a departmental internal audit |
|
|
|
|
|
In participating in a departmental external audit |
|
|
|
|
|
In audit process and scheduling cycle |
|
|
|
|
|
Environment |
|
|
|
|
|
Reporting of areas within the workplace that do not meet environmental requirements |
|
|
|
|
|
Communication: | ||||
|
Liaising with clinical staff in times of downtime that will affect service delivery |
|
|
|
|
|
Implementation of contingency arrangements when authorised by appropriate senior colleague. |
|
|
|
|
|
Maintaining updates with clinical colleagues at time of business continuity. |
|
|
|
|
|
Identifying the information clinical colleagues will need to receive and provide to ensure clinical needs are met or where there will be a shortfall. |
|
|
|
|
5 Career pathways, skill development and continued professional development
5.1 The career pathways presented in this technical bulletin are intended to prescribe an approach towards successfully establishing a decontamination career in NHS healthcare science. They demonstrate the variety of roles available, experience required and competencies needed for professional development, plus the resources available to support these careers.
5.2 Agenda for Change (AfC) is the pay and conditions structure the NHS uses for staff, except for very senior managers (VSM).
5.3 To progress up the AfC banding structure, NHS staff must develop both their experience and skills to meet the criteria outlined in the job profile and job description in Chapter 4. Table 1 indicates some of the generic skills expected by employees at each banding level.
Continuing professional development
5.4 It is essential as healthcare scientists that decontamination personnel engage in continuing professional development (CPD) throughout their careers. This involves proactively taking steps to maintain and enhance knowledge, skills and experience beyond initial qualifications. Actively undertaking CPD will ensure that staff:
- stay up-to-date with the latest standards, technologies and best practices in decontamination
- develop new competencies and skills to advance career progression
- maintain high quality standards and compliance with regulations
- provide the best possible service and patient safety outcomes.
5.5 There are various activities that can count towards CPD for decontamination staff. These include:
- attending training courses, workshops and conferences
- undertaking formal qualifications highlighted in Table 1
- reading relevant journals, publications and guidance documents
- participating in online learning and webinars
- becoming a member of the IDSc
- getting involved with professional networks and organisations such as the IDSc
- cross-training to develop wider experience and skills
- mentoring or buddying schemes with colleagues
- taking on new project work or expanding one’s role
- job shadowing and observational placements.
5.6 CPD activities must be recorded and maintained in a portfolio in compliance with the CPD standards of the Academy for Healthcare Science (AHCS).
5.7 Managers have a responsibility to support CPD by building time and resources into schedules, setting development goals and creating a learning culture, as well as linking CPD to career progression.
5.8 Overall, professional development is an investment that leads to positive outcomes in maintaining high standards of patient safety and service quality. A commitment to CPD enables decontamination staff to continuously develop their expertise over the long term.
6 Registration
6.1 There is currently no mandatory or statutory requirement for registration of decontamination personnel or a requirement for membership to a professional organisation. Therefore, there is no overarching organisation that is responsible for their professional and personal performance. This situation allows organisations to employ people with no defined qualifications. In HTM 01-01 it suggests that it would be best practice for senior decontamination staff (for example the User) to be members of a relevant professional body such as the IDSc (HTM 01-01, Part A, p.25).
IDSc technical certificate
6.2 The IDSc worked with the AHCS to develop a directory of personnel who had been successful in attaining the IDSc technical certificate in 2017. This directory is updated twice yearly and individuals remain on the directory IDSc chartered membership
6.3 An additional directory was developed by AHCS in 2019 for IDSc chartered members. This continues to be available via the IDSc website for employers that have stipulated that a chartered member of IDSc is a requirement for managerial job roles.
6.4 These directories are voluntary but individuals registered on them are required to meet the code of conduct standards of the AHCS.
AHCS non-accredited registers
6.5 The AHCS has recently published non-accredited registers for HCS Assistants and HCS Associates showing individuals who have successfully completed the Level 2 or Level 4 Diploma in Healthcare Science apprenticeship, respectively. On receipt of their Education and Skills Funding Agency (ESFA) certificate of completion, the apprentice can then apply to the AHCS to be added to the relevant non-accredited register. For clarity, an “accredited” register is accredited by the Professional Standards Authority, a “non-accredited” register is managed by the AHCS.
6.6 Further work is now being undertaken by the IDSc and AHCS to extend the non-accredited registers for decontamination personnel that have achieved the IDSc Technical Certificate qualification, and subsequently a relevant apprenticeship. Inclusion on the register means that registrants will be subject to the guidance, rules and regulatory processes of the AHCS.
6.7 The aim of this work is to move towards accredited registration of decontamination personnel in line with other healthcare science practitioners. The benefits will be an appropriately qualified and regulated workforce that will be accountable to work within the Good Scientific Practice (GSP) guidelines and the Standards of Proficiency for Healthcare Science Assistants (SPHSA) or Healthcare Science Associates (HSA).
6.8 This is a significant change and will take a three-to-five-year period to achieve. Clarity for the requirements of the qualifications, training and competency of personnel at each level in this document will assist in the process. A minimum qualification for each role within decontamination is detailed in Table 1.
6.9 This would also apply to the roles within the decontamination of flexible endoscopes where the same roles are described in HTM 01-06 Part A.
References
Acts and Regulations
- Health and Social Care Act 2008 (Regulated Activities) Regulations 2014
- Medical Devices Regulations. Si 2002 No. 618
Codes of Practice
Standards
- ISO 13485. Medical devices: quality management systems: requirements for regulatory purposes. International Organization for Standardization, Geneva.
Health Service Safety Investigations Body (HSSIB) guidance
NHS England guidance
- Estates and Facilities Workforce action plan: building, developing and engaging our people. 15 June 2022.
- Health Building Note 13. Sterile services department
- Health Technical Memorandum 01-01: Management and decontamination of surgical instruments (medical devices) used in acute care
- Health Technical Memorandum 01-06: Decontamination of flexible endoscopes
- NHS Long Term Workforce Plan. June 2023
Department of Health and Social Care guidance
Academy for Healthcare Science guidance
- Good Scientific Practice (GSP) standards
- Improving quality, protecting patients: standards of proficiency for healthcare science practitioners. July 2014
Other publications
Acknowledgements
- Trevor Garcia, National Chairman, Institute of Decontamination Sciences
- Helen Campbell, Decontamination, Education and Consultancy
- John Prendergast, Senior Decontamination Engineer, NHS Wales Shared Services Partnership – Specialist Estates Services
Publication reference: PRN00978

