Foreword
Genomics will be at centre of the next generation of healthcare and is already being used to guide a personalised approach to medicines optimisation. In the NHS, this is underpinned by the world leading NHS Genomic Medicine Service that was launched in 2018 with the aim of embedding genomic medicine into mainstream care.
As set out in Accelerating genomic medicine in the NHS, published in 2022, advances in genomics are enabling personalised medicine developments through improvements in predicting, preventing and diagnosing disease, and the use of precision medicines. For example, with genomic sequencing technologies we can understand whether a tumour sample has a known genomic mutation that precision medicines are targeted against and whether the presence of specific genetic alterations in an individual may affect the response to a specific medicine (pharmacogenomics). Together this information is guiding choice and dose of medication as well as enabling access to clinical trials and supporting the development of innovative precision medicines.
To harness the power of genomics in the NHS, healthcare professionals need sufficient, up-to-date knowledge of genomics in their field and the skills and confidence to discern when testing may be relevant for their patients. Collaboration and multiprofessional networks need to grow and evolve to enable the implementation of new genomic pathways, and to make genomics informed medicines optimisation a part of routine care.
Genomic medicine is a cross-cutting discipline with touchpoints in every specialty and profession. The mission of the NHS England National Genomics Education Programme is to support everyone in the healthcare system to access the genomics education and training they need – from those who need a little knowledge, to those who need a lot, and offer that support in an equitable and accessible way.
The upskilling of the pharmacy workforce in genomics, alongside the multiprofessional team, is crucial for the effective integration of genomic medicine into mainstream NHS healthcare and to the use of precision and targeted medicines including advanced therapeutic medicinal products. Their role in supporting genomics informed medicines optimisation is clear and this strategic framework describes how we are aiming to develop the pharmacy workforce for this role.
We are starting from a strong base, with expert clinical leadership from the pharmacy clinical leads across the seven NHS Genomic Medicine Service alliances who are at or working towards consultant pharmacist level practice in genomics. This sits alongside the pharmacy expertise within NHS England. However, to fully realise the potential of genomics for our patients and the public, the wider pharmacy workforce needs the relevant knowledge and skills so that they can deliver and support these services within their local teams.
We need to consider the application of genomics within prescribing practice as many pharmacists are already able to prescribe independently and all newly qualified pharmacists will from 2026. In addition, we should give pharmacy professionals the opportunity to develop specific and high-level expertise in genomics to make an even greater contribution to the NHS.
To achieve this, pharmacy genomics workforce education and training should become routine. At a national, regional and local level we need to link priorities through working together, reviewing the principles of medicines optimisation, and reflecting on how genomics can support these principles. This will equip pharmacy professionals as part of the multiprofessional team to provide leadership on medicines optimisation, including clinical implementation, education and training, and research and evaluation.
This strategic framework sets out a 3-year approach to integrate genomic medicine into pharmacy education and training and workforce development. It will empower the pharmacy workforce to use the increasingly available genomic tools to support medicines optimisation and deliver the benefits for patients.
Professor Dame Sue Hill, Chief Scientific Officer for England and Senior Responsible Officer for Genomics in the NHS
David Webb, Chief Pharmaceutical Officer for England
Richard Cattell, Deputy Chief Pharmaceutical Officer for England and Chair of the Pharmacy Workforce Group for Genomics
Professor Kate Tatton-Brown, Clinical Director for NHS England National Genomics Education Programme
1. Genomic medicine in the NHS
Genomic medicine is an emerging discipline that combines the use of genomic information (an individual’s or tumour’s complete set of genetic material) with other diagnostic and clinical data as part of clinical care. Powerful new genomic testing technologies are already transforming medicine and healthcare, and the NHS is a leader in their development and at the forefront of embedding their use into routine clinical care.
An entire human genome can now be sequenced in just a few days. This faster, cheaper sequencing gives unprecedented access to genomic data, and this is increasingly being used to diagnose disease, predict the probability of someone developing a condition, and optimise treatment for individuals and their families.
Medicines are the most common therapeutic intervention in healthcare, yet the efficacy and safety of many drugs show considerable interpersonal variation. Genomics offers the opportunity to move away from a ‘one-size-fits-all’ towards a more tailored treatment approach.
Advances in personalised medicine, driven by genomics, range from novel therapies through to optimisation of existing medicines. These include:
- Gene therapies – utilising cutting-edge technology to deliver tailor-made genetic material into a patient’s cells to treat disease.
- Targeted treatments – based on an increased understanding of the genomic basis for disease and diagnosis.
- Pharmacogenomics – guiding treatment decisions and dosing using genomic information to predict response to medicines.
- Repurposing – insights into genomic variations, biological pathways and mechanisms of disease can enable identification of new uses for existing medicines.
- Predicting resistance to medicines – considering genomic characteristics of tumours and infectious organisms (eg bacteria and viruses) to predict resistance to treatment.
Figure 1: Personalised medicine. Adapted from NHS England. Improving outcomes through personalised medicine, 2016
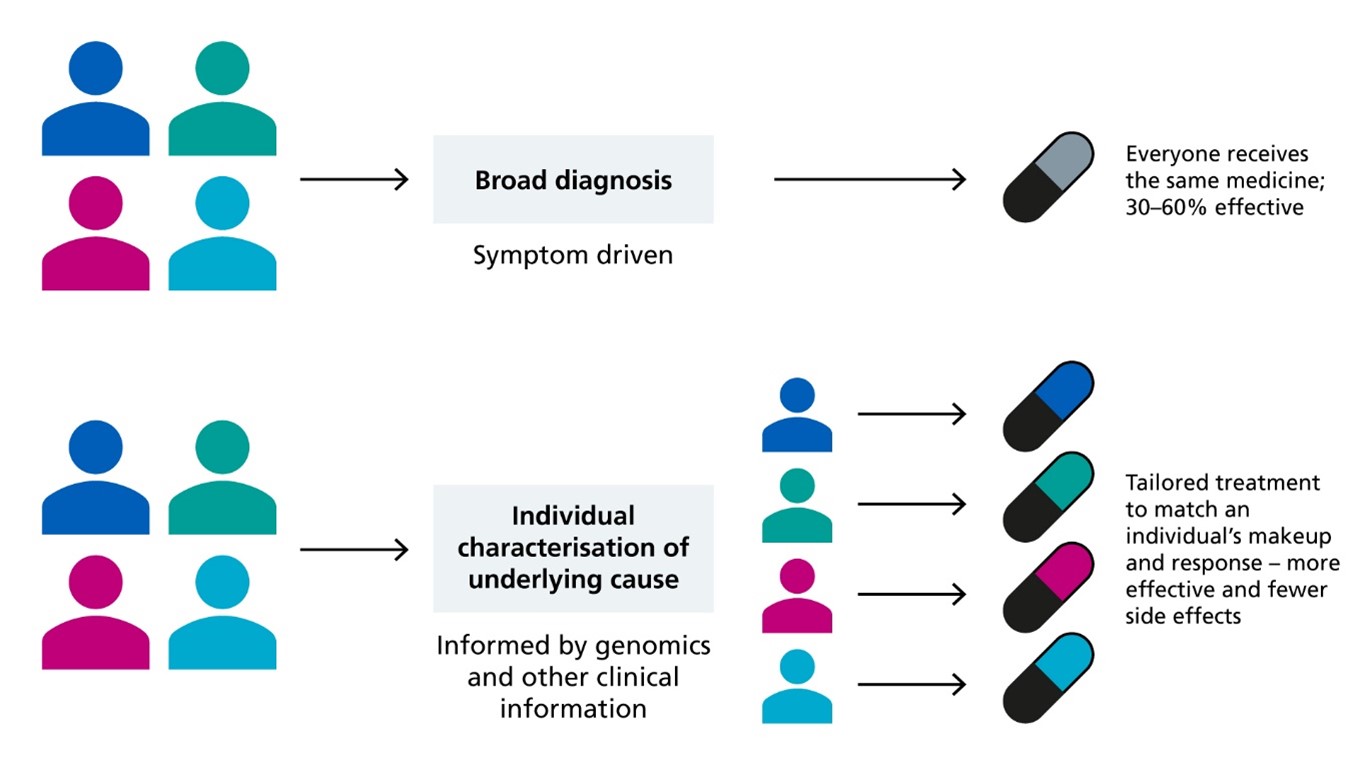
This diagram illustrates two different approaches to use of medicines. The first shows how if all patients with the same condition receive the same first line treatment it may be only 30% to 60% effective. The second illustration shows how comprehensive genomic and diagnostic characterisation can identify different subtypes of patients with a given condition and allow the tailoring of treatment to match an individual’s make-up for greater effectiveness and fewer side effects.
An intrinsic part of the Genome UK policy (2020) is to incorporate the latest genomics advances into routine healthcare to improve the diagnosis, stratification and treatment of illness. NHS England established the NHS Genomic Medicine Service (NHS GMS) in 2018 to drive this ambition.
Other government strategies and policies, such as the NHS Long Term Plan, Topol review, Rare diseases framework and Genome UK, and the NHS Long Term Workforce Plan outline the importance of and drivers for advancing genomics across mainstream healthcare. Significantly, in October 2022, the Genomics Unit in NHS England released Accelerating genomic medicine in the NHS. This outlines the 5-year ambition to accelerate the use of genomic medicine across the NHS and the four priority areas for the NHS GMS to realise the potential of genomics for the population we serve:
- Embedding genomics across the NHS, through a world-leading innovative service model from primary and community care through to specialist and tertiary care.
- Delivering equitable genomic testing for improved outcomes in cancer, rare, inherited and common diseases, and to enable precision medicine and reduce adverse drug reactions.
- Enabling genomics to be at the forefront of the data and digital revolution, ensuring genomic data can be interpreted and informed by other diagnostic and clinical data.
- Evolving the service through cutting-edge science, research and innovation to ensure that patients can benefit from rapid implementation of advances.
2. Genomics education and training of the pharmacy workforce
Pharmacists and pharmacy technicians in all areas of healthcare need appropriate education, training and leadership to harness the benefits of genomic medicine for personalised care in their practice and ensure equitable access to appropriate testing and subsequently optimised treatments. The recently published Regional arrangements for medicines optimisation in the NHS in England emphasises the importance of aligning across genomic medicine and medicines optimisation programmes, as well as the need for pharmacy leadership in genomic medicine informed prescribing.
This strategic framework will provide a widespread and agreed approach as to how the pharmacy workforce can learn and understand more about this area, and then build on this education and training in their day-to-day roles providing optimised treatments to patients. It is anticipated that the outlined areas of focus and timelines will be expanded over time, in line with service development and the definition of professional roles and responsibilities to support these.
2.1 The evolving role of pharmacy professionals in genomic medicine
The pharmacy workforce provides expertise in the safe and effective use of medicines, and they have a responsibility to understand all factors that influence response to medicines, including genomics.
A pharmacy workforce skilled in consultation, interpretation, regulation and use of genomics is required so that genomics can be safely integrated into mainstream medicines optimisation. Therefore, there is an immediate need to ensure that all pharmacy professionals across all sectors gain competence in the fundamentals of genomics relevant to their role and current practice. Pharmacy professionals working in specialist genomic services and early implementation areas will require additional expertise and role-specific competencies for guiding dosing and selection of medicines based on genomic sequencing information. It is also evident that genomics will increasingly impact on the wider pharmacy workforce and patient pathways, particularly as evidence on genomics informed medicines optimisation expands and informs future implementation across more areas of the NHS (NHS Long Term Workforce Plan, 2023).
Implementation of genomics informed medicines optimisation services at scale will require careful consideration and evaluation as evidence continues to develop. This work is underpinned by several important themes, including the approach to testing, use of informatics, equitable access and improved patient safety, and needs to be driven by collaboration across multiple professions and with patient and public involvement. Pharmacy professionals are well placed to drive forward the use of genomic medicine in existing and new medicines pathways to optimise benefits for patients, but require the appropriate skills to do so. Moreover, the need for continuous monitoring and review of real-world implementation represents an opportunity for pharmacy to support translational research for the use of genomics within medicines optimisation.
Examples of how pharmacy professionals are already supporting the mainstreaming of genomic medicine in the NHS are given in appendix 1.
2.2 The National Genomics Education Programme
The National Genomics Education Programme exists to deliver and advise on learning and development opportunities that prepare the current and future NHS workforce to make the best use of genomics in their practice. The programme works closely with higher education institutes, partner organisations, professional groups/specialties and subject matter experts to provide profession and role-specific education and training packages. The programme has four workstreams:
- Identify NHS workforce needs.
- Build and join networks across the country.
- Help develop and educate the NHS workforce.
- Increase awareness of genomics across healthcare.
2.3 The NHS England Pharmacy Workforce Group for Genomics
The National Genomics Education Programme, in partnership with the NHS England Genomics Unit, established the NHS England Pharmacy Workforce Group for Genomics in 2021. This group reports to the NHS England Joint Genomics Workforce Steering Group, which is chaired by the NHS England Genomics Education Programme and the NHS England Genomics Unit.
The group’s mandate is to deliver on the following key priorities:
- Oversee the implementation of this national strategic framework outlining workforce development and education and training support and approaches across different sectors and stages of pharmacy professional training.
- Provide a forum for national collaboration and co-ordination of pharmacy workforce planning and education activity related to genomics.
- Support development of competency frameworks and career frameworks relevant to the pharmacy workforce, including recommendations on the role of pharmacy professionals in pharmacogenomics (via additional working groups).
- Support the review and implementation of undergraduate and postgraduate curricula and recommendations for integrating genomics, including development of the educational framework and curriculum requirements for initial pharmacist education and training.
- Advise on and support wider multiprofessional discussions on the healthcare workforce in genomics.
Membership includes pharmacy and genomics leadership from professional, educational and regulatory bodies, as well as representation from across sectors and professions.
As roles, responsibilities and requirements for pharmacy professionals expand, the NHS England Pharmacy Workforce Group for Genomics and other key stakeholders will continue to review resource planning to build on this framework for workforce development.
2.4 Developing a strategic approach to pharmacy genomics education and training
Pharmacy professionals will continue to provide medicines expertise across the health and social care system, offering advice and clinical leadership as part of a multiprofessional team. As genomic medicine evolves and becomes part of everyday practice, their strong clinical leadership in genomics informed medicines optimisation will enable them to support patients and work in partnership with clinical colleagues to effectively personalise medicine choices. Currently, this is supported by a number of pharmacy professionals with early and specialist interest in genomics. Moving forward, the aim is to enable their review of individuals’ genomic information, via diagnostic and pharmacogenomic testing, as part of routine medicines optimisation.
The strategic framework has been informed by the NHS England Pharmacy Workforce Group for Genomics’ wide stakeholder engagement. In a round table event and wider discussions the following points were considered:
- How to identify pharmacy workforce needs; by considering future clinical pathways and what skills and competencies may be required from the pharmacy workforce.
- How to build and join professional networks across the country.
- How to educate and develop the pharmacy workforce; by considering what our professional leads and networks can currently offer and agreeing the best way to ensure accessibility and suitability across the wider pharmacy workforce.
- How to increase awareness of genomics across the pharmacy profession; by committing as a group to next steps and priorities.
The strategic framework below sets out the recommended approach to applying the National Genomic Education Programme’s four workstreams to the pharmacy workforce over the next 3 years. It is intended to enable all pharmacy professionals to make a significant contribution to the mainstreaming of genomic medicine in the NHS for patients.
It aligns with the priorities and timeline of the NHS England Accelerating genomic medicine in the NHS strategy and the development of the NHS Genomic Medicine Service. The timeline is also consistent with the reformed foundation pharmacist programme due to be in place from 2025/26 and the anticipated review of pharmacy technician initial education and training.
3. The strategic framework
Strategic aim 1: Integrate awareness of genomics as part of pharmacy practice
Why is this important now?
The use of genomics to inform medicines optimisation is becoming more widespread across healthcare and pharmacy teams are increasingly encountering genomics in their daily practice.
It is critical that all pharmacy professionals have an awareness of genomics and understand its relevance to medicines, so that they can use genomic information as appropriate within their roles, advise patients and signpost when required.
What do we need to achieve our objectives?
Present:
Encourage pharmacy stakeholders to raise awareness of, and champion the integration of, genomics into pharmacy practice.
1 to 3 years:
- Demonstrate the importance and relevance of genomics in pharmacy practice and medicines optimisation to the pharmacy professions and multiprofessional teams, eg via best practice case studies.
- Enable a consistent level of awareness and knowledge of genomic medicine for all pharmacy professionals so that they can support patients appropriately.
Ongoing
- Support the development of pharmacy genomics champions to drive practice development and raise awareness of genomics across the pharmacy professions and multiprofessional networks.
What tools and resources will we use to achieve our objective?
- The NHS England Pharmacy Workforce Group for Genomics and its subgroups will provide a forum for engagement, communication and partnership working. The group will also facilitate sharing of best practice case studies, expertise and resources across pharmacy networks and organisations.
- Social media and communication strategies to support the dialogue between the National Genomics Education Programme, pharmacy education providers, pharmacy professional networks and the pharmacy workforce to promote genomics education and training resources.
- Close collaboration with the NHS Genomic Medicine Service alliances, which include chief pharmacists and Genomic Medicine Service alliances pharmacy clinical leads – working at or towards consultant level – providing system leadership to drive pharmacy workforce awareness of genomics (Groves and others, 2022).
- Collaboration with professional pharmacy networks and organisations to ensure the inclusion of genomics in their objectives and priorities, to highlight the relevance of genomics to pharmacy professionals.
What will success look like?
- Pharmacy professionals have an awareness of genomics and its relevance to medicines use. They are aware of the National Genomic Education Programme and increasingly use its education resources.
What are we working towards?
- Growth of pharmacists and pharmacy technicians within genomic champion roles to advocate and raise awareness of genomics within their networks and locality.
- Readiness of the pharmacy workforce to integrate genomics as part of routine professional practice, with pharmacy professionals aware of how genomics applies to their individual roles and responsibilities.
- Appreciation of the advances in genomic medicine and benefits to patient care and medicines optimisation.
Strategic aim 2: Build and join networks
Why is this important now?
Pharmacy and multiprofessional networks are already providing both individuals and services with guidance and resources to facilitate the integration of genomic medicine into medicines optimisation, and they are an important means for many pharmacy professionals to receive genomic medicine updates in a supportive environment. They also provide a forum for the recognition, sharing and celebration of best practice in genomic medicine, and facilitate the adoption of genomic technologies to deliver personalised medicine.
Importantly these networks must also engage with patient and public networks to ensure that the patient voice shapes the direction of pharmacy workforce development in line with their needs.
Pharmacy leadership and the professional expertise of pharmacists and pharmacy technicians will increasingly be required to shape practice and education in genomics informed medicines optimisation. As well as using existing pharmacy networks, broader genomic medicine, multiprofessional and patient networks must be engaged to ensure work to embed genomics is collaborative as part of safe and effective person-centred care.
What do we need to do to achieve our objective?
Present:
- Collaboration between pharmacy professional leadership, representative bodies and organisations, and in partnership with wider multiprofessional colleagues, patients and the public to ensure a nationally coherent approach to pharmacy genomics workforce development, education and training.
1 to 3 years:
- Enable growth and development of pharmacy clinical genomics expertise to lead and shape services, as well as to link research, education and clinical networks.
- Continue to drive the embedding of genomic medicine into mainstream pharmacy practice via existing medicines optimisation networks (eg communities of practice and pharmacy professional groups).
Ongoing:
- Promote a ‘do once, learn and share’ approach, facilitating the dissemination of genomics expertise nationwide and reducing duplication.
What tools and resources will we use to achieve our objective?
- The NHS England Pharmacy Workforce Group for Genomics and its subgroups will provide a forum for engagement, communications and partnership working. The group will also facilitate sharing of best practice, expertise and resources across pharmacy networks and the wider multiprofessional genomics education networks.
- Harnessing existing pharmacy and multiprofessional networks to share knowledge, foster collaboration and share education resources and best practice to further genomics education and training of network members.
- Collaboration with the NHS Genomic Medicine Service alliances, which include chief pharmacists and Genomic Medicine Service alliances pharmacy clinical leads – working at or towards consultant level – who provide system leadership to support pharmacy workforce education and training across networks (Groves and others, 2022), as well as established patient and public involvement groups.
- Use of national, regional and local clinical networks supporting pharmacists, pharmacy technicians and their multiprofessional colleagues to generate engagement and support them in starting to use genomic medicine in their practice.
What will success look like?
- Genomics featuring in professional pharmacy networks and communities of practice objectives and priorities.
- Recognised pharmacy genomics groups with multiprofessional engagement.
- Availability of shared tools and resources, reducing duplication of effort across networks.
- Genomics-focused networks that recognise and demonstrate the inclusion of diverse perspectives, knowledge and understanding and advocate an inclusive and equitable approach to genomic medicine.
What are we working towards?
- Local and regional workforce development activity driven by networks aligned to national genomics and pharmacy priorities.
- Recognised network of pharmacy leaders in genomics driving and shaping practice developments and education for pharmacy professionals and multiprofessional colleagues in areas where genomics impacts the safe and effective use of medicines.
Strategic aim 3: Identify pharmacy genomics workforce needs
Why is this important now?
To empower pharmacy professionals to be at the forefront of embedding genomic medicine into mainstream NHS care, it is vital to identify pharmacy workforce needs, aligned with baseline knowledge and competencies.
Pharmacy professionals with an appropriate level of genomic medicine knowledge and skills will enable patients and communities to get the best from their medicines.
What do we need to achieve our objective?
Present:
- Review the current genomic medicine literacy in the pharmacy workforce, at all career stages and in all areas of pharmacy practice.
- Assess the genomic education and training needs of the pharmacy workforce based on current roles and professional responsibility for the effective and safe use of medicines.
1 to 3 years:
- Horizon scan for future genomic clinical pathways related to medicines use and identify the corresponding education and training needs for pharmacy professionals within various roles.
- Stratify knowledge and competency requirements for general and specialist roles as the use of genomic medicine in different services develops.
- Build on requirements for specialties where precision medicines may be commonly used (eg cancer, rare and inherited disease), to ensure pharmacy professionals can support advances in these areas.
Ongoing:
- Periodic evaluation and refinement of education and training needs to ensure the professions are sufficiently equipped to deliver future services as they develop.
What tools and resources will we use to achieve our objective?
- Clinical pathway initiative (CPI): This is a multiprofessional competency-based framework produced by the National Genomics Education Programme. It is built around genomic patient pathways and aligned to education, training and workforce needs. CPI projects can be used to identify pharmacy workforce gaps and development needs to optimise the delivery of specific genomic patient pathways involving medicines. It may also be used to inform more general medicines optimisation workforce needs.
- Pharmacy workforce data should be collected and analysed to understand the education and training needs of the pharmacy workforce and inform effective workforce planning.
- Collaboration with service commissioners to monitor service change and support the identification of education and training needs of pharmacy professionals for future services.
What will success look like?
- Production of a comprehensive view of the current pharmacy workforce education and training needs in genomics, and clear recommendations for pharmacy education and training offers and workforce planning based on their needs, genomic technological advances, and patient input and collaboration.
- Development of multiprofessional CPI projects around genomic patient pathways and aligned with pharmacy education and training initiatives.
What are we working towards?
- Defined roles and responsibilities for pharmacists and pharmacy technicians in genomic medicine pathways of care, in line with developing roles and professional responsibilities.
- Mapped education and training requirements for new genomic services with resources to support pharmacy professionals providing genomic medicine services.
Strategic aim 4: Educate and develop the pharmacy workforce
Why is this important now?
The development of appropriately skilled and competent pharmacy professionals in genomics relies on the education, training, professional development and support they receive throughout their learning journey.
Pharmacy professionals must have the appropriate knowledge, skills and experience to deliver genomics informed services, for the benefit of communities and patients. It is therefore essential that genomics is appropriately integrated across the continuum of pharmacist and pharmacy technician education and training, including pre-registration, post-registration and specialist training pathways.
The core concepts of genomics should be integrated into education and training pathways. Some pharmacists and pharmacy technicians will also require role-specific education and training based on their role and area of practice.
It is also important to consider the application of genomics within prescribing practice as many pharmacists already prescribe independently and need to be able to apply genomics appropriately within their prescribing practice; this will expand to all newly qualified pharmacists from 2026.
What do we need to do to achieve our objective?
Present:
- Facilitate the suitable use of genomics education resources available via the National Genomics Education programme and other education providers, including proactive learning (from bite-sized resources through to Master’s programmes) and reactive learning (accessed at the point of need) (see appendix 2).
- Enable the integration of genomics across initial education and training pathways and align with educational reforms for pharmacists and pharmacy technicians.
1 to 3 years:
- Facilitate review and implementation of curriculum requirements, educational frameworks, key educational resources and credentialing pathways to support the pharmacy workforce in managing genomic pathways of care.
- Enable the integration of genomics across post-registration education and training pathways.
- Increase availability of good practice exemplars and case studies for genomics in pharmacy practice, showcasing implementation of genomic medicine and offering practical guidance on applying genomics to patient care.
- Develop access to resources and support available for educators to deliver genomics effectively in their teaching programmes at varying levels of complexity.
Ongoing:
- Work with service commissioners to monitor service change and growth to ensure the right levels of pharmacy workforce provision, skills, knowledge and experience to implement and deliver genomics informed medicines optimisation, as part of a multiprofessional team.
- Enable pharmacy roles in research to support priorities linked to service needs in genomics informed medicines optimisation.
What tools and resources will we use to achieve our objective?
- The National Genomics Education Programme provides a full range of educational resources and courses (see appendix 2) to facilitate the delivery of genomics education and training, eg GeNotes.
- The Centre for Pharmacy Postgraduate Education offers an introductory learning programme in genomics and pharmacogenomics for pharmacy professionals.
- The standards for the initial education and training of pharmacists include genomics, facilitating the training of undergraduate pharmacy students and trainee pharmacists in this area of expertise.
- The Royal Pharmaceutical Society provides a credentialing process for defining and assuring post-registration standards of patient-focused pharmacy practice. Incorporating genomics into professional standards will be an important consideration as genomics plays an increasingly relevant role in medicines safety and optimisation.
What will success look like?
- Alignment of core genomics knowledge, skills and experience standards for pharmacy professionals to those used in other professions, to promote interdisciplinary collaboration and consistency in patient care.
- Increased availability of, and opportunity for, interprofessional learning and development in genomics.
- Support for educators and mentors delivering genomics as part of training programmes, such as the availability of an educator’s toolkit including case studies.
- Increased uptake of the National Genomics Education Programme’s resources and those of other pharmacy education providers.
- Availability of and widespread engagement with case studies across forums.
What are we working towards?
- A structured continuum of professional learning and development in genomics for pharmacists and pharmacy technicians from pre-registration to post-registration.
- Pharmacists and pharmacy technicians are supported to develop the appropriate genomic competencies relevant to their role and aligned with the curriculum and credentialling structure for post-registration practice.
4. Challenges to adopting genomics in mainstream pharmacy practice
The main barriers that may be encountered when implementing this strategic framework are listed below (adapted from the Academy of Medical Royal College’s Genomics generic syllabus). Recognition of these barriers, and action to resolve them, will support the integration of genomics across the continuum of pharmacist and pharmacy technician education and training.
- Genomic needs differ across sectors and clinical specialties. While a good understanding of the fundamentals of genomics is essential for all practising pharmacists and pharmacy technicians, certain specialty areas of practice with advanced applications of genomics (eg oncology) will require development of additional specialty-specific capabilities.
- Many practising pharmacists and pharmacy technicians do not yet understand the relevance of genomics to their roles. While the evidence is growing for the adoption of genomics into medicines optimisation, mainstream clinical implementation is currently predominantly in specialist areas currently (eg cancer and rare disease).
- Availability of case studies. To integrate genomics into curricula, students and trainees will need exposure to case studies showing how genomics is being employed in the safe and effective use of medicines. While ‘real-world’ examples are currently limited, opportunities for experiential leaning will increase as services develop.
- Availability of appropriate mentors/supervisors. The pharmacy workforce supporting trainees in meeting learning outcomes are unlikely currently to have the necessary exposure to or knowledge of genomics they will need.
- Defined roles and responsibilities. The specifics of pharmacist and pharmacy technician roles in genomic patient pathways are evolving as services develop and increase. It is anticipated that role specifics will likely be determined as clinical practice progresses in line with increasing patient access to genomics informed medicines optimisation services.
As highlighted above, genomic medicine services are still developing and may not yet be in everyday practice. This strategic framework is aligned with the NHS England genomic medicine strategy and highlights areas of focus over the next 3 years that will support all pharmacy professionals as these services evolve with the mainstreaming of genomic medicine into medicines pathways.
Appendix 1: How are pharmacy professionals supporting the mainstreaming of genomic medicine in the NHS?
Case study 1: Education and shared decision-making to help patients understand the benefits and limitations of precision medicine
Pharmacy professionals play an important role in informing patients about the concept of precision medicine, including its benefits and potential applications. Through education and increasing awareness, pharmacists and pharmacy technicians can ensure that patients from diverse backgrounds have access to information about precision medicine and its relevance in holistic patient care. For example, Macmillan Cancer Support has developed a patient leaflet on a novel type of cancer treatment called histology independent therapies (HITs) in collaboration with several other cancer charities and organisations.
Two HIT drugs are currently available, larotrectinib and entrectinib, for the treatment of cancers with the NTRK gene fusion mutation, which leads to uncontrolled cell growth. Neurotrophic tyrosine receptor kinase (NTRK) fusion is rare but can be more common in certain cancers, such as paediatric cancers. Clinicians should be aware that patients can be tested for this mutation and if it is detected that HIT drug is a treatment option.
National Genomics Education programme: Genomic test supports next-generation cancer drugs
Case study 2: Multiprofessional team working to identify toxicity-related variants in the dihydropyrimidine dehydrogenase (DPYD) gene prior to starting fluoropyrimidine chemotherapy
Fluoroyprimidine chemotherapy (eg capecitabine and 5-fluorouracil) is used in the treatment of several cancers at all stages. It is generally well tolerated but severe adverse drug reactions do occur in 5% to 10% of patients and these are occasionally fatal. Testing for variation in the DPYD gene can help identify patients deficient in the enzyme dihydropyrimidine dehydrogenase and at risk of developing fluoroyprimidine-associated toxicity. The test is performed on a standard blood sample by NHS genomic laboratory hubs in England using simple targeted testing technology.
Pharmacy teams play a crucial role in interpreting DPYD pharmacogenomic test results when screening prescriptions for capecitabine and 5-fluorouracil prior to administration. They collaborate with other healthcare professionals and patients to make informed decisions about medication dose and selection. If a patient has a DPYD gene variant associated with reduced enzyme activity, alternative treatment or lower dose may be considered to reduce the risk of severe side effects, as recommended in national guidance. Pharmacy teams can also support dose adjustments for further cycles of chemotherapy in patients who tolerate treatment well.
UK Systemic Anti-Cancer Therapy Board: Personalised medicine approach for fluoropyrimidine-based therapies
Case study 3: Collaborative care for people with monogenic diabetes
Monogenic diabetes is caused by a genetic change affecting pancreatic beta cells; it is thought to affect 12,000 people in England and accounts for around 1 in 50 cases of diabetes. However, as it is challenging to differentiate from Type 1 or 2 diabetes, some patients with monogenic diabetes are inappropriately treated with insulin.
Availability of genomic testing via the NHS Genomic Medicine Service means that patients can receive an accurate diagnosis and tailored treatment. This may involve switching from insulin to sulfonylureas, which often results in better diabetic control and increased patient convenience.
The pharmacy workforce needs to be aware of monogenic diabetes and its management to ensure that these patients receive optimal care. They can also collaborate with other healthcare professionals such as endocrinologists, diabetes specialist nurses and genetic counsellors to ensure comprehensive and co-ordinated care for individuals with monogenic diabetes. For pharmacist prescribers specialising in diabetes care, additional competencies may also be required to support patient referral for genetic testing and for prescribing optimal treatment.
National Genomics Education Programme: NHS targets better diabetes care with genomic testing
DiabetesGenes: Tests for diabetes subtypes
Case study 4: Familial hypercholesterolemia genetic testing as part of lipid optimisation
As the understanding of the genomic basis for rare and common diseases expands, pharmacists and pharmacy staff managing genetic conditions will need the skills to order tests and deliver results safely and ethically to patients and their families.
Familial hypercholesterolaemia (FH) is one of the most common inherited genetic conditions. Without early detection and treatment, FH can lead to cardiac disease and mortality at a young age. Central commissioning of testing through the NHS Genomic Medicine Service means that appropriately trained healthcare professionals can order FH genetic testing for their patients.
Pharmacists in primary and secondary care have established roles in lipid optimisation and cardiovascular disease management. In some areas pharmacists have received genetics training and are now delivering FH testing to patients.
Centre for Pharmacy Postgraduate Education: Familial hypercholesterolaemia: supporting people better – focal point
National Genomics Education programme: Transformation project: Familial hypercholesterolaemia
Case study 5: Genomics in antimicrobial stewardship and infectious disease
Pharmacists and pharmacy technicians working in antimicrobial stewardship increasingly have access to genomic data on microorganism identification and antibiotic susceptibility and can use this to help guide prescribers in their choice of antibiotics.
Genomics is to identify infectious organisms and track outbreaks, such as identification of new coronavirus variants. It can also be used to determine which antibiotics a bacterium is susceptible to, often faster than conventional culture methods. For instance, whole genome sequencing of mycobacterium tuberculosis provides susceptibility data within a week, rather than 4 to 6 weeks, and as such patients can be prescribed appropriate antibiotics faster.
Genomic testing can provide information that helps avoid adverse drug reactions, such as to the HIV antiretroviral medication abacavir. It can also indicate increased risk of reactions to antibiotics, such as hearing loss with aminoglycosides.
Medicines and Healthcare products Regulatory Agency: Aminoglycosides (gentamicin, amikacin, tobramycin, and neomycin): increased risk of deafness in patients with mitochondrial mutations
Stocco G, Lucafo M, Decorti G. Pharmacogenomics of antibiotics. International Journal of Molecular Sciences 2020; 21(17): 5,975. DOI: 10.3390/ijms21175975
Public Health England: England world leaders in the use of whole genome sequencing to diagnose TB
Case study 6: Pharmacy-led research and the implementation of pharmacogenomics
Clinical guidance is available for a some drug–gene pairs, but for many there remains a translational gap to the use of pharmacogenomic testing in practice in the NHS. One reason for this is the need for more evidence for testing, including the cost-effectiveness of this. There is also a need to ensure research includes people from different ancestral backgrounds to ensure the generalisability and applicability of pharmacogenomics information across diverse populations.
The pre-emptive pharmacogenomic testing for preventing adverse drug reactions (PREPARE) study (Swen and others, 2023) demonstrated that pre-emptive pharmacogenomic panel testing (testing for variants in multiple genes on a single test) can reduce adverse drug reactions. However, the introduction of a new pharmacogenomic service will require continued evaluation, with patient and public input, to explore issues around uptake and acceptance of testing, impact on health inequalities as well as ethical, legal and social issues.
The lifelong relevance of pharmacogenomic information means that clinical decision support (CDS) systems will be crucial to maximise the clinical and cost-effectiveness of pharmacogenomic test results; there remains a need for research into the optimal integration of CDS alerts into electronic health records and prescribing systems.
Pharmacy professionals will have a key role in service evaluation and research as pharmacogenomic services are rolled out, so that evidence can be developed to assess how best to embed pharmacogenomics in routine practice and enable continuous, cyclical improvements to patient care. Pharmacy staff have been involved in the design and delivery of the NHS PROGRESS study (Pharmacogenetics Roll Out – Gauging Response to Service) into implementing pharmacogenomics in primary care and led by the North West Genomic Medicine Service Alliance.
The NHS PROGRESS Study: Implementing pharmacogenetics in primary care (animation)
Further examples of pharmacy professionals supporting with mainstreaming of genomic medicine are available on the NHS Futures platform: pharmacy genomics workforce roundtable.
Appendix 2: Genomics education and training resources
NHS England: NHS Genomic Medicine Service
NHS England: National genomic test directory. Specifies which genomic tests are commissioned by the NHS in England, the technology they use and the eligible patients.
NHS England National Genomics Education Programme
1. ‘Bite-sized’ learning
2. Genomics 101 courses – these courses take about 30 minutes and issue a certificate of completion
- Genomics in healthcare
- From genes to genome
- Taking and drawing a genetic family history
- From gene to protein
- Inheriting genomic information
- Talking genomics
- Dominant, recessive and beyond: how genetic conditions are inherited
- Investigating the genome part 1: the process
- Investigating the genome part 2: the tests
3. Online courses and expert webinars
- National Genomics Education Programme: The LinkAGE (Linking Academia and Genomics Education) webinar series
- St George’s University Hospital and St George’s, University of London: The genomics era: the future of genetics in medicine
- University of Exeter: Genomic medicine: transforming patient care in diabetes
4. GeNotes: Genomic notes for clinicians
- GeNotes: Genomic notes for clinicians has quick, concise information to help healthcare professionals make the right genomic decisions at each stage of a clinical pathway.
5. Master’s in genomic medicine framework
- Continued personal and professional development (CPPD) modules, a postgraduate certificate or diploma, or a full Master’s degree.
- NHS professionals can apply for course funding, which is currently available for standalone CPPD modules up to March 2024. Please refer to the Master’s in genomic medicine for further information.
The Centre for Postgraduate Pharmacy Education (CPPE) has an e-learning package that introduces pharmacogenomics and its application, and explores the opportunities it can bring to patient-centred care. Please note that this CPPE Programme is only available to General Pharmaceutical Council registered pharmacy professionals, which includes trainee pharmacists.
- Introduction to genomics in pharmacy: CPPE
- Familial hypercholesterolaemia: supporting people better – focal point
Appendix 3: Glossary
Clinical geneticist: a medically trained doctor who specialises in diagnosing and managing patients with genomic conditions through a combination of medical knowledge and a specialist understanding of molecular biology.
Deoxyribonucleic acid (DNA): the chemical that contains, or ‘encodes’, genetic information. DNA is made up of four different chemical bases.
Gene: a segment of DNA that contains the biological instructions for the production of a polypeptide chain, usually a specific protein or component of a protein.
Genomics: the study of the genomes of individuals and organisms that examines both the coding and non-coding regions. This term is also used when talking about related laboratory and bioinformatic techniques. The study of genomics in humans focuses on areas of the genome associated with health and disease.
Genomic medicine: genomic medicine (or healthcare) is the use of genomic information and technologies to determine disease risk and predisposition, diagnosis and prognosis, and the selection and prioritisation of therapeutic options.
Genomics informed medicines optimisation: genomic information can facilitate the selection of optimal treatments; this may be achieved through diagnostic tests (eg familial hypercholesterolemia), identifying specific disease pathways which then enables the use of targeted treatments (such as in cancer), or the repurposing of existing medicines (such as in some rare diseases where specific treatments do not currently exist). It may also involve the testing of specific genes to predict an individual’s response to a medication, in relation to likely effectiveness or adverse effects.
Histology independent treatment: a treatment that targets all solid tumours with a certain genomic mutation, regardless of where the primary tumour is in the body.
Inherited condition: a condition caused by a genetic variant that has been passed down from parent to child.
National genomic test directory: a directory that specifies which genomic tests are commissioned by the NHS in England, the technology by which they are available and the patients who will be eligible to access each test.
National Genomics Education Programme: exists to deliver and advise on learning and development opportunities that prepare current and future NHS professionals to make the best use of genomics in their practice.
NHS Genomic Laboratory Hub: responsible for co-ordinating genomic testing services, across one of seven regions in England.
NHS Genomic Medicine Service Alliance: Responsible for overseeing and co-ordinating the embedding of genomics into mainstream clinical care, across one of seven regions in England.
Personalised medicine: medical care targeted towards an individual or group of individuals, which uses knowledge of genetic, environmental and lifestyle factors to determine suitable methods of prevention, diagnosis and treatment of disease.
Pharmacogenomics: the use of genetic and genomic information to tailor pharmaceutical treatment to an individual.
Precision medicines: medicines targeted towards an individual or group of individuals, which uses knowledge of genetic, environmental and lifestyle factors to determine suitable methods of prevention, diagnosis and treatment of disease.
Rare and inherited disease: a disease that affects less than 1 in 2,000 of the general population (EU definition). In the UK, approximately 3.5 million people will be affected by a rare disease at some point in their life.
Sequencing: a technique used in laboratories to determine the order of bases in deoxyribonucleic acid (DNA) .
Solid tumour: a mass of tumours, which represent approximately 90% of adult human cancers.
Publications reference: PRN00558

