Chapter 1: Standard infection control precautions (SICPs)
Contents
1.1 Patient placement/assessment for infection risk
1.2 Hand hygiene
1.3 Respiratory and cough hygiene
1.4 Personal protective equipment (PPE)
1.5 Safe management of care equipment
1.6 Safe management of the care environment
1.7 Safe management of linen
1.8 Safe management of blood and body fluid spillages
1.9 Safe disposal of waste (including sharps)
1.10 Occupational safety: prevention of exposure (including sharps injuries)
Standard infection control precautions (SICPs) are to be used by all staff, in all care settings, at all times, for all patients whether infection is known to be present or not, to ensure the safety of those being cared for, staff and visitors in the care environment.
SICPs are the basic infection prevention and control measures necessary to reduce the risk of transmitting infectious agents from both recognised and unrecognised sources of infection.
Sources of (potential) infection include blood and other body fluids, secretions or excretions (excluding sweat), non-intact skin or mucous membranes and any equipment or items in the care environment that could have become contaminated.
The application of SICPs during care delivery is determined by assessing risk to and from individuals. This includes the task, level of interaction and/or the anticipated level of exposure to blood and/or other body fluids.
To protect effectively against infection risks, SICPs must be used consistently by all staff. SICPs implementation monitoring must also be ongoing to ensure compliance with safe practices and to demonstrate ongoing commitment to patient, staff and visitor safety as required by the Health and Safety Executive and the care regulators, the Care Quality Commission.
There are 10 elements of SICPs:
- patient placement/assessment of infection risk
- hand hygiene
- respiratory and cough hygiene
- personal protective equipment
- safe management of the care environment
- safe management of care equipment
- safe management of healthcare linen
- safe management of blood and body fluids
- safe disposal of waste (including sharps)
- occupational safety/managing prevention of exposure (including sharps)
1.1 Patient placement/assessment for infection risk
Patients must be promptly assessed for infection risk on arrival at the care area, eg inpatient/outpatient/care home, (if possible, prior to accepting a patient from another care area) and should be continuously reviewed throughout their stay.
This assessment should influence placement decisions in accordance with clinical/care need(s).
Patients who may present a cross-infection risk include those:
- with diarrhoea, vomiting, an unexplained rash, fever or respiratory symptoms
- known to have been previously positive with a multi-drug resistant organism (MDRO), eg MRSA, CPE
- who have been an inpatient in any hospital in the UK or abroad or are a known epidemiological link to a carrier of CPE.
The National Infection Prevention and Control Isolation Prioritisation Tool provides a systematic framework that can be used to assist in the prioritisation of isolation rooms as part of multidisciplinary assessment.
NB the Isolation Prioritisation tool MUST not be used for patients with a suspected High Consequence Infectious Disease (HCID).
Further information can be found in the patient placement literature review.
1.2 Hand hygiene
Hand hygiene is considered one of the most important ways to reduce the transmission of infectious agents that cause healthcare associated infections (HCAIs).
Clinical hand-wash basins must:
- be used for that purpose only and not used for the disposal of other liquids
- have mixer taps, no overflow or plug and be in a good state of repair
- have wall mounted liquid soap and paper towel dispensers.
Hand hygiene facilities should include instructional posters.
Before performing hand hygiene:
- expose forearms (bare below the elbow). If disposable over-sleeves are worn for religious reasons, these must be removed and disposed of before performing hand hygiene, then replaced with a new pair*
- remove all hand and wrist jewellery. The wearing of a single, plain metal finger ring, eg a wedding band, is permitted but should be removed (or moved up) during hand hygiene. A religious bangle can be worn but should be moved up the forearm during hand hygiene and secured during patient care activities
- ensure fingernails are clean and short, and do not wear artificial nails or nail products
- cover all cuts or abrasions with a waterproof dressing.
*refer to NHS England uniforms and workwear guidance (Appendix B) for more information on the use of over-sleeves and longer-sleeved uniforms.
To perform hand hygiene:
Wash hands with non-antimicrobial liquid soap and water if:
- hands are visibly soiled or dirty
- caring for patients with vomiting or diarrhoeal illnesses
- caring for a patient with a suspected or known gastrointestinal infection, eg norovirus or a spore-forming organism such as clostridioides difficile.
In all other circumstances, use alcohol-based handrubs (ABHRs) for routine hand hygiene during care.
ABHRs must be available for staff as near to the point of care as possible. Where this is not practical, personal ABHR dispensers should be used, eg within the community, domiciliary care, mental health units etc. In settings where personal ABHR dispensers are deemed unsuitable due to staff safety concerns, organisations can consider alternative products and are responsible for ensuring safe systems of work, including the completion of a documented risk assessment approved through local governance procedures. Organisations must confirm the efficacy and suitability of the product (i.e., that it conforms with the relevant standards and is appropriate for the intended use) with the product manufacturer. Any differences in use and application, including volume, contact and disinfection time, of an alternative product compared with ABHR should be identified as part of this assessment and appropriate implementation plans should include education and supporting materials for staff.
Where running water is unavailable, or hand hygiene facilities are lacking, staff may use hand wipes followed by ABHR and should wash their hands at the first opportunity.
Perform hand hygiene:
- before touching a patient.
- before clean or aseptic procedures.
- after body fluid exposure risk
- after touching a patient; and
- after touching a patient’s immediate surroundings.
Always perform hand hygiene before putting on and after removing gloves.
For how to wash hands, see the step-by-step guide in appendix 1 of this document.
For how to hand rub, see the step-by-step guide in appendix 2 of this document.
Skin care
- dry hands thoroughly after hand washing, using disposable paper towels
- use an emollient hand cream regularly eg during breaks and when off duty
- do not use or provide communal tubs of hand cream in the care setting
- staff with skin problems should seek advice from occupational health or their GP and depending on their skin condition and the severity may require additional interventions or reporting.
Surgical hand antisepsis
Surgical scrubbing/rubbing (this applies to those undertaking surgical and some invasive procedures):
- perform surgical scrubbing/rubbing before donning sterile theatre garments or at other times, eg before inserting central vascular access devices
- remove all hand and wrist jewellery (including wedding band)
- nail brushes should not be used for surgical hand antisepsis
- nail picks (single-use) can be used if nails are visibly dirty
- soft, non-abrasive, sterile (single-use) sponges may be used to apply antimicrobial liquid soap to the skin if licensed for this purpose
- use an antimicrobial liquid soap licensed for surgical scrubbing or an ABHR licensed for surgical rubbing (as specified on the product label)
- ABHR can be used between surgical procedures if licensed for this use or between glove changes if hands are not visibly soiled.
For surgical scrubbing (not rubbing), follow the step-by-step guide in appendix 3 of this document.
For surgical rubbing (not scrubbing), follow the step-by-step guide in appendix 4 of this document.
For hand hygiene posters/leaflet, refer to the resources section of NIPCM.
Further information in the hand hygiene literature reviews:
- Hand washing, hand rubbing and indications for hand hygiene
- Hand hygiene products
- Skin care
- Surgical hand antisepsis in the clinical setting
1.3 Respiratory and cough hygiene
Respiratory and cough hygiene is designed to minimise the risk of cross transmission of known or suspected respiratory illness (pathogens):
- cover the nose and mouth with a disposable tissue when sneezing, coughing, wiping and blowing the nose; if unavailable use the crook of the arm
- dispose of all used tissues promptly into a waste bin
- wash hands with non-antimicrobial liquid soap and warm water after coughing, sneezing, using tissues, or after contact with respiratory secretions or objects contaminated by these secretions
- where there is no running water available or hand hygiene facilities are lacking, staff may use hand wipes followed by ABHR and should wash their hands at the first available opportunity
- keep contaminated hands away from the eyes nose and mouth.
Staff should promote respiratory and cough hygiene helping those (eg, elderly, children) who need assistance with this, eg providing patients with tissues, a dedicated receptacle i.e. waste bag for used tissues and hand hygiene facilities as necessary.
Further information can be found in cough etiquette/respiratory hygiene in the hospital setting literature review.
1.4 Personal protective equipment (PPE)
Before undertaking any procedure, staff should assess any likely exposure to blood and/or other body fluids, non-intact skin or mucous membranes and wear personal protective equipment (PPE) that protects adequately against the risks associated with the procedure. The principles of PPE use set out below are important to ensure that PPE is used correctly to ensure patient and staff safety. Avoiding overuse or inappropriate use of PPE is a key principle that ensures this is risk-based and minimizes its environmental impact. Where appropriate, consideration should be given to the environmental impact of sustainable or reusable PPE options versus single-use PPE while adhering to the principles below.
All PPE must be:
- located close to the point of use. PPE for healthcare professionals providing care in the community and domiciliary care providers must be transported in a clean receptacle
- stored to prevent contamination in a clean, dry area until required (expiry dates must be adhered to)
- single-use only unless specified by the manufacturer
- changed immediately after each patient and/or after completing a procedure or task
- disposed of after use into the correct waste stream, eg domestic waste, offensive (non-infectious) or clinical waste
- discarded if damaged or contaminated.
NB Reusable PPE such as goggles/face shields/visors, must be decontaminated after each use according to manufacturer’s instruction.
Gloves must be:
- worn when exposure to blood and/or other body fluids, non-intact skin or mucous membranes is anticipated or likely
- changed immediately after each patient and/or after completing a procedure/task even on the same patient, and hand hygiene performed
- changed if a perforation or puncture is suspected
- appropriate for use, fit for purpose and well-fitting
- never decontaminated with ABHR or soap between use
- low risk of causing sensitisation to the wearer
- appropriate for the tasks being undertaken, taking into account the substances being handled, type and duration of contact, size and comfort of the gloves, and the task and requirement for glove robustness and sensitivity.
Sterile gloves must be worn:
- when sterility is required in an operating theatre, and
- for some aseptic techniques eg insertion of central venous catheters, insertion of peripherally inserted central catheters, insertion of pulmonary artery catheters and spinal, epidural and caudal procedures
NB Double gloving is NOT recommended for routine clinical care. However, it may be required for some exposure prone procedures, eg orthopaedic and gynaecological operations, when attending major trauma incidents or as part of additional precautions for high consequence infectious disease management.
Further information can be found in the gloves literature review.
If worn, oversleeves must be:
- changed immediately after each patient and/or after completing a procedure/task even on the same patient, and hand hygiene performed
- removed and disposed of if visibly contaminated or soiled.
Aprons must be:
- worn to protect uniform or clothes when contamination is anticipated or likely
- changed between patients and/or after completing a procedure or task.
Full body gowns or fluid-resistant coveralls must be:
- worn when there is a risk of extensive splashing of blood and/or body fluids, eg operating theatre, ITU
- worn when a disposable apron provides inadequate cover for the procedure or task being performed
- changed between patients and removed immediately after completing a procedure or task
- sterile when sterility is required in an operating theatre and for some aseptic techniques eg for insertion of central venous catheters, insertion of peripherally inserted central catheters, insertion of pulmonary artery catheters and spinal, epidural and caudal procedures.
Further information can be found in the aprons/gowns literature review.
Eye or face protection (including full-face visors) must:
- be worn if blood and/or body fluid contamination to the eyes or face is anticipated or likely, eg by members of the surgical theatre team and always during aerosol generating procedures; regular corrective spectacles are not considered eye protection
- not be impeded by accessories such as piercings or false eyelashes
- not be touched when being worn.
Further information can be found in the eye/face protection literature review.
Fluid resistant surgical face masks (FRSM):
Surgical face masks are required:
- as a means of source control, eg to protect the patient from the wearer during sterile procedures such as surgery, and
- to protect the wearer when there is a risk splashing or spraying of blood, body fluids, secretions or excretions onto the respiratory mucosa.
- as an element of PPE for droplet precautions (see section 2.4 and appendices 5b and 6).
FRSM must be:
- worn (with eye protection) if a full-face visor is not available and spraying or splashing of blood, body fluids, secretions or excretions onto the respiratory mucosa (nose and mouth) is anticipated or likely (Type IIR)
- worn to protect patients from the operator as a source of infection, eg when performing surgical procedures or epidurals or inserting a central vascular catheter (CVC) (Type II (not classed as an FRSM) or Type IIR)
- well-fitting and fit for purpose, fully covering the mouth and nose (manufacturers’ instructions must be followed to ensure effective fit and protection)
- removed or changed:
‒ at the end of a procedure/task
‒ if the mask’s integrity is breached, eg from moisture build-up after extended use or from gross contamination with blood or body fluids
‒ in accordance with manufacturers’ specific instructions.
Further information can be found in the surgical face masks literature review.
Footwear must be:
- visibly clean, non-slip and well-maintained, and support and cover the entire foot to avoid contamination with blood or other body fluids or potential injury from sharps
- removed before leaving a care area where dedicated footwear is used, eg theatre; these areas must have a decontamination schedule with responsibility assigned.
Further information can be found in the footwear literature review.
Headwear
Headwear is not routinely required in clinical areas unless part of theatre attire or to prevent contamination of the environment such as in clean rooms.
Headwear must be:
- worn in theatre settings and clean rooms, eg central decontamination unit
- well-fitting and completely cover the hair
- changed or disposed of between clinical procedures/lists or tasks and if contaminated with blood and/or body fluids
- removed before leaving the theatre or clean room
- individuals with facial hair must also cover this in areas where headwear is required, eg wear a snood.
NB Headwear worn for religious reasons such as turbans, kippot veils, headscarves must not compromise patient care and safety. These must be washed and/or changed daily or immediately if contaminated and comply with additional attire requirements, for example, in theatres.
Further information can be found in the headwear literature review.
For the recommended method of putting on and removing PPE, refer to appendix 6.
1.5 Safe management of care equipment
Care equipment is easily contaminated with blood, other body fluids, secretions, excretions and infectious agents. Consequently, it is easy to transfer infectious agents from communal care equipment during care delivery.
Care equipment is classified as either:
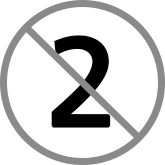 single use: equipment which is used once on a single patient then discarded. This equipment must never be re-used. The packaging will carry the symbol of the number two in a circle with a diagonal cross
single use: equipment which is used once on a single patient then discarded. This equipment must never be re-used. The packaging will carry the symbol of the number two in a circle with a diagonal cross- single patient use: equipment which can be reused on the same patient and may require decontamination in-between use such as nebuliser masks
- reusable invasive equipment: used once then decontaminated, eg surgical instruments and solid state reusable equipment, such as, flexible endoscopes and transducers
- reusable non-invasive equipment: (often referred to as communal equipment) – reused on more than one patient following decontamination between each use, eg commode, patient transfer trolley.
NB Needles and syringes are single use devices, they should never be used more than once or reused to draw up additional medication. Never administer medications from a single-dose vial or intravenous (IV) bag to multiple patients.
Before using any sterile equipment check that:
- the packaging is intact
- there are no obvious signs of packaging contamination
- the expiry date remains valid
- any sterility indicators are consistent with the process being completed successfully.
Decontamination of reusable non-invasive care equipment must be undertaken:
- between each use/between patients
- after blood and/or body fluid contamination
- at regular predefined intervals as part of an equipment cleaning protocol
- before inspection, servicing or repair.
If providing domiciliary care, equipment should be transported safely and decontaminated as above before leaving the patient’s home.
Always adhere to Control of Substances Hazardous to Health (COSHH) risk assessments and manufacturers’ guidance for use and decontamination of all care equipment.
- all reusable non-invasive care equipment must be decontaminated between patients/clients using either approved detergent wipes or
detergent solution, in line with manufacturers’ instructions, before being stored clean and dry. - decontamination protocols must include responsibility for; frequency of; and method of environmental decontamination
- an equipment decontamination status certificate will be required if any item of equipment is being sent to a third party, eg for inspection, servicing or repair
- guidance should be sought from the infection, prevention and control team prior to procuring, trialling or lending any reusable non-invasive equipment
- medical devices and other care equipment must have evidence of planned preventative maintenance programmes.
For how to decontaminate reusable non-invasive care equipment see Appendix 7.
For decontamination of surgical instruments see HTM01-01 decontamination of surgical instruments.
Further information can be found in the management of patient care equipment literature review.
1.6 Safe management of the care environment
The care environment must be:
- visibly clean, free from non-essential items and equipment to facilitate effective cleaning
- well maintained, in a good state of repair and with adequate ventilation for the clinical specialty.
Always adhere to COSHH risk assessments for product use and processes for decontamination of the care environment.
Routine cleaning
- the environment should be routinely cleaned in accordance with the National Cleaning Standards
- use of detergent wipes is acceptable for cleaning surfaces/frequently touched sites within the care area
- a fresh solution of general-purpose neutral detergent in warm water is recommended for routine cleaning. This should be changed when dirty or when changing tasks
- routine disinfection of the environment is not recommended however, 1,000ppm available chlorine should be used routinely on sanitary fittings
- staff groups should be aware of their environmental cleaning schedules for their area and clear on their specific responsibilities
- cleaning protocols should include responsibility for, frequency of, and method of environmental decontamination.
Further information can be found in the safe management of the care environment literature review.
1.7 Safe management of linen
Healthcare laundry must be managed and segregated in accordance with HTM 01-04 . Healthcare linen is categorised as:
- Clean linen – linen washed and ready to be used.
- Used (soiled and fouled) linen – used linen, irrespective of state, which on occasion may be contaminated by blood or body fluids, and
- Infectious linen – linen that has been used by a patient who is known or suspected to be infectious.
Storage and handling of clean linen:
- Hand hygiene should be performed prior to handling clean linen.
- Clean linen should be removed from plastic bags before storage to prevent the growth of Bacillus cereus.
- Clean linen should be stored above floor level in a designated area, preferably an enclosed cupboard that is clean, dry and cool.
- If clean linen is not stored in a cupboard, then the trolley used for storage must be designated for this purpose and completely covered with an impervious covering/or door that is able to withstand decontamination.
- Clean linen storage areas should be dedicated for the purpose and appropriately designed to prevent damage to linen and to allow for the rotation of stocks.
- Clean linen should be physically separated from used/infectious linen when in storage and during transport.
Storage and handling of used (previously known as soiled/fouled linen) and infectious linen:
- Staff handling used and/or infectious linen must wear appropriate PPE (see section 1.4).
- Hand hygiene must be performed after handling used and/or infectious linen.
- Ensure a laundry receptacle is available as close as possible to the point of use for immediate linen deposit.
- Used items of linen should be removed one by one and placed in the used linen hamper/stream.
- do not:
- rinse, shake or sort linen on removal from beds/trolleys
- place used linen on the floor or any other surfaces eg a locker/table top
- re-handle used linen once bagged
- overfill laundry receptacles (not more than 2/3 full); or
- place inappropriate items in the laundry receptacle eg used equipment/needles
- Infectious linen must not be sorted but should be rolled together and sealed in a water-soluble bag (entirely water soluble ‘alginate’ bag or impermeable bag with soluble seams), which is then placed in an impermeable bag immediately on removal from the bed and secured before leaving a clinical area.
- Linen should be placed in an impermeable bag immediately on removal from the bed or before leaving a clinical department
- Linen bags/receptacles must be tagged (eg, hospital ward/care area) and dated
- Store all used/infectious linen in a designated, safe, lockable area while awaiting collection. Collection schedules must be acceptable to the care area and there should be no build-up of linen receptacles
- All linen that is deemed unfit for re-use, eg, torn or heavily contaminated, should be categorised at the point of use and returned to the laundry for assessment and disposal.
Linen used during patient transfer, eg, blankets, should be categorised at the point of destination.
Linen from patients infected with, or at high risk of having, Hazard Group 4 organisms (haemorrhagic fever viruses such as Lassa Fever) should be disposed of at the point of use as Category A waste and must not be returned to a laundry.
Further information can be found in the safe management of linen literature review.
For how to manage linen at care area level see Appendix 8.
1.8 Safe management of blood and body fluid spillages
Spillages of blood and other body fluids may transmit blood borne viruses.
Spillages must be treated immediately by staff trained to undertake this safely.
Responsibilities for the management of blood/body fluid spills must be clear within each area/care setting.
For management of blood and body fluid spillages see Appendix 9.
If an organisation locally approves a product for use in the management of blood and body fluid spills, the organisation is responsible for ensuring safe systems of work, including the completion of a documented risk assessment approved through local governance procedures. Organisations must confirm the efficacy and suitability of the product (i.e., that it conforms with the relevant standards and is appropriate for the intended use) with the product manufacturer.
A locally approved product which conforms to: EN17126, EN13727, EN14348, EN14476, EN13697, EN14885, EN13704, EN1650, EN1276 and EN13624 may be used for the management of blood and body fluid spills.
Further information can be found in the management of blood and body fluid literature review.
Healthcare providers should ensure that any polymer gel for non-patient use (eg spill kits, controlled drug destruction, use by cleaning staff) is kept secure and away from patients. See National Patient Safety Alert – National Patient Safety Alert – Superabsorbent polymer gel granules (2019) NatPSA/2019/002/NHSPS.
1.9 Safe disposal of waste (including sharps)
Health Technical Memorandum (HTM 07-01) contains the regulatory waste management guidance for all health and care settings (NHS and non-NHS) in England and Wales including waste classification, segregation, storage, packaging, transport, treatment and disposal.
Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 outline the regulatory requirements for employers and contractors in the healthcare sector in relation to the safe disposal of sharps.
Definitions
Healthcare (including clinical) waste:
Clinical waste means waste from a healthcare activity (including veterinary healthcare) that:
- contains viable micro-organisms or their toxins which are known or reliably believed to cause disease in humans or other living organisms. For example, if a patient is known or suspected to be infected, or colonised, by an infectious agent. Clinical judgement should be applied in the assessment of waste and should consider the infection status of a patient and the item of waste produced.
- contains or is contaminated with a medicine that contains a biologically active pharmaceutical agent, or
- is a sharp, or a body fluid or other biological material (including human and animal tissue) containing or contaminated with a dangerous substance within the meaning of Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures, as amended from time to time.
Offensive waste is waste that:
- is not clinical waste,
- is not infectious, but may contains body fluids, secretions or excretions,
- is non-hazardous, and
- falls within waste codes 18 01 04 if from healthcare, or 20 01 99 if from municipal sources.
Table 1: Categories of waste and segregation at source
| Category | Segregation | Treatment/disposal |
| Offensive (non-infectious) | Yellow bag with black stripe (tiger) bag | Energy from waste, landfill or other permitted processes |
| Clinical waste (infectious only) | UN approved orange bag, UN approved box or sharps container | For alternative treatment |
| Healthcare waste contaminated with non-hazardous pharmaceuticals or chemicals) | UN approved yellow bag, UN approved box or sharps container | For incineration or other permitted process |
| Waste contaminated with cytotoxic or cytostatic medication | UN approved purple bag, UN approved box or sharps container | For incineration |
| Non-hazardous pharmaceuticals (no sharps) | Blue box/container | For incineration or other permitted process |
| Anatomical waste/full blood bag and blood preserves | UN approved red lidded container | For incineration only |
| Domestic | Black/clear bags | Energy from waste, recovery or landfill |
| Recycling | Clear, green or other colour bag | Recycling |
Safe waste disposal at care area level:
Always dispose of waste:
- immediately and as close to the point of use as possible; and
- into the correct segregated colour coded rigid container or sharps box if a sharp
- Liquid waste, eg, suction canisters, must be rendered safe by adding a polymer gel or compound to the container prior to placing in an orange lidded leak proof bin or yellow lidded leak proof bin if contaminated by pharmaceuticals.
- waste bags must be no more than 2/3 full and no more than the UN approved weight and must be securely tied using a plastic tie or secure knot using a ‘swan neck’ to close. Waste must be traceable back to ward/care area or department, this may be achieved by writing on bags (prior to use), attaching sticky labels or uniquely numbered tags with the post code on them.
- store all waste in a designated, safe, lockable area while awaiting collection. Collection schedules must be acceptable to the care area and there should be no build-up of waste receptacles.
- Local guidance on management of waste at care level, eg, domiciliary settings should be followed.
Sharps containers (for safety devices, refer to section 1.10)
Sharps containers must:
- have a handle (small community boxes do not require a handle) and temporary closure mechanism, employed when box is not in use
- be disposed of when the manufacturers’ fill line is reached
- be labelled with point of origin and date of assembly and disposal. Where re-usable sharps containers are used, organisations must have a protocol in place to assure themselves of safe use and reprocessing.
Further information can be found in the Health Technical Memorandum (HTM 07-01).
1.10 Occupational safety: prevention of exposure (including sharps injuries)
The Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 outline the regulatory requirements for employers and contractors in the healthcare sector in relation to: arrangements for the safe use and disposal of sharps; provision of information and training to employees; investigations and actions required in response to work related sharps injuries.
There is a potential risk of transmission of a BBV (blood bourne virus) from a significant occupational exposure and staff must understand the actions they should take when a significant occupational exposure incident takes place. There is a legal requirement to report all sharps injuries and near misses to line managers/employers.
A significant occupational exposure is:
- a percutaneous injury eg injuries from needles, instruments, bone fragments, or bites which break the skin; and/or
- exposure of broken skin (abrasions, cuts, eczema, etc); and/or
- exposure of mucous membranes including the eye from splashing of blood or other high risk body fluids.
For the management of an occupational exposure incident see Appendix 10.
Safety devices
Health and Safety (Sharp Instruments in Healthcare) Regulations 2013 are concerned with reducing and eliminating the number of ‘sharps’ related injuries which occur within healthcare. Its basic guidance is:
- avoid unnecessary use of sharps
- if use of medical sharps cannot be avoided, source and use a ‘safer sharp’ device;
- if a safer sharp device is not available then safe procedures for working with and disposal must be in place eg sticky mats, sharps bins, safety procedures and training.
Sharps handling must be assessed, kept to a minimum and eliminated, if possible, with the use of approved safety devices.
- manufacturers’ instructions for safe use and disposal must be followed
- needles must not be re-sheathed/recapped or disassembled after use
- sharps must not be passed directly hand to hand
- used sharps must be discarded at the point of use by the person generating the waste
- always dispose of needles and syringes as 1 unit
- if a safety device is being used safety mechanisms must be deployed before disposal.
When transporting sharps boxes for community use these must be transported safely with the use of temporary closures.
Further information can be found in occupational exposure management (incl. sharps) literature review.
| << Introduction | Chapter 2 >> |
