The saving babies’ lives care bundle v3.2 – summary of changes
- updated implementation guidance to reflect the Clinical Negligence Scheme for Trusts (CNST) Maternity Incentive Scheme Safety Action 6 (Implementing version 3 of the Saving Babies’ Lives Care Bundle)
- revised sections on equity and midwifery continuity of carer (Principles to be considered alongside implementing version 3)
- changed wording of interventions 2.4, 2.10 and 2.12; removed outcome measure 2e (Element 2)
- clarified which blood pressure monitors meet NHS standards (Element 2)
- updated intervention 3.3 and outcome measures 3a and 3c (Element 3)
- revised interventions 4.3, 4.4 and 4.5 (Element 4)
- updated interventions 5.9 and 5.11; removed intervention 5.14 and measures 5d and 5h (Element 5)
- renumbered and revised interventions 5.18 to 5.26 and measures 5b and 5h (Element 5)
- added note on fetal fibronectin test supply issues (Element 5 – Implementation)
- updated interventions 6.2, 6.3 6.5 and 6.12 to align with Hybrid Closed Loop strategy (Element 6)
- revised measures 6b, 6d and 6f; added explanatory sections on Hybrid Closed Loop (Element 6)
- removed original Appendix B and replaced with signposting to public health guidance (Principles to be considered alongside implementing version 3)
- updated Appendix C Figure 1: Algorithm for using uterine artery Doppler as a screening tool for risk of early onset FGR (Appendix C)
- updated Appendix B Table 1: Clinical risk assessment for pre-eclampsia as indications for aspirin in pregnancy (Appendix B)
Summary
The Saving Babies’ Lives Care Bundle (SBLCB) provides evidence-based best practice for providers and commissioners of maternity care across England, to reduce perinatal mortality.
The NHS has worked hard towards meeting the national maternity safety ambition, to halve rates of perinatal mortality from 2010 to 2025 and achieve a 20% reduction by 2020. Office for National Statistics (ONS) data showed a 25% reduction in stillbirths in 2020, but against the same baseline only 20% in 2021 during the COVID-19 pandemic. Much has been achieved in the past few years, but more recent data shows there is more to do to achieve the ambition in 2025.
Version 3 of the Saving Babies’ Lives Care Bundle (SBLCBv3) has been co-developed with clinical experts including frontline clinicians, Royal Colleges and professional societies; service users and maternity voices partnerships; and national organisations including charities, the Department of Health and Social Care (DHSC) and a number of arm’s length bodies (see Appendix A: Acknowledgements).
Building on the achievements of previous iterations, version 3 refreshes all existing elements, drawing on national guidance, such as that from the National Institute for Health and Care Excellence (NICE) and the Royal College of Obstetricians and Gynaecologists (RCOG) Green Top guidelines, and frontline learning to reduce unwarranted variation where the evidence is insufficient for NICE and RCOG to provide guidance. It adds a new element on the management of pre-existing diabetes in pregnancy, based on data from the National Pregnancy in Diabetes (NPID) Audit.
This means there are now 6 elements of care:
Element 1 focuses on reducing smoking in pregnancy by implementing NHS-funded tobacco dependence treatment services within maternity settings, in line with the NHS Long Term Plan and NICE guidance. This includes carbon monoxide testing and asking women about their smoking status at the antenatal booking appointment and, as appropriate, throughout pregnancy. Women who smoke should receive an opt-out referral for in-house support from a trained tobacco dependence adviser who will offer a personalised care plan and support throughout pregnancy.
Element 2 covers fetal growth: risk assessment, surveillance and management. It builds on the widespread adoption of mid-trimester uterine artery Doppler screening for early-onset fetal growth restriction (FGR) and placental dysfunction. Element 2 seeks to further improve FGR risk assessment by mandating the use of digital blood pressure measurement. It recommends a more nuanced approach to late FGR management to improve the assessment and care of mothers at risk of FGR and lower rates of iatrogenic late preterm birth.
Element 3 is focused on raising awareness of reduced fetal movement (RFM). It encourages increasing awareness among pregnant women of the importance of reporting RFM and seeks to ensure providers have protocols based on best available evidence in place to manage care for women who report RFM. Induction of labour before 39 weeks’ gestation is only recommended where there is evidence of fetal compromise or other concerns in addition to a history of RFM.
Element 4 promotes effective fetal monitoring during labour through ensuring all staff responsible for monitoring the fetus are competent in using the techniques of intermittent auscultation and/or CTG in relation to the clinical situation, use the buddy system and escalate accordingly when concerns arise or risks develop. This includes staff who are brought in from other clinical areas to support a busy service, as well as locum, agency and bank staff.
Element 5 on reducing preterm birth recommends 3 intervention areas to reduce adverse fetal and neonatal outcomes: improving the prediction and prevention of preterm birth and optimising perinatal care when preterm birth cannot be prevented. All providers are encouraged to draw on the learning from the British Association of Perinatal Medicine toolkits and the wide range of resources from other successful regional programmes (for example, PERIPrem resources, MCQIC).
Element 6 covers the management of pre-existing diabetes for women with Type 1 or Type 2 diabetes, as the most significant modifiable risk factor for poor pregnancy outcomes. This element recommends multidisciplinary team pathways and an intensified focus on glucose management within maternity settings, in line with the NHS Long Term Plan and NICE guidance. It includes clear documentation of assessing glucose control digitally, using HbA1c to risk stratify and provide additional support / surveillance (National Diabetes Audit data), and consistently offering access to evidence-based continuous glucose monitoring and pregnancy-specific hybrid closed loop technology to improve glucose control.
In addition to the provision of safe and personalised care, achieving equity and reducing health inequalities is a key aim for all maternity and neonatal services and is essential to achieving the national maternity safety ambition. In developing each element in SBLCBv3, actions to improve equity have been considered, including for babies from Black, Asian and mixed ethnic groups and for those born to mothers living in the most deprived areas, in accordance with the NHS equity and equality guidance.
As part of the Three year delivery plan for maternity and neonatal services, NHS trusts have been responsible for implementing SBLCBv3 by March 2024 and integrated care boards for agreeing a local improvement trajectory with providers, along with overseeing, supporting and challenging local delivery.
SBLCBv3 also sets out the important wider principles to consider during implementation. These reflect best practice care and following them in conjunction with the 6 elements is recommended, but are not mandated by the SBLCB.
Forewords
ONS data suggests that because of the improvement in the perinatal mortality rate since 2010, at least 900 more families will return home with a healthy baby. That is a great achievement, and all those who work in maternity and neonatal services should be incredibly proud of our progress towards the national maternity safety ambition.
The recent rise in the perinatal mortality rate is likely to relate to the direct and indirect effects of the COVID-19 pandemic and is a stark reminder that there will always be challenges to reducing stillbirths and neonatal deaths. The trajectory to meet the national maternity safety ambition was unlikely to be a simple linear progression, particularly as the factors that lead to avoidable perinatal mortality are many and varied. We should all acknowledge that while we have reduced avoidable deaths, there is more to be done.
If we are to meet the national ambition for a 50% reduction in the stillbirth and neonatal mortality rates by 2025, we need to address longstanding inequitable outcomes associated with ethnicity and levels of deprivation. While it is clear that some solutions lie beyond the control of the health sector, our services must do everything possible to mitigate against the wider social determinants of health to continue to drive down the perinatal mortality rate.
The need to continuously iterate and improve care is why we have developed version 3 of the Saving Babies’ Lives Care Bundle at pace. Clinical experts, professional bodies, charities, service users and national regulators have collaborated to develop national best practice. However, it is important to remember that the care bundle is just one of a series of interventions to help reduce perinatal mortality and preterm birth and shouldn’t be implemented in isolation. The Three year delivery plan for maternity and neonatal services describes more broadly how providers should continue to implement best practice care wherever possible and a set of wider principles are included in the care bundle.
Despite the recent set back in perinatal mortality rates the stillbirth rate was still 19% lower in 2021 than in 2010 and the neonatal mortality rate 30% lower. Thank you to all those who work tirelessly to drive improvement in our maternity services, whether they be NHS employees, parents or charities. I am confident that the collaborative approach modelled by the Saving Babies’ Lives Care Bundle will continue to deliver improvements in outcomes and reduce the number of parents who have to face the tragedy of perinatal bereavement.
Donald Peebles, National Clinical Director for Maternity, NHS England
On behalf of the Royal College of Midwives (RCM), I welcome the publication of version 3 of the Saving Babies’ Lives Care Bundle. We continue to support the ambition to achieve a 50% reduction in stillbirths and maternal and neonatal deaths by 2025. The care bundle to date has made a vital contribution to achieving this.
The RCM knows that the relationships that professionals form in the workplace, in their teams and with women, are key to safety and preventing the avoidable tragedies of stillbirth and the death of babies. We are therefore pleased to see continued emphasis on professionals working together and with women to help them make choices about their care and reduce the risks to their baby.
Gill Walton, Chief Executive, Royal College of Midwives
As Saving Babies’ Lives Care Bundle (SBLCBv3) enters its 3rd edition and its 7th year, it continues to innovate and drive forward quality improvement in key areas of maternity care. We welcome the addition of an element covering diabetes in pregnancy and the continued development of the other successful 5 elements. This version builds on versions 1 and 2, to focus on supporting those caring for pregnant women and to help support women to make choices about their care and reduce unnecessary intervention.
Whenever a new guideline is introduced, it will always have limitations and there will be compromises to be made, influenced by lack of current evidence and resource requirements to support successful implementation. However, the premise of the bundle is to reduce variation and provide a framework for continuous improvement. This will be supported by ongoing learning from evaluation of the bundle and is key to its success and value.
The Saving Babies’ Lives Care Bundle is part of a number of initiatives to improve maternity care and safety. However, there are areas that we urgently need to address if we are to ensure a continued reduction in perinatal mortality for all women and babies. We must, therefore, harness the expertise and experience of obstetricians and specialists in fetal and maternal medicine, frontline maternity teams, academics and policy-makers to tackle inequality and the social determinants of health in the pregnant population.
The British Maternal and Fetal Medicine Society is honoured to have worked closely on all 3 versions of SBLCB and fully supports the initiatives within this new version and the opportunity to work to deliver improvements in maternity care.
Kate Morris, President, British Maternal and Fetal Medicine Society
Every day, maternity services support thousands of women and their families through pregnancy and childbirth. The majority of those using maternity services have good outcomes and report a positive experience of care but maternity care is complex and, unfortunately, adverse events occur.
Recent independent inquiries into maternity care have emphasised the importance of continued learning and action on improving safety. The Royal College of Obstetricians and Gynaecologists warmly welcomes the publication of version 3 of the Saving Babies’ Lives Care Bundle, which will support further progress towards a 50% reduction in the rate of stillbirths, neonatal mortality and serious brain injury and a reduction of preterm births from 8% to 6% in the UK by 2025, as set in the NHS Long Term Plan.
Maternity care is delivered through multiprofessional teams working together to support all women, requiring a wide range of skills, knowledge and expertise, and a supportive context in which these can be applied. By implementing the evidence-based, best practice elements of the care bundle, local maternity teams can ensure women receive personalised care that will continue to reduce perinatal mortality.
Importantly, each element of the care bundle includes action to improve equity, including for babies from Black, Asian and mixed ethnic groups and for those born to mothers living in the most deprived areas. Maternity systems must continue work to embed these into their local action plans.
The care bundle aligns with and complements a range of other important maternity safety initiatives and tools, including wider work being taken forward through the Maternity Transformation Programme as well as initiatives such as the Avoiding Brain Injury in Childbirth (ABC) programme.
The Royal College of Obstetricians and Gynaecologists will continue to work with partners, including other Royal Colleges, national policy-makers and safety leaders, to support the NHS to implement these together, to improve the quality and safety of care that women and babies receive in the UK.
Dr Ranee Thaker, President, Royal College of Obstetricians and Gynaecologists
Having achieved the first national maternity safety ambition milestone of reducing by 20% the perinatal mortality rate by 2020 the focus is now on achieving the further 30% reduction by 2025. This will require accelerated progress in the face of having probably dealt with the ‘easier’ problems to prevent and manage.
Activities on multiple fronts are going to be required, which is why, among other actions, the full implementation in all trusts of the Saving Babies’ Lives Care Bundle is needed. This, the 3rd version of the care bundle, is a welcome reminder of the 5 elements from version 2 and the introduction of a 6th new element to improve diabetic management in pregnancy for women with Type 1 and Type 2 diabetes.
As demonstrated in the National Diabetes in Pregnancy Audit, monitoring and managing tight glycaemic control from pre-pregnancy and throughout pregnancy is key to reducing the risks of adverse outcomes including congenital anomalies and perinatal death. The 2022 MBRRACE-UK Maternal Confidential Enquiry illustrated the risks to both diabetic pregnant women and their babies of poorly managed diabetic ketoacidosis.
The steps outlined in Element 6 of the new version of the care bundle provide practical advice for service delivery to support improved management for this high-risk group of mothers and babies. Achieving the improvements that could be realised from the full implementation of all 6 elements of the new version will provide some of the essential pieces of the jigsaw of activities still needed to further reduce the national rate of perinatal deaths.
Professor Jenny Kurinczuk, Professor of Perinatal Epidemiology, Director, National Perinatal Epidemiology Unit, National Programme Lead MBRRACE-UK/PMRT, University of Oxford
Despite falls in perinatal mortality in recent years, too many parents and families are still devastated by the death of their baby. The exact impact of COVID-19 is as yet unclear but it’s very possible that the pandemic has had a significant negative impact not just on women and pregnant people’s experiences of maternity, but crucially on outcomes for both them and their babies. Importantly, the government is unlikely to meet the national maternity safety ambition to halve stillbirths and neonatal baby deaths by 2025.
Coming in the wake of further investigations into poor care such as the Ockenden and East Kent reports, this 3rd version of the Saving Babies’ Lives Care Bundle has urgency towards ensuring better, safer care. This new version maintains the focus of version 2 but adds another crucially important element around caring for women with Type 1 and Type 2 diabetes who we know to be at 4–5 times increased risk of losing their baby. This version also encourages awareness among those who are pregnant of the importance of early warning signals that something may be wrong, such as noticing and reporting reduced fetal movement.
An innovation in this version is an assurance tool to help trusts track their progress in implementation, thereby removing the need to have regular implementation surveys.
Listening to bereaved parents’ experiences is vital in understanding why babies die, and learning from every baby’s death is an essential part of the continual improvement that underpins this care bundle. Parents tell us that if lessons can be learned from the death of their baby it can help them live with their grief, providing an important and lasting legacy.
This updated, 3rd version of the care bundle carries essential knowledge for every healthcare professional who supports and works with those who are pregnant. It helps address inequalities with the same emphasis of continuity of carer, especially for those from Black and minority ethnic backgrounds and those living in areas of social deprivation. When the worst happens, it ensures standards in bereavement care, in line with the National Bereavement Care Pathway.
We welcome its implementation and believe that it provides an opportunity to protect babies’ lives in the future.
Clea Harmer, Chief Executive, Sands
Tommy’s work is dedicated to reducing rates of pregnancy complications and baby loss as we know the heartbreak and devastation this causes far too many parents and families. Despite ambitious targets, the rates of stillbirth and preterm birth are not falling as quickly as we would have hoped, and indeed the stillbirth rates sadly rose in 2021. It is also clear that variation in care continues, and not all women and birthing people have the same chance of taking home a healthy baby – the outcome every family deserves.
So, we warmly welcome version 3 of the Saving Babies’ Lives Care Bundle as a vital resource for all professionals involved in supporting people to have a safe and healthy pregnancy and birth.
Research is continually advancing understanding and growing evidence and it is vital this is translated into improvements in care. While this guidance has been produced before the evaluation of version 2, we support the fast tracking of new evidence so that everyone can benefit as quickly as possible, and potentially more babies’ lives can be saved.
We know from the MBRRACE data that some communities continue to experience much poorer outcomes than others. This is unacceptable and we’re therefore particularly pleased that version 3 has been reviewed from an inequity standpoint and highlights the promotion of equity and equality as an important principle to apply when implementing the care bundle. It is also positive to see that continuity of carer is explicitly noted as a key intervention to improve equitable outcomes.
A key addition to version 3 is the management of diabetes in pregnancy. The number of women and birthing people with diabetes is on the rise and perinatal mortality rates for pregnant people with Type 1 and Type 2 diabetes have remained very high for the last 5 years. This practical guidance should standardise pathways and join these up with other aspects of maternity care to reduce risk.
The care bundle also contains a renewed focus on reduced fetal movement (RFM). This is such an important message given the relationship between episodes of RFM and stillbirth, and the vital role of timely hospital attendance and fetal monitoring. Everyone must feel they can and should contact their hospital if they are worried that their baby’s movements have changed.
This version of the bundle is another important step on the journey to safer pregnancy and birth. We know that when all maternity units follow these actions, fewer families will face the heartbreak and devastation of pregnancy complications and loss.
Kath Abrahams, Chief Executive, Tommy’s
Preterm birth causes 78% of deaths in the neonatal period (first 28 days of life; National Child Mortality Database Thematic Report: The contribution of Newborn Health to Child Mortality across England, July 2022) and is also a major contributor to childhood disability and poorer neurodevelopmental outcome. Interventions to reduce the impact of prematurity on morbidity and mortality must therefore be a major focus to move towards the national maternity safety ambition to halve the rate of stillbirth, neonatal death, maternal death and serious intrapartum brain injury by 2025.
BAPM therefore welcomes expansion of Element 5 in version 3 of the Saving Babies’ Lives Care Bundle, which aims to reduce preterm birth where possible and optimise perinatal care where preterm birth cannot be prevented. Given the importance of the interventions in improving outcomes, BAPM strongly encourages trusts to ensure that appropriate time is allocated to the neonatal medical and nursing leads of the preterm birth team, in addition to the maternity and obstetric leads, to allow rapid implementation.
Eleri Adams, President, British Association of Perinatal Medicine
Progress towards the national maternity safety ambition
In 2015, the Secretary of State for Health announced a national maternity safety ambition to halve the rates of stillbirths, neonatal and maternal deaths and intrapartum brain injuries by 2030, with a 20% reduction by 2020. In 2017, the date to achieve the ambition was brought forward to 2025 and the ambition was extended to include a reduction in the rate of preterm births from 8% to 6%.
Office for National Statistics (ONS) data (shown in Figure 1 below) demonstrated a 25% reduction in stillbirths between 2010 and 2020, from 5.1 to 3.8 per 1,000 births, exceeding the 2020 milestone for stillbirths. It is not entirely clear why stillbirth rates increased during the COVID-19 pandemic, but it is likely that the direct effects of coronavirus as well as the indirect impact of the pandemic on accessing maternity services played a part in the increase of the stillbirth rate from 3.8 per 1,000 births in 2020 to 4.1 in 2021. The neonatal mortality rate also increased between 2020 and 2021 from 1.3 to 1.4 per 1,000 live births at 24 weeks’ gestation and over. Despite the significant challenges faced by the NHS, these rates remain 19% and 30% lower respectively than in 2010. This equates to more than 900 families returning home with a healthy baby in 2021, than if the rates had not changed from 2010.
Figure 1: National maternity safety ambition – Summary of progress on stillbirths
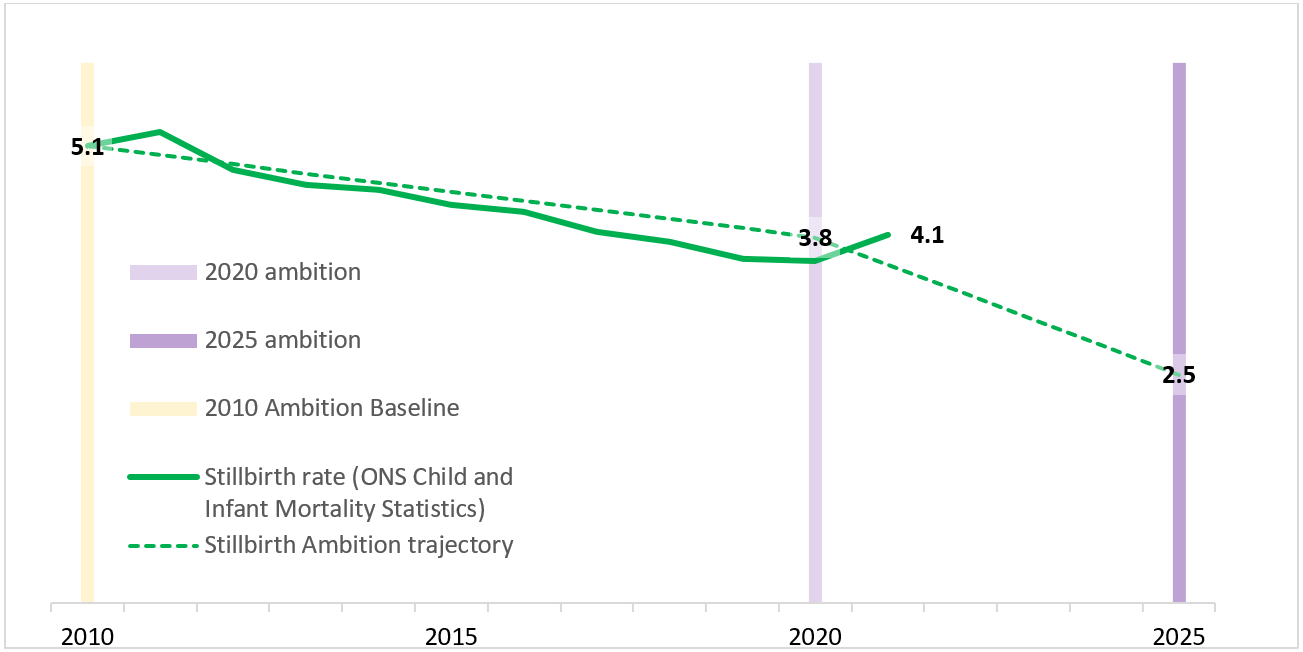
The data on serious brain injury shows that rate of occurrence during or soon after birth fell by 9% between 2014 and 2019 to 4.25 per 1,000 live births. Further reduction is needed to meet the 2025 ambition, a rate of 2.16 per 1,000 live births.
Despite the achievements of the past few years and in light of the recent setbacks, there is clearly much more to be done to achieve the ambition by 2025. In particular, there is a need to address inequitable outcomes associated with ethnicity and deprivation. MBRRACE-UK perinatal mortality surveillance report for births in 2020 showed that the lowest stillbirth rates were for babies of White ethnicity from the least deprived areas, at 2.78 per 1,000 total births. The highest stillbirth rates were for babies of Black African and Black Caribbean ethnicity from the most deprived areas, at around 8 per 1,000 total births. The pattern is similar for neonatal deaths. Maternity services must do everything possible to mitigate the wider social determinants of health, to continue to drive down the perinatal mortality rate.
Progress of the Saving Babies’ Lives Care Bundle
The first version of the Saving Babies’ Lives Care Bundle (SBLCBv1) was published in March 2016, and focused predominantly on reducing the stillbirth rate. An independent evaluation in 2018 showed a decrease in stillbirths in participating trusts, concluding that, despite being one of many concurrent interventions, it was highly plausible that SBLCBv1 had contributed to the reduction.
The evaluation informed the development of version 2 (SBLCBv2). Launched in March 2019, SBLCBv2 aimed to go further in reducing stillbirth while also minimising unnecessary intervention. In response to the expansion of the national maternity safety ambition in 2017, it introduced Element 5 on reducing preterm birth and further decreasing perinatal mortality.
The SBLCB is now a universal innovation in the delivery of maternity care in England and continues to drive quality improvement to reduce perinatal mortality. It has been included for several years in the NHS Long Term Plan, NHS planning guidance, the Standard Contract and the Clinical Negligence Scheme for Trusts Maternity Incentive Scheme (CNST MIS), with every maternity provider expected to have fully implemented SBLCBv2 by March 2020.
ONS and MBRRACE-UK data demonstrates the urgent need to continue reducing preventable mortality. Published 4 years after SBLCBv2, SBLCBv3 has been developed through a collaboration of frontline clinical experts, service users and key stakeholder organisations. All existing elements have been updated, incorporating learning from CNST MIS and insights from NHS England’s regional maternity teams. SBLCBv3 aligns with national guidance from the National Institute for Health and Care Excellence (NICE) and the Royal College of Obstetricians and Gynaecologists (RCOG) Green Top guidelines where available but it also aims to reduce unwarranted variation where the evidence is insufficient for NICE and RCOG to provide guidance. SBLCBv3 also includes a new element on optimising care for women with pregnancies complicated by diabetes.
While SBLCBv3 would ideally be informed by the evaluation of SBLCBv2, this has been delayed due to the pandemic. Stakeholders agreed that improvements to best practice could not be delayed when evidence is readily available for improvements to several elements. The evaluation of SBLCBv2 remains a priority and will be published in 2025. Findings will inform ongoing iterations of the SBLCB.
SBLCBv3 should not be implemented in isolation, but as one of a series of important interventions to help reduce perinatal mortality, morbidity and preterm birth. It is important that providers continue to implement best practice care whenever possible, including by following NICE guidance and using the National Maternity and Neonatal Recommendations Register to assess their compliance with recommendations from confidential enquiries and other key national reports.
Implementing version 3 of the Saving Babies’ Lives Care Bundle
As part of the three year delivery plan for maternity and neonatal services, all NHS maternity providers have been responsible for fully implementing SBLCBv3 by March 2024.
‘Full implementation’ of the Saving Babies Lives Care Bundle means implementing all interventions for all 6 elements.
Overseeing implementation
To comply with Safety Action 6 of the Clinical Negligence Scheme for Trusts Maternity Incentive Scheme (CNST MIS), trusts must provide assurance to their board and integrated care board (ICB) that they are on track to achieve compliance with all 6 elements of SBLCBv3, through quarterly quality improvement discussions with their ICB. Up to 3 (and at least 2) discussions should be evidenced for the relevant year, with discussions to include:
- details of element-specific improvement work being undertaken including evidence of generating and using the process and outcome metrics for each element
- progress against locally agreed improvement aims
- evidence of sustained improvement where high levels of reliability have already been achieved
- regular review of local themes and trends with regard to potential harms in each of the 6 elements
- sharing of examples and evidence of continuous learning by individual trusts with their local ICB and neighbouring trusts, publishing these on FutureNHS platform where appropriate
While the Three year delivery plan set out that SBLCBv3 should be fully implemented by March 2024, providers that have still to do so can achieve compliance with Safety Action 6 if the ICB confirms it is assured that all best endeavours – and sufficient progress – have been made towards full implementation, in line with the locally agreed improvement trajectory.
To support compliance, a national implementation tool is available for trusts to use if they wish on the FutureNHS platform. The tool can support providers to baseline current practice against SBLCBv3, agree a local improvement trajectory with their ICB and track progress locally in accordance with that trajectory. It will be updated in line with SBLCBv3.2 and existing data sets with the aim of reducing the burden of manual audits.
Trusts should be capturing SBLCB data as far as possible in their maternity information systems/electronic patient records, for submission to the Maternity Service Data Set (MSDS). A technical glossary assists MSDS v2.0 data submitters and details (where relevant) the data items, values and (where relevant) SNOMED CT terms that can be included in MSDS v2.0 submissions. Currently, some of the process and outcome indicators in the SBLCB cannot be captured in MSDS. Work is underway to integrate more process and outcome indicators as part of the development of MSDS v2.5.
Where trusts choose not to use the implementation tool to evidence compliance, they can provide a declaration signed by their executive medical director that SBLCBv3 is fully or will be in place as agreed with the ICB.
While there will be no routine, deadline-based submissions of data to the national NHS England team for the purposes of assurance, the national team will review any data stored on trust implementation tools on an ad-hoc basis to assess national progress in implementation.
Organisational roles and responsibilities
Successful implementation of SBLCBv3 requires providers, commissioners and networks to collaborate successfully. National levers, including NHS planning guidance, the NHS Standard Contract and Safety Action 6 in the CNST MIS, will be updated in to reflect the following organisational responsibilities:
- Providers are responsible for implementing SBLCBv3, including baselining current compliance, developing an improvement trajectory and reporting on implementation with their ICB as agreed locally. They are also responsible for submitting data nationally relating to key process and outcome measures for each element.
- ICBs are responsible for agreeing a local improvement trajectory with providers, along with overseeing, supporting and challenging local delivery. Where there is unresolved clinical debate about a pathway, providers may wish to agree a variation to an element of the SBLCB with their ICB. As an integral part of integrated care systems (ICSs), local maternity and neonatal systems (LMNSs) are accountable to ICBs and have the system’s maternity and neonatal expertise to support planning and provide leadership for improvement, facilitating peer support, and ensuring that learning from implementation and ongoing provision of the SBLCBv3 is shared across the system footprint.
- Clinical networks and regional maternity teams are responsible for providing support to providers, ICBs and LMNSs to enable delivery and achieve expected outcomes. It is important that specific variations from the pathways described in the SBLCBv3 are agreed as acceptable clinical practice by their clinical network.
Principles to be considered alongside implementing version 3
It has been necessary to restrict the scope of the SBLCBv3 to ensure it is deliverable. Nevertheless, it is just one of a series of important interventions to reduce perinatal mortality and preterm birth. The following principles should be considered alongside implementing the care bundle.
Promoting equity
Equity means that all groups attain the health outcomes of the most advantaged group. To help achieve equity, action must be universal but with a scale and intensity proportionate to the level of disadvantage; this is known as ‘proportionate universalism’.
While England is one of the safest countries in the world to give birth, stillbirth and neonatal mortality rates are higher for service users from Black and Asian ethnic groups and those living in the most deprived areas.
Maternity and neonatal services need to consider equity when implementing the SBLCB. They should:
- ensure that the needs of different groups are met, with support increasing as health inequalities increase. This requires use of quantitative and qualitative data about the local population and their health needs, along with co-production, to improve care pathways and implementation processes
- respond to each person’s unique health and social situation, so that care is personalised
Continuous improvement activity for each element of the SBLCB will routinely require consideration of access, experience and outcomes in relation to protected characteristics and other factors influencing inequalities, such as deprivation. Pathways and processes should be changed or additional supportive activity carried out to improve equity.
During the booking appointment, record if parents are in a close relative marriage, as this may indicate an increased risk for certain genetic conditions: while there is an increased risk of congenital anomalies in some couples, around 90% of couples remain unaffected. A detailed family history should be taken to identify potential genetic conditions and, if a potential genetic condition is identified, the antenatal screening midwife and the regional genetics service should be consulted to establish whether a referral is appropriate. Early identification empowers women to make informed reproductive choices.
To find out more, take the e-learning module Close relative marriage: equitable access to genetic information and services, which is available to all health and care staff.
MSDS consanguinity data quality guidance provides instructions on how to submit data regarding consanguinity and pregnancy to the MSDS.
Enhanced midwifery continuity of carer (EMCoC)
EMCoC remains an important intervention to address inequalities in experience and outcomes for Black and Asian women and women from economically disadvantaged groups. In line with the Core20PLUS5 strategy, local maternity and neonatal systems, regional and national colleagues will continue to support trusts with sufficient staffing to focus rollout of EMCoC to neighbourhoods with high numbers of women from Black, Asian and mixed ethnic groups and women living in deprived areas.
To support this, NHS England is providing funding to ICBs to operate EMCoC teams. Maternity services are advised to roll out these teams where safe staffing is in place, to provide improved care for women living in the most deprived 10% of neighbourhoods. EMCoC teams have additional staffing to provide more holistic support for women in these areas, who are more likely to experience adverse outcomes during pregnancy and birth.
Informed choice and personalised care
Evidence shows that better outcomes and experiences, as well as reduced health inequalities, are possible when pregnant women can actively shape their care and support. Personalised care means pregnant women have choice and control over the way their care is planned and delivered, based on best available evidence, ‘what matters’ to them and their individual strengths, needs and preferences. Pregnant women receiving maternity care make informed decisions. They and their maternity professionals discuss evidence-based options together, exploring preferences, benefits, risks and consequences to enable a safe and positive experience.
For any given situation where a decision needs to be made, women are supported by their maternity professionals to understand their options and the potential benefits, harms and consequences of each. They have all the information they need for shared decision-making and give consent, in line with the Montgomery ruling.
Linked to this principle, the following areas are particularly relevant to implementing a number of elements:
Informing women of the long-term outcomes of early-term birth
One of the key interventions in Elements 2 and 3 of the SBLCBv3 is offering early birth for women at risk of stillbirth. It is important that this intervention is not extended to pregnancies that are not at risk.
The Avoiding Term Admissions Into Neonatal units (Atain) programme identified that babies born at 37–38 weeks’ gestation were twice as likely as babies born at later gestations to be admitted to a neonatal unit. There are also concerns about long-term outcomes following early-term birth (defined as at 37–38 weeks’ gestation): These concerns relate to potential long-term adverse effects on the baby from birth before reaching maturity because, for example, the baby’s brain continues to develop at term. Birth results in huge changes to the baby’s physiology. For example, the arterial partial pressure of oxygen increases by a factor of 3 to 4 within minutes following birth and it is plausible that earlier exposure to these changes could alter long-term development of the child’s brain and data exists to support this possibility. One example is the risk that the child will subsequently have a record of special educational needs (SEN). The risk of this outcome is about 50% among infants born at 24 weeks of gestational age and it progressively falls with increasing gestational age at birth, only to bottom out at around 40–41 weeks.
Figure 2: Prevalence of special educational needs by gestation at birth
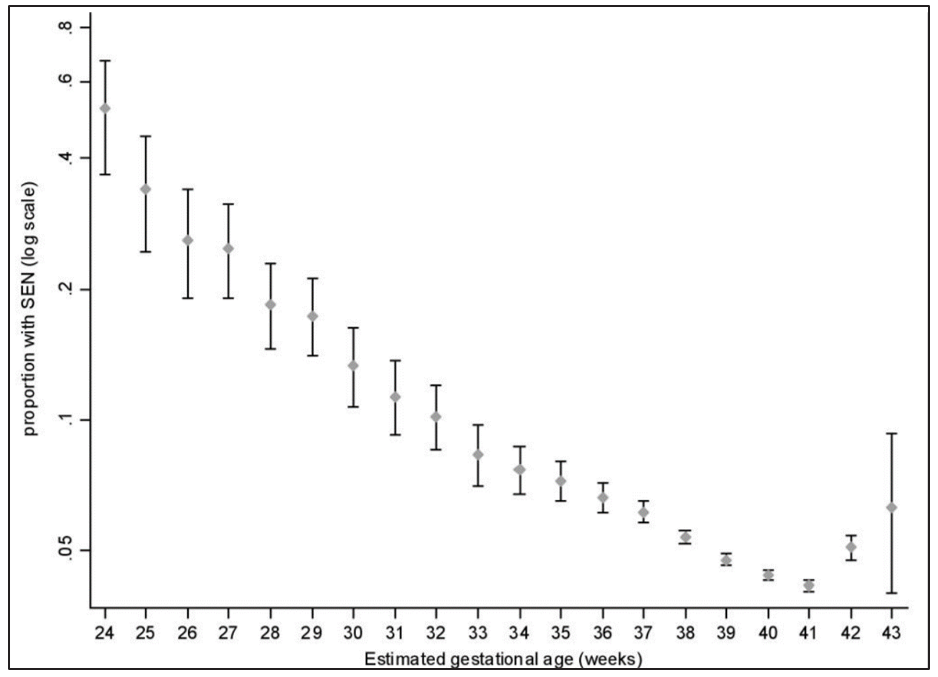
After adjusting for maternal and obstetric characteristics and expressed relative to birth at 40 weeks, the risk of SEN was increased by 36% [95% CI (confidence interval) 27–45] at 37 weeks, by 19% (95% CI 14–25) at 38 weeks and by 9% (95% CI 4–14) at 39 weeks. The risk of subsequent SEN was 4.4% at 40 weeks. Hence, assuming causality, there would be one additional child with SEN for every 60 inductions at 37 weeks, for every 120 inductions at 38 weeks and for every 250 inductions at 39 weeks compared with the assumption that they would otherwise have been born at 40 weeks. Recent data from the UK Millennium Cohort Study confirmed the finding that children born at early-term gestational ages (37–38 weeks) were more likely not to achieve the expected level of attainment in primary school but, interestingly, there was no association between early-term birth and poorer attainment at secondary school. Moreover, as the current data is based on the observed gestational age at birth, the negative associations with later outcome may be explained by the factors that determined early-term birth rather than a direct effect of gestational age. However, induction of labour before 39 weeks should continue to be considered as a significant medical intervention that requires appropriate justification.
Considering how the risks of induction of labour change with gestational age
For uncomplicated pregnancies NICE guidance on induction of labour should be followed. In all cases of induction, it is important that women are given a clear explanation of why they are being offered induction and that the risks, benefits and alternatives are discussed.
At 39+0 weeks’ gestation and beyond, induction of labour is not associated with an increase in caesarean section, instrumental vaginal birth, fetal morbidity or admission to the neonatal intensive care unit. The NICE guidance and data from the ARRIVE study provide contradictory evidence as to whether induced labours are associated with a longer hospital stay or more painful labours. Induction of labour may also increase the workload of the maternity service, which has the potential to impact on the care of other women.
Safe and healthy pregnancy information to enable women and their families to make informed choices
It is important that women have access to high quality information before and during their pregnancy to enable them to reduce risks to their baby. The Office for Health Inequalities and Disparities and Sands have developed key messages:
Summary of safe and healthy pregnancy key messages
Pre-pregnancy:
- choose when to start or grow your family by using contraception
- consult with your GP if taking medication for long-term conditions (for example, diabetes, hypertension, epilepsy) as your medication may have to change prior to pregnancy
- eat a healthy balanced diet and be physically active to enter pregnancy at a healthy weight
- take a daily supplement of 400 micrograms (400 µg) folic acid before conception (some women will require a higher dose of 5 mg as advised by a healthcare professional)
- ensure that you are up to date with routine vaccinations, for example measles, rubella, coronavirus (COVID-19), flu
- find out if you think you or your partner could be a carrier for a genetic disorder
- stop smoking and/or exposure to second-hand smoke
- reduce/stop alcohol consumption
During pregnancy:
- continue to take 400 µg folic acid until the 12th week of pregnancy (some women will require a higher dose of 5 mg as advised by a healthcare professional)
- pregnant women should have 10 µg of vitamin D a day
- you may be advised to take aspirin from 12 weeks of pregnancy
- alcohol – the safest advice is to not drink alcohol; if you are concerned, talk to your midwife or doctor, and help and advice is available for you
- don’t smoke and avoid second-hand smoke; support is available to help with this
- do tell your midwife if you use illegal street drugs or other substances; help is available for you.
- eat healthily and be physically active to maintain a healthy weight while pregnant
- maintain oral hygiene. Free dental care is available to all pregnant women and up to a year after the birth
- recommended vaccinations and boosters: seasonal flu, pertussis (whooping cough), coronavirus (COVID-19)
- always check with your pharmacist, midwife or doctor about medicines and therapies used in pregnancy, even if you have taken them for a long time on prescription or think they are harmless
- avoid contact with people who have infectious illnesses, including diarrhoea, sickness, childhood illnesses or any rash-like illness
- reduce the risk of cytomegalovirus, toxoplasmosis, Mpox infections, etc
- attend all antenatal appointments
- contact the maternity service promptly if you are worried about reduced fetal movements, vaginal bleeding, watery or unusual discharge, signs of pre-eclampsia or itching. Don’t wait!
- in later pregnancy (after 28 weeks), it is safer to go to sleep on your side than on your back
More detailed information on how women can plan, prepare, and look after themselves before and during pregnancy can be found on NHS.uk and the Safer Pregnancy website developed by Sands.
Working within networks for more specialist care
In several specialist fields, maternity services are working within networks so that women and babies with complex needs have consistent access to the most specialist care, while also encouraging local expertise and ensuring that care remains as close as possible to home.
While a networked model has been in place for a number of years in fetal medicine and in neonatal care, NHS England announced the creation of 14 maternal medicine networks, which are now in operation across England.
All providers should be engaging in these networks and contributing to the development of joint protocols and ways of working. In this vein, new elements of best practice should not be implemented in isolation locally. Providers should consider what implications or opportunities this presents for ways of working agreed within wider maternal medicine, fetal medicine or neonatal operational delivery networks.
Implementing relevant NICE guidance
Integrated care systems (ICSs)/ICBs are under an obligation in public law to have regard for NICE guidance and to provide clear reasons for any general policy that does not follow NICE guidance.
Providers and commissioners are encouraged to implement NICE guidance relating to antenatal, intrapartum and postnatal care. In particular, implementation of the NICE guidance on the management of diabetes in pregnancy, hypertension in pregnancy, multiple pregnancy and service provision for women with complex social factors is key to addressing some of the most significant contributors to perinatal mortality.
Best practice care in the event of a stillbirth or neonatal death
Despite the reduction in stillbirth rates sadly thousands of parents each year will experience the devastation of their baby dying before, during or shortly after birth. A best practice pathway for the clinical management of women experiencing stillbirth is available on the North West Coast (NWC) Strategic Clinical Network website.
Sands has developed a National Bereavement Care Pathway (NBCP) to help ensure that all bereaved parents are offered equal, high quality, individualised, safe and sensitive bereavement care when they experience pregnancy loss or the death of a baby.
The national Perinatal Mortality Review Tool (PMRT) is used to support hospital reviews by providing a standardised, structured process so that what happened at every stage of the pregnancy, birth and after, from booking through to bereavement care, is carefully considered by staff reviewing care. This online tool may help staff understand why a baby has died and whether there are any lessons to be learned to save lives in future.
Continuous improvement and maternity and neonatal services
SBLCBv3 maintains the continuous improvement approach. Each element focuses on a small number of outcomes, now with fewer process measures. Implementation of the elements will require a more comprehensive evaluation of each organisation’s processes and pathways and an understanding of where improvements can be made.
Each organisation will be expected to look at its performance against the outcome measures for each element with a view to understanding where improvement may be required. We have provided suggested areas for improvement in each element, but these lists are not meant to be exhaustive.
There is an expectation that as well as organisations reporting on their implementation of each element, there will be complimentary reporting of ongoing improvement work (with associated detail of interventions and improvement in process measures and outcomes) for each element. An integral component of this improvement work will be a focus on learning from incidents or enquiry. Harm may have occurred in relation to implementation of or non-compliance with an element of the SBLCB. The use of the Perinatal Mortality Review Tool will complement the investigation and learning in this context.
Element 1: Reducing smoking in pregnancy
Element description
Reducing smoking in pregnancy by identifying smokers with the assistance of carbon monoxide (CO) testing and ensuring in-house treatment from a trained tobacco dependence adviser (TDA)* is offered to all pregnant women who smoke, using an opt-out referral process.
*The TDA role is also known under alternative names, including smoking cessation adviser, stop smoking adviser and smoking cessation practitioner. Irrespective of role name or grade, this role is underpinned by appropriate training to deliver tobacco dependence treatment interventions (see 1.10).
Interventions
1.1 CO testing offered to all pregnant women at the antenatal booking and 36-week antenatal appointment.
1.2 CO testing offered at all other antenatal appointments to groups identified in NICE guidance [NG209].
1.3 Whenever CO testing is offered, it should be followed up with an enquiry about smoking status with the CO result and smoking status recorded.
1.4 Instigate an opt-out referral for all women who have an elevated CO level (4 ppm or above), who identify themselves as smokers or who have quit in the last 2 weeks for treatment by a trained TDA within an in-house tobacco dependence treatment service.
1.5 Nicotine replacement therapy (NRT) should be offered to all smokers and provision ensured as soon as possible.
1.6 The tobacco dependence treatment includes behavioural support and NRT, initially 4 weekly sessions following the setting of the quit date then regularly (as required, however as a minimum monthly) throughout pregnancy to support the woman to remain smokefree.
1.7 Feedback is provided to the pregnant woman’s named maternity healthcare professional regarding the treatment plan and progress with their quit attempt (including relapse). Where a woman does not book or attend appointments there should be immediate notification back to the named maternity healthcare professional.
1.8 Any staff member using a CO monitor should have appropriate training on its use and discussion of the result.
1.9 All staff providing maternity care to pregnant women should receive training in the delivery of very brief advice (VBA) about smoking, making an opt-out referral and the processes within their maternity pathway (for example, referral, feedback, data collection).
1.10 Individuals delivering tobacco dependence treatment interventions should be fully trained to National Centre for Smoking Cessation and Training (NCSCT) standards.
Continuous Learning
1.11 When analysing patient safety incidents, maternity care providers should review smoking status throughout pregnancy and determine whether the appropriate pathway of care for this was followed.
1.12 Maternity providers should regularly review (a minimum of quarterly) their smoking-related data to understand performance and develop improvement plans (this list is designed to provide a steer and is not exhaustive):
a. Identification of women who smoke – determine any factors that would optimise CO testing rates and enquiry about smoking status from both the provider/pathway and service-user perspective and make changes to pathways and processes as appropriate.
b. Training of staff – ensure all staff involved in identification, referral and treatment of women who smoke and provision of VBA are appropriately trained.
c. Engagement – determine and address any barriers to engagement with treatment services or compliance with treatment interventions from both the provider/pathway and service-user perspective.
d. Referral – determine and address any factors that are influencing opt-out referral from both the provider/pathway and service-user perspective.
e. Quit rates – consider the pathway holistically to determine which steps can be optimised to facilitate quit attempts and successful quits.
f. Relapse – determine factors that are contributing to relapse and whether additional support or changes to pathways may address these.
g. Inequalities – consider all the above by protected characteristics and other variables influencing inequalities, such as factors related to deprivation. Make changes to pathways and processes, or carry out additional supportive activity, to address any inequity or inequalities identified.
1.13 To monitor quality and effectiveness of pathways, maternity services should set ambitions for their pathway with regular review (a minimum of quarterly) of data and targeted quality improvement work to ensure they are being achieved.
1.14 Based on highly performing areas, stretching ambitions to achieve effective implementation of the full element may include:
a. 95% of women where CO measurement and smoking status is recorded at their booking appointment
b. 95% of women where CO measurement and smoking status is recorded at their 36-week appointment
c. 95% of smokers have an opt-out referral at booking for treatment by a TDA within an in-house service
d. 85% of all women referred for tobacco dependence treatment engage with the programme (have at least one session and receive a treatment plan)
e. 60% of those referred for tobacco dependence treatment set a quit date
f. 60% of those setting a quit date successfully quit at 4 weeks
g. At least 85% of quitters should be CO verified
1.15 Individual providers should examine their outcomes in relation to other providers or systems with similar smoking prevalence or populations. National benchmarking is available through the Maternity Services Dashboard and will be available to ICS/LMNS as the tobacco dependence patient-level collection is established.
Process Indicators
1a. Percentage of women where there is a record of:
i. CO measurement at booking appointmen
ii. CO measurement at 36-week appointment
iii. smoking status** at booking appointment
iv. smoking status** at 36-week appointment
1b. Percentage of smokers* who have an opt-out referral at booking to an in-house / in-reach tobacco dependence treatment service.
1c. Percentage of smokers* who are referred for tobacco dependence treatment who set a quit date.
Outcome Indicators
1d. Percentage of smokers* at antenatal booking who are identified as CO verified non-smokers at 36 weeks.
1e. Percentage of smokers* who set a quit date and are identified as CO verified non-smokers at 4 weeks.
*A ‘smoker’ is a pregnant woman with an elevated CO level (≥4 ppm) and identifies themselves as a smoker (smoked within the last 14 days) or has a CO level <4 ppm but identifies as a smoker (smoked within the last 14 days).
**Smoking status relates to the outcome of the CO test (>4 ppm) and the enquiry about smoking habits.
Rationale
Smoking increases the risk of pregnancy complications, such as stillbirth, preterm birth, miscarriage, low birthweight and sudden infant death syndrome (SIDS). Whether or not a woman smokes during her pregnancy has a far-reaching impact on the health of a child throughout their life. Studies have found that the risk of a number of poor pregnancy outcomes can be reduced to that of a non-smoker if a successful quit is achieved early in pregnancy. Others show increased risk with any smoking in pregnancy and increasing risk with continued smoking. This reinforces the need to support women to quit smoking as early as possible in pregnancy to reduce the risk of poor pregnancy outcomes.
Smoking at time of delivery (SATOD) rates have declined since the release of previous versions of the SBLCB, albeit at a slower rate than required to meet the government’s 2022 national ambition of 6% (and ultimately a smokefree generation by 2030). Although there is significant variation, the national SATOD rate was 9.1% in 2021/22, demonstrating that further work is required to reduce smoking during pregnancy.
This element is evidence based and provides a practical approach incorporating the NHS Long Term Plan pathway for a smokefree pregnancy and core elements of NICE guidance. It builds on the previous version of the SBLCB’s focus on CO testing to support identification of smokers, with referral to in-house tobacco dependence treatment services and ensuring that effective treatment is available to all pregnant women who smoke. Research indicates that pregnant women expect their maternity provider to ask about smoking and if the issue is not raised, this can be incorrectly interpreted as smoking not being a problem for the pregnancy. Learning from frontline services demonstrates a need for a treatment offer that is part of the maternity pathway and the woman’s maternity journey. This should include processes for referral, treatment and feedback that are timely and optimise engagement, with dedicated leadership within the maternity service that has oversight of the full pathway. To support this, all clinicians with whom the woman comes into contact during pregnancy should give consistent messages.
This element has a positive impact on the other SBLCB elements. Reducing smoking in pregnancy will reduce instances of fetal growth restriction, intrapartum complications and preterm birth. This demonstrates the complementary and cumulative nature of the SBLCB approach.
This element also reflects the wider prevention agenda, impacting positively on the health of babies and the long-term outcomes for families and society.
Implementation guidance
Key factors for effective implementation include:
In-house pathways: Clinical leadership, delivery and oversight of the service and its outcomes remains with maternity. Services are considered as in-house when the woman’s care for treating their tobacco dependence remains within the maternity service – that is, is not referred out to another provider like a local authority stop smoking service.*
* In-reach services where a third party, such as the local authority stop smoking service, provide services as part of the maternity team with the patient staying under the care and management of the maternity service would count as in-house.
Opt-out referral pathways: Effective pathways are in place to ensure that as soon as a smoker is identified there is rapid referral to the TDA on an opt-out basis. Immediate referral and consultation with the TDA is the ideal, but as a minimum the woman should be contacted within 1 working day and seen (ideally face to face) by a TDA within 5 working days.
Nicotine replacement therapy (NRT): Pathways should ensure provision of long and short-acting NRT at the earliest opportunity to facilitate quitting at an optimal time to improve perinatal outcomes and maximise engagement with referral and treatment services. Ideally, this should be at the earliest opportunity when maternity care has started, even if prior to a formal booking appointment.
Recent quitters: The definition of a smoker includes those who have smoked within the past 2 weeks. However, it is good clinical practice to offer support to all women who have quit smoking since conception given that changes in 1st trimester pregnancy symptoms may affect smoking habits.
CO testing: All pregnant women should be offered CO testing at the antenatal booking and 36-week antenatal appointment, with testing offered at all other antenatal appointments after booking to groups identified within NICE guidance [NG209]. All staff providing antenatal care should have access to a CO monitor and training in how to use it and interpretation of results. Appropriate procurement processes should be in place for obtaining CO monitors and associated consumables (for example, tubes and batteries).
CO verified non-smokers at 4 weeks: This corresponds to the 4-week quit that is regularly captured by stop smoking services and is a comparable indicator that can be used to assess the quality of the intervention.
CO levels: The most common reason for a raised CO level is smoking. However exposure may come from other sources such as second-hand smoke, faulty boilers, faulty heating/cooking appliances or car exhausts (and can happen at home or at the workplace). If women have raised CO levels and are non-smokers, environmental exposure from a source in the home should be considered and the women should be advised to contact the Gas Emergency Line on 0800 111 999 for further advice. Referral for further medical advice should be sought if symptoms are consistent with CO poisoning. For NICE guidance on air pollution and vulnerable groups, see recommendation 1.7.7. in NICE guidance [NG70].
Tobacco dependence treatment: Following an initial appointment where a quit is initiated, weekly face-to-face appointments with the TDA should take place for at least 4 weeks after the quit date is set, followed by regular appointments (as required but monthly as a minimum) throughout the pregnancy. Treatment includes behavioural support and a combination of long and short-acting NRT. A recommended delivery model pathway is available on the Prevention Programme’s FutureNHS webpages.
Recording data: There should be routine recording of the CO test result and smoking status for each pregnant woman on maternity information systems (MIS), reporting through to the tobacco dependence patient-level collection and, where appropriate, the Maternity Service Data Set (MSDS).
Review and act on local data: Use available tools (for example, the Maternity Services Dashboard’s clinical quality improvement metrics) to review the current situation with smoking and data quality, compare with other nearby or demographically similar trusts and identify if your trust is an outlier and/or where improvements can be made.
Vaping: In its position statement on support to quit smoking in pregnancy, the Royal College of Midwives states that “E-cigarettes contain some toxins, but at far lower levels than found in tobacco smoke. If a pregnant woman and birthing person who has been smoking chooses to use an e-cigarette (vaping) and it helps her to quit smoking and stay smokefree, she should be supported to do so”. More information is available via the Smoking in Pregnancy Challenge Group.
System-wide action: Action to help pregnant women stop smoking should be supplemented by wider activity across the local system to reduce smoking rates among women, partners and other household or family members. This includes reducing smoking rates in women pre-conception, in addition to working with neonatal care and health visiting services to ensure there are links with local stop smoking services to support quitting postnatally. Local tobacco control networks alongside LMNSs, ICSs and regional teams should be able to support with integrating activity to reduce smoking prevalence in all population groups, which can impact on reducing maternal smoking.
Implementation resources
Resources: The NHS Long Term Plan delivery model for smokefree pregnancy provides details of the pathway and treatment programme that should be delivered in this element. This can be found on the FutureNHS webpages with additional resources and shared materials.
Information and links to further resources are also available from the Maternal and Neonatal Health Safety Collaborative. Action on Smoking and Health (ASH) produces annual briefings for ICSs showing the impact of smoking, using data at ICS level. The briefings include national data on maternal smoking and other clinical areas broken down to ICS level, and signpost to current resources and information.
The Smoking in Pregnancy Challenge Group produces a range of resources and information for healthcare professionals working to reduce maternal smoking. Those with an interest can also join the Smokefree Pregnancy Information Network administered by ASH, which will provide up to date information throughout the year. For more information contact admin@smokefreeaction.org.uk.
Training – tobacco dependence adviser: Those providing tobacco dependence treatment interventions are specialist advisers and should have successfully completed NCSCT smoking practitioner training (or local training to the to the required NCSCT standard) and a speciality course for smoking in pregnancy (requires registration and log-in), including opportunities to observe good practice; to ensure they have the knowledge and skills to deliver the treatment. TDAs should receive annual refresher training. NCSCT-derived competency frameworks are also available on the FutureNHS platform.
Training – all maternity healthcare professionals: All multidisciplinary staff providing maternity care for pregnant women should receive training on how to use a CO monitor (see CO testing section), the delivery of very brief advice (VBA) about smoking, making an opt-out referral and the processes within their maternity pathway (for example, referral, feedback, data collection). Annual refresher training should align with the core competency framework. VBA on smoking in pregnancy training can be accessed via NCSCT e-learning or eLearning for Health Hub.
Element 2: Fetal growth: risk assessment, surveillance and management
Element description
Risk assessment and management of babies at risk of or with fetal growth restriction (FGR).
Interventions
Reduce the risk of FGR where possible.
2.1 Assess all women at booking to determine if prescription of aspirin is needed using an appropriate algorithm (for example, Appendix B) agreed with the local ICSs and regional maternity team.
2.2 Recommend vitamin D supplementation to all pregnant women.
2.3 Assess smoking status and manage findings as per Element 1.
Monitor and review the risk of FGR throughout pregnancy.
2.4 Perform a risk assessment for FGR by 14 weeks’ gestation using an agreed pathway (for example, Appendix C). Risk assessment in multiparous women should include the calculation of previous birthweight centiles. If a woman is a late booker or transfer of care, then a risk assessment should be completed using the risk assessment system of the new location trust. The pathway and centile calculator used should be agreed by both the local ICSs and the regional maternity team.
2.5 During risk assessment trusts are encouraged to use information technology platforms to facilitate accurate recording and correct classification of risk by staff. No single provider is recommended, but technology platforms should not prevent compliance with Element 2 guidance and should follow national recommendations on the use of fundal height and fetal growth charts.
2.6 As part of the risk assessment for FGR, blood pressure should be recorded using a digital monitor that has been validated for use in pregnancy (see ‘Implementation’ below).
2.7 Women who are designated as high risk for FGR (for example, see Appendix C) should undergo uterine artery Doppler assessment between 18+0 and 23+6 weeks’ gestation.
2.8 The risk of FGR should be reviewed throughout pregnancy and maternity providers should ensure that processes are in place to enable the movement of women between risk pathways dependent on current risk.
2.9 When an ultrasound-based assessment of fetal growth is performed, trusts should ensure that robust processes are in place to review which risk pathway a woman is on and agree a plan of ongoing care.
2.10 Women who are at low risk of FGR should have serial measurement of symphysis fundal height (SFH) at each antenatal appointment after 24+0 weeks’ gestation (no more frequently than every 2 weeks). The first measurement should be carried out by 28+6 weeks. Measurements should be plotted or recorded on charts by clinicians trained in their use.
2.11 Staff who perform SFH measurement should be competent in measuring, plotting (or recording), interpreting appropriately and referring when indicated. Only staff who perform SFH measurement need to undergo training in SFH measurement.
2.12 Women who are undergoing planned serial scan surveillance should cease SFH measurement after serial surveillance begins. SFH measurement should also cease if women are moved onto a scan surveillance pathway in later pregnancy for a developing pregnancy risk (for example, late-onset FGR).
2.13 Women who are at increased risk of FGR should have ultrasound surveillance of fetal growth at 3–4-weekly intervals until delivery (see RCOG guidance and Appendix C).
Provide the correct surveillance when FGR is suspected and delivery at the right time.
2.14 When FGR is suspected an assessment of fetal wellbeing should be made including a discussion regarding fetal movements (see Element 3) and if required computerised cardiotocograph (cCTG). A maternal assessment should be performed at each contact this should include blood pressure measurement using a digital monitor that has been validated for use in pregnancy and a urine dipstick assessment for proteinuria. In the presence of hypertension NICE guidance on the use of PlGF/sflt1 testing should be followed.
2.15 Umbilical artery Doppler is the primary surveillance tool for FGR identified prior to 34+0 weeks and should be performed as a minimum every 2 weeks. Maternity care providers caring for women with early FGR identified prior to 34+0 weeks’ gestation should have an agreed pathway for management that includes fetal medicine network input (for example, through referral or case discussion by phone). Further information is provided in Appendix C.
2.16 When FGR is suspected, the frequency of review of estimated fetal weight (EFW) should follow the guidance in Appendix C or an alternative that has been agreed with local ICSs following advice from the provider’s clinical network and/or regional team.
2.17 Risk assessment and management of growth disorders in multiple pregnancy should comply with NICE guidance or a variant that must be agreed by both the local ICSs and the regional maternity team.
2.18 All management decisions regarding the timing of FGR infants and the relative risks and benefits of iatrogenic delivery should be discussed and agreed with the mother. When the EFW is <3rd centile and there are no other risk factors (see 2.20), initiation of labour and/or delivery should occur at 37+0 weeks’ gestation and no later than 37+6 weeks’ gestation.
2.19 In fetuses with an EFW between the 3rd and <10th centile, delivery should be considered at 39+0 weeks’ gestation. Birth should be achieved by 39+6 weeks’ gestation. Other risk factors should be present for birth to be recommended prior to 39 weeks (see 2.20).
2.20 Fetuses who demonstrate declining growth velocity from 32 weeks’ gestation are at increased risk of stillbirth from late-onset FGR. Declining growth velocity can occur in fetuses with an EFW >10th centile. Evidence to guide practice is limited and guidance (see Appendix C) is currently based on consensus opinion. In fetuses with declining growth velocity and EFW >10th centile, the risk of stillbirth from late-onset FGR should be balanced against the risk of late preterm delivery. In infants where declining growth velocity meets criteria (see Appendix C), delivery should be planned from 37+0 weeks’ gestation unless other risk factors are present. Risk factors that should trigger review of timing of birth are: reduced fetal movements, any umbilical artery or middle cerebral artery Doppler abnormality, cCTG that does not meet criteria, maternal hypertensive disease, abnormal sFlt1: PlGF ratio/free PlGF or reduced liquor volume. Opinion on timing of birth for these infants should be made in consultation with specialist fetal growth services or fetal medicine services depending on trust availability.
Continuous learning
Learning from excellence and error or incidents
2.21 Trusts should determine and act on all themes related to FGR that are identified from investigation of incidents, perinatal reviews and examples of excellence.
2.22 Trusts should provide data relating to the following to their boards and share this with their ICS:
a. percentage of babies born <3rd birthweight centile >37+6 weeks’ gestation
b. ongoing case note audit of <3rd birthweight centile babies not detected antenatally and born after 38+0 weeks’ gestation, to identify areas for future improvement (at least 20 cases per year or all cases if fewer than 20 occur)
c. percentage of babies born >39+6 and <10th birthweight centile to provide an indication of detection rates and management of small for gestational age (SGA) babies
d. percentage of babies >3rd birthweight centile born <39+0 weeks’ gestation
2.23 Use the Perinatal Mortality Review Tool (PMRT) to calculate the percentage of perinatal mortality cases annually where the identification and management of FGR was a relevant issue. Trusts should review their annual MBRRACE perinatal mortality report and report to their ICS on actions taken to address any identified deficiencies.
2.24 Individual trusts should examine their outcomes in relation to similar trusts to understand variation and inform potential improvements.
2.25 Individual trusts should provide data on the distribution of FGR outcomes with relation to maternal reported ethnicity.
2.26 Maternity providers are encouraged to focus improvement in the following areas:
a. appropriate risk assessment for FGR and other conditions associated with placental dysfunction and robust referral processes to appropriate care pathways following this
b. appropriate prescribing of aspirin in line with this risk assessment in women at risk of placental dysfunction
c. review of ultrasound measurement quality control. Trusts are encouraged to comply with British Medical Ultrasound Society (BMUS) guidance on audit and continuous learning with relation to 3rd trimester assessment of fetal wellbeing
d. Trusts will share evidence of these improvements with their board and ICS and demonstrated continuous improvement in relation to process and outcome measures
Process indicators
2a. Percentage of pregnancies where a risk status for FGR is identified and recorded at booking. (This should be recorded on the provider’s maternity information system (MIS) and included in the MSDS submission to NHS England once the primary data standard is in place.)
2b. Percentage of pregnancies where an SGA fetus (between 3rd and <10th centiles) is detected antenatally and this is recorded on the provider’s MIS and included in its MSDS submission to NHS England.
2c. Percentage of perinatal mortality cases annually where the identification and management of FGR was a relevant issue (using the PMRT).
Outcome indicators
2d. Percentage of babies <3rd birthweight centile born >37+6 weeks’ gestation (this is a measure of the effective detection and management of FGR).
Rationale
There is strong evidence linking undiagnosed FGR to stillbirth. Therefore, antenatal detection of growth restricted babies is vital and has been shown to reduce stillbirth risk significantly because it gives the option to consider timely delivery of the baby.
Update for version 3
The previous versions of this element have made a measurable difference to antenatal detection of FGR across England. Version 2 resulted in the widespread uptake of uterine artery Doppler screening for the first time outside tertiary centres in England and a significant improvement in the quality of care provided to pregnant women in all types of maternity setting. By introducing more nuanced risk assessment we have sought to reduce intervention while maintaining the focus on delivering babies at risk. In this version we seek to clarify this further so that all members of staff caring for pregnant women have clear, practical guidance. Our new title – Fetal growth: risk assessment, surveillance and management – reflects this.
Important changes in this update are:
- following a review by the Chief Scientific Officer team, only digital measurement of blood pressure is now recommended for risk assessment and monitoring of FGR
- The previous definition of suboptimal fetal growth of <20 g/day in the late 3rd trimester has been too didactically interpreted and has therefore been removed. A prospectively tested method of identifying suboptimal fetal growth in babies >10th centile remains elusive so suggestion for replacement is contained within Appendix C
- RCOG guidance on fundal height and EFW charts is still awaited so trusts may continue to use a range of charts. However, charts that are appropriate for plotting EFW and birthweight are recommended for reporting to reduce discrepancies
Risks and benefits of early-term delivery
It is well recognised that preterm birth is associated with both short and long-term sequelae for the infant. The distinction between preterm and term birth is based on the 37+0-week threshold. However, like any threshold on a continuous scale, the separation into 2 groups is arbitrary. Some of the risks associated with preterm birth are still apparent at ‘early-term’ gestation, defined as 37 and 38 weeks. The association with short-term morbidity can be captured by analysing the risk of admission of the infant to the neonatal unit. One of the best UK analyses compared the risk of neonatal unit admission associated with induction of labour at the given week with the comparison group of all women delivered at a later week of gestation (Table 1).
Table 1: Neonatal unit admission according to week of gestational age, comparing induction of labour and expectant management
|
Week of gestational age | Neonatal admission per 1,000 |
Adjusted odds ratio (95% CI) | |
|
|
Induction of labour |
Delivered later |
|
|
37 |
176 |
78 |
2.01 (1.80–2.25) |
|
38 |
113 |
74 |
1.53 (1.41–1.67) |
|
39 |
93 |
73 |
1.17 (1.07–1.20) |
|
40 |
80 |
73 |
1.14 (1.09–1.20) |
|
41 |
66 |
84 |
0.99 (0.93–1.05) |
However, delivery of the baby early prevents the subsequent risk of antepartum stillbirth. As antepartum stillbirth is the major single cause of perinatal death at term, earlier delivery will prevent perinatal death. The same paper also reported data on the risk of extended perinatal mortality associated with earlier induction (Table 2).
Table 2: Extended perinatal mortality according to week of gestational age, comparing induction of labour and expectant management
|
Week of gestational age | Extended perinatal mortality per 1,000 |
Adjusted odds ratio (95% CI) | |
|
|
Induction of labour |
Delivered later |
|
|
37 |
0.9 |
2.3 |
0.15 (0.03 –0.68) |
|
38 |
0.8 |
2.0 |
0.23 (0.09–0.58) |
|
39 |
0.6 |
1.9 |
0.26 (0.11–0.62) |
|
40 |
0.8 |
1.8 |
0.39 (0.24–0.63) |
|
41 |
0.7 |
2.2 |
0.31 (0.19–0.49) |
The dilemma is that early-term delivery reduces the risk of a very rare but serious adverse event (stillbirth or neonatal death) while increasing the risk of much more common but less severe adverse events. Decision-making balances the risks of causing mild harm to relatively large numbers of infants, to prevent serious harm to a relatively small number. For example, using the data above, at 37 weeks’ gestation, 10 inductions will lead to 1 additional baby being admitted for neonatal care, but it will require >700 inductions to prevent each perinatal death. Hence, current care is aimed at targeting early term induction to those who are at increased risk of perinatal death.
Implementation
Element 2 recognises that there are a range of expert opinions on some interventions and allows some flexibility in the choice of pathways. The pathways in Appendix C have been widely implemented but are not mandated. If an alternative pathway is chosen it should be agreed with local ICSs following advice from the provider’s clinical network and/or regional team as to whether the pathway is acceptable, to prevent idiosyncratic care.
To implement this element effectively trusts should:
- ensure that all pregnant women are assessed for their risk of placental dysfunction with the associated potential for FGR in early pregnancy
- ensure that a robust training programme and competency assessment is included in any processes designed to detect FGR – for example, measurement of SFH, use and interpretation of charts, ultrasound scanning for growth and uterine artery Doppler measurement to detect early-onset FGR
- agree a plan so that all blood pressure monitors in use with pregnant women comply with guidance from the Chief Scientific Officer. Please note that in addition to the lists of monitors currently recommended in this guidance, STRIDE-BP is also considered to maintain an academically rigorous list of monitors clinically validated for use in pregnancy, and monitors on this list will be considered compliant for the purposes of the SBLCB. Plans and timescales for procurement must be in view of local resources, with priority given to the replacement of analogue / aneroid blood pressure monitors. In the meantime, the use of non-compliant devices should be raised in the service risk register
- agree which charts will be used antenatally to measure fetal growth and ensure that these charts are based on EFW reference ranges
- electronic ultrasound database and MIS suppliers should provide EFW centile charts and birthweight centile charts with reference curves for the 3rd and 10th centiles. Providers using paper EFW and birthweight centile charts should ensure that the charts have reference curves for the 3rd and 10th centiles. The baby’s actual birthweight should be assessed for audit purposes using the same methodology used antenatally – that is, based on EFW reference not a birthweight reference scale, to ensure consistency
The RCOG SGA guideline advises that fetal biometry surveillance scans do not need to be performed more frequently than every 3 weeks unless potential abnormalities in fetal growth are identified, in which case scans may need to be performed more frequently (see intervention 2.7). Ultrasound surveillance of biometry in at-risk fetuses should continue until delivery.
Providers with capacity may wish to use assessment of middle cerebral artery Doppler pulsatility indices in addition to umbilical artery Doppler to help identify and act on potential fetal compromise in later pregnancy (after 34+0 weeks’ gestation).
Version 2 of the MSDS enables the recording of antenatally detected SGA using local criteria and the recording of fetal biometry, EFW and birthweight.
Trusts submitting data to the MSDS will be able to view the percentage annually of <10th centile and <3rd centile births in each gestational week of the 3rd trimester in their unit. This data will allow trusts to compare outcomes with similar units and to monitor the performance of their SGA and FGR detection programmes over time.
Element 3: Raising awareness of reduced fetal movement
Element description
Raising awareness among pregnant women of the importance of reporting reduced fetal movements (RFM) and ensuring providers have protocols based on best available evidence in place to manage care for women who report RFM.
Interventions
3.1 Information from practitioners, accompanied by an advice leaflet on RFM (for example, RCOG or Tommy’s leaflet available in multiple languages), based on current evidence, best practice and clinical guidelines, to be available to all pregnant women by 28+0 weeks of pregnancy and fetal movement discussed at every subsequent contact.
3.2 Use a checklist such as the one suggested under ‘implementation’ below to manage care of pregnant women who report RFM, in line with national evidence-based guidance (for example, RCOG Green Top guideline 57).
Continuous learning
3.3 Maternity care providers should examine their outcomes in relation to the interventions, trends and themes within their own incidents and perinatal mortality reviews where the presentation and/or management of RFM is considered to have been a contributory factor.
3.4 Maternity care providers should ensure that inequalities (particularly relating to ethnicity and deprivation) are being adequately addressed when there are incidents relating to presentation with or management of RFM.
3.5 Individual trusts should examine their outcomes in relation to similar trusts to understand variation and inform potential improvements.
3.6 Maternity providers are encouraged to focus improvement in the following areas:
a. signposting to information regarding RFM to pregnant women by 28+0 weeks of pregnancy
b. appropriate care according to local guidance in relation to risk stratification and ongoing care for women presenting with RFM
c. ensuring appropriate use of induction of labour when RFM is the only indication (for example, induction of labour for RFM alone is not recommended prior to 39+0 weeks’ gestation)
Process indicators
3a. Percentage of women who attend with RFM who are clinically eligible who have a computerised CTG.
3b. Proportion of women who attend with recurrent RFM who had an ultrasound scan by the next working day to assess fetal growth.
Outcome indicators
3c. Percentage of stillbirths and neonatal deaths which had issues associated with RFM management identified using PMRT.
3d. Rate of induction of labour when RFM is the only indication before 39+0 weeks’ gestation.
Rationale
Enquiries into stillbirth have consistently described a relationship between episodes of RFM and stillbirth, from the 8th Confidential Enquiry into Stillbirths and Deaths in Infancy report published in 2001 to the MBRRACE-UK reports into antepartum and intrapartum stillbirths respectively. In all these case reviews unrecognised or poorly managed episodes of RFM have been highlighted as contributory factors to avoidable stillbirths. Issues relating to inadequate investigation or management of RFM are identified in 16% of perinatal mortality reviews. In addition, a growing number of studies, including an individual patient data meta-analysis, have confirmed a correlation between episodes of RFM and stillbirth. This relationship increases in strength when women have multiple episodes of RFM in late pregnancy (after 28 weeks’ gestation). There is no accepted definition of recurrent RFM; one UK region has successfully adopted a consensus definition of 2 or more episodes of RFM occurring within a 21-day period after 26 weeks’ gestation. The relationship between RFM and stillbirth appears to be mediated by placental insufficiency.
Implementation
A systematic review found that interventions to encourage awareness of fetal movement in pregnant women were associated with a reduction in perinatal death, neonatal intensive care unit (NICU) admissions and Apgar scores of <7 at 5 minutes of age and were not associated with increases in caesarean births or induction of labour. The effect of encouraging fetal movement awareness or clinical management of RFM on stillbirth is uncertain.
It is possible that altering clinical management after RFM will cause an increase in ultrasound scans and obstetric intervention, such as induction of labour and caesarean birth. The AFFIRM study found that a care package that recommended all women have an ultrasound assessment of fetal biometry, liquor volume and umbilical artery Doppler following presentation with RFM after 26 weeks’ gestation and offered induction of labour for recurrent episodes of RFM after 37 weeks’ gestation did not significantly reduce stillbirths, but was associated with an increase in induction of labour and caesarean births. However, this care pathway reduced the number of small for gestational age (SGA) babies born at or after 40 weeks’ gestation. Other studies found raised awareness of fetal movements, with no mandated management package, did not increase caesarean births.
There are no trials of computerised CTG (cCTG) following presentation with RFM. However, cCTG is recommended as it provides an objective assessment of fetal wellbeing and may be completed more quickly than conventional CTG. It is also recommended over and above visualised CTG due to its potential to reduce the risks of human error.
If a cCTG has been performed and is normal and there are no other indications for an ultrasound scan, then a scan is not required for a first presentation of RFM, but should be offered for women reporting recurrent RFM. If an appropriate scan has been performed within the previous 2 weeks and was normal, a repeat scan is not required.
Before 39 weeks’ gestation, induction of labour or caesarean birth is associated with small increases in perinatal morbidity and neurodevelopmental delay. Thus, a recommendation for birth needs to be individualised and based on evidence of fetal compromise (for example, abnormal CTG, EFW <10th centile or oligohydramnios) or other concerns (for example, concomitant maternal medical disease, such as hypertension or diabetes, or associated symptoms such as antepartum haemorrhage).
At 39 weeks’ gestation and beyond, induction of labour is not associated with an increase in caesarean birth, birth with forceps or ventouse, fetal morbidity or admission to the NICU. Therefore, expediting birth by induction of labour (for women in whom this is not contraindicated) could be discussed (risks, benefits and mother’s wishes) with women presenting with a single episode of RFM after 38+6 weeks’ gestation. The patient decision aid for timing of induction of labour should be used.
It is important that women presenting with recurrent RFM are additionally informed of the association with an increased risk of stillbirth. After 39+0 weeks’ gestation they should be given the option of expediting birth (by the most appropriate route for them) for RFM alone. The decision to offer expedited birth should consider whether the timing of this can be achieved within the safe capacity of the unit.
Suggested checklist for the management of reduced fetal movements (RFM)
1. Ask
- confirm there is maternal perception of RFM?
- how long has there been RFM?
- is this the first episode?
- when were movements last felt?
2. Act
- auscultate fetal heart (hand-held Doppler/Pinnard) to confirm fetal viability
- assess fetal growth by reviewing growth chart, perform symphysis fundal height (SFH) if not performed within last 2 weeks (if not on an ultrasound surveillance pathway already)
- perform CTG to assess fetal heart rate in accordance with national guidelines (ideally computerised CTG should be used)
- ultrasound scan for fetal growth, liquor volume and umbilical artery Doppler only need to be offered on first presentation of RFM if there is an indication for scan [for example, the baby is small for gestational age (SGA) on clinical assessment]
- ultrasound scan for fetal growth, liquor volume and umbilical artery Doppler should be offered to women presenting with recurrent RFM after 28+0 weeks’ gestation
- scans are not required if there has been a growth scan in the previous 2 weeks
- in cases of RFM after 38+6 weeks’ gestation, discuss induction of labour with all women and offer birth to those with recurrent RFM after 38+6 weeks’ gestation
3. Advise
- convey results of investigations to the mother. Mother should be encouraged to reattend if she has further concerns about RFM
- in the event of being unable to auscultate the fetal heart, arrange immediate ultrasound assessment
Element 4: Effective fetal monitoring during labour
Element description
Effective fetal monitoring during labour.
Interventions
4.1 All staff who care for women in labour are required to undertake annual training and competency assessment on knowledge and skills required for effective fetal monitoring via intermittent auscultation (IA) (midwives) and electronic fetal monitoring (midwives and obstetricians).
4.2 At the onset of every labour, there is a structured risk assessment undertaken which informs the clinicians recommendation of the most appropriate fetal monitoring method at the start of labour. This risk assessment should be revisited throughout labour as part of a holistic review.
4.3 Regular (at least hourly) systematic review of maternal and fetal wellbeing is recommended. This should be accompanied by a clear guideline for escalation if concerns are raised using this structured process. All staff to be trained in the review system and escalation protocol.
4.4 A separate clinical review should be undertaken to help provide an objective holistic review, for example ‘Fresh Eyes’. This can also include obstetric reviews on ward rounds and when escalating clinical concerns to senior decision-makers. This should be undertaken at least hourly when CTG monitoring is used and at least 4 hourly when IA is utilised, unless there is a trigger to provide a holistic review earlier.
4.5 Identify a dedicated lead midwife (minimum of 0.4 WTE) and lead obstetrician (minimum 0.1 WTE) with demonstrated fetal monitoring expertise to focus on and champion best practice in fetal monitoring. Capacity should be sufficient to be visible, lead and educate across all sites and feed actively into continuous improvement and learning.
Continuous learning
4.6 Maternity care providers should examine their outcomes in relation to the interventions, trends and themes in their own incidents where fetal monitoring was likely to have been a contributory factor.
4.7 Individual trusts should examine their outcomes in relation to similar trusts to understand variation and inform potential improvements.
4.8 Maternity providers are encouraged to focus improvement in the following areas:
a. risk assessment of the woman/fetus at the beginning and regularly during labour
b. interpretation and escalation of concerns over fetal wellbeing in labour
Process indicators
4a. Percentage of staff who have received training on CTG interpretation and intermittent auscultation, human factors and situational awareness.
4b. Percentage of staff who have successfully completed mandatory annual competency assessment.
4c. Fetal monitoring lead roles appointed.
Outcome indicators
4d. Percentage of intrapartum stillbirths, early neonatal deaths and cases of severe brain injury* where failures of intrapartum monitoring are identified as a contributory factor.
* using the severe brain injury definition in Gale et al (2018)
Rationale
As well as reducing stillbirth rates, there is a need to reduce avoidable fetal morbidity related to brain injury causing conditions such as hypoxic-ischaemic encephalopathy and cerebral palsy. These conditions have a huge emotional and financial impact on families. They also have significant economic consequences for the health and social care system from the costs of care to support those with an avoidable brain injury throughout their lives and the litigation understandably brought by families when something goes wrong during labour.
The importance of good fetal monitoring during labour to the birth of a healthy baby is underlined by the data in the RCOG’s Each baby counts report. Fetal monitoring was identified in 74% of babies as a critical contributory factor where improvement in care may have prevented the outcome.
The report identified problems with fetal monitoring using IA, including inappropriate assignment of women to ‘low risk’ and delays in responding to abnormalities and switching to CTG monitoring when appropriate. There was also a failure to follow national guidelines about technique and frequency of IA and a failure to recognise transition between the stages of labour.
In the case of a high-risk labour where continuous monitoring is needed, CTG is the best clinical tool available for this as it is a well-established method of confirming fetal wellbeing and identification of potential fetal hypoxia. However, CTG interpretation is a high-level skill and is susceptible to variation in judgement between clinicians and by the same clinician over time. These variations can lead to inappropriate care planning and subsequently impact on perinatal outcomes.
The RCOG’s report identified failure to initiate CTG when indicated, failure to record a good quality CTG, inadequate CTG interpretation and failure to communicate the findings to senior staff in a timely manner. It recommended:
- a regular/rolling programme of training in the use of electronic fetal monitoring
- simple guidelines on the interpretation of electronic fetal monitoring
- clear lines of communication when an abnormal CTG is suspected
- guidelines on appropriate management in situations where the CTG is abnormal
Many of the findings and recommendations from the Each baby counts report are echoed in the 2017 MBRRACE-UK Perinatal Confidential Enquiry that focused on term, singleton, intrapartum stillbirth and intrapartum-related neonatal death. Recommendations that have now been incorporated into this element of the SBLCB include the use of a risk assessment tool on admission and then throughout labour to guide the nature, frequency and interpretation of fetal monitoring, as well as determining the optimal form of training and competency assessment.
In addition, both reports identify that CTG or IA monitoring cannot be used in isolation and are only part of a complex assessment of fetal wellbeing – “Failure to recognise an evolving problem, or the transition from normal to abnormal, was a common theme. It was rarely due to a single issue, more commonly appearing to arise from a more complex failure of situational awareness and ability to maintain an objective overview of a changing situation” (MBRRACE-UK Perinatal Confidential Enquiry). There is, therefore, a real need for all staff to undertake multidisciplinary training that includes situational awareness, human factors and communication. The importance of ensuring situational awareness is present in teams performing complex tasks is also highlighted in the Each baby counts report from 2015.
Implementation
Trusts should be able to demonstrate that all qualified staff who care for women in labour are competent to interpret IA (midwives) and CTG (midwives and obstetricians) in relation to the clinical situation, use the buddy system and escalate accordingly when concerns arise or risks develop. This includes staff who are brought in from other clinical areas such as the postnatal ward and the community to support a busy service, as well as locum, agency and bank staff (medical or midwifery).
Intervention 4.1: Owing to a lack of validated packages, it is not possible to be prescriptive about the exact nature of either training packages or competency assessment. However, it is recommended that trusts mandate annual human factor training for all staff working in a maternity setting. The content of human factor training must be agreed within the LMNS.
Principles for training packages are included in Appendix D. These should include psychological safety and upholding civility in the workplace, and ensuring staff are enabled to escalate clinical concerns.
Intervention 4.2: The 2017 MBRRACE-UK Perinatal Confidential Enquiry report recommended the national development of a standardised risk assessment tool. As this is not yet available the procedure should comply with fetal monitoring guidelines.
Intervention 4.3: The principle underlying this intervention is that fetal wellbeing is assessed regularly (at least hourly) during labour and documented using a structured proforma. This review should be more than recording the fetal heart rate using IA or categorisation of the CTG (Appendix D).
Intervention 4.4: A discussion between the midwife caring for the woman and another midwife or doctor should occur at least 4 hourly when undertaking IA and at least hourly when using CTG monitoring. The discussion should include the fetal heart rate (IA or CTG), review of antenatal risk factors such as concurrent reduced fetal movements, fetal growth restriction, previous caesarean section and intrapartum risk factors such as meconium, suspected infection, vaginal bleeding or prolonged labour, and should lead to escalation if indicated (Appendix D).
Intervention 4.5: Some trusts may choose to extend the remit of the practice development midwife to fulfil the role of fetal monitoring lead, whereas others may wish to appoint a separate clinician. The critical principle is that the fetal monitoring leads have dedicated time within their remit to support staff working in intrapartum care to provide high quality intrapartum risk assessments and accurate fetal heart rate interpretation using either IA or CTG. The role should contribute to building and sustaining a safety culture in intrapartum care with all staff committed to continuous improvement.
Element 5: Reducing preterm births and optimising perinatal care
Element description
Reducing the number of preterm births and optimising perinatal care when preterm birth cannot be prevented.
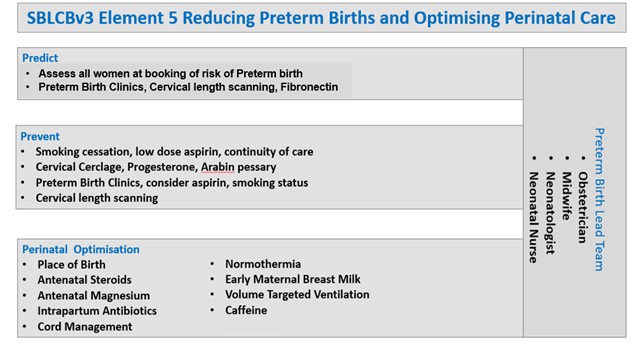
Element description
Preterm birth lead team
- obstetrician
- midwife
- neonatologist
- neonatal nurse
Predict
- assess all women at booking of risk of preterm birth
- preterm birth clinics, cervical length scanning, fibronectin
Prevent
- smoking cessation, low dose aspirin, continuity of care
- cervical cerclage, progesterone, arabin pessary
- preterm birth clinics, consider aspirin, smoking status
- cervical length scanning
Perinatal optimisation
- place of birth
- antenatal steroids
- antenatal magnesium
- intrapartum antibiotics
- cord management
- normothermia
- early maternal breast milk
- volume targeted ventilation
- caffeine
Interventions
Preterm birth lead team
5.1 Each provider trust should have:
a. an obstetric consultant lead for preterm birth, delivering care through a specific preterm birth clinic or within an existing fetal medicine service
b. an identified local preterm birth / perinatal optimisation midwife lead
c. a neonatal consultant lead for preterm perinatal optimisation
d. an identified neonatal nursing lead for preterm perinatal optimisation
5.2 Each preterm birth lead team should have clear audit and quality improvement (QI) pathways for preterm birth prevention, prediction and perinatal optimisation, and should engage in shared learning and QI with local preterm birth clinical networks, LMNSs and neonatal operational delivery networks.
Prediction
5.3 Assessment of all women at booking for their risk of preterm birth and stratification to low, intermediate and high-risk pathways using the criteria in Appendix E. It is recognised that there are imperfections in the predictability of preterm birth on the basis of history; the use of digital algorithms and tools (for example, the Tommy’s app) may also be useful to support assessment.
5.4 In the assessment of women presenting in suspected preterm labour, evaluated digital tools are now available (QUIDS, QUiPP) to improve predictive accuracy of triage and enable collaborative decision-making.
5.5 Networked trusts should agree on the use of these tools within their ICS/LMNS.
5.6 Multiple pregnancy – risk assessment and management in multiple pregnancy should comply with NICE guidance or a variant that has been agreed with the local ICS following advice from the provider’s regional maternity team.
Prevention
All women:
5.7 Assess smoking status (see Element 1) and implement appropriate intervention to ensure the pregnancy is smokefree before 15 weeks.
5.8 Assess all women at booking to determine if a prescription of aspirin is appropriate using the algorithm in Appendix B or an alternative that has been agreed with the LMNS or ICS following advice from the provider’s clinical network.
5.9 Symptomatic women require assessment and use of decision-assist tools such as the QUiPP and QUIDS apps. Due to the recent discontinuation of fetal fibronectin (FFN) cassettes, interim clinical adjustments are in place. Please follow this link for the latest guidance while we await further national steer on point of care testing. Transvaginal cervix scanning (TVCS) may also be used with or without quantitative FFN. Further advice may be sought from UK Preterm Clinical Network, BAPM or NICE Clinical guideline 55.
Women at intermediate or high risk:
5.10 Assess each woman with a history of preterm birth to determine whether this was associated with placental disease and discuss prescribing aspirin with her.
5.11 Test for asymptomatic bacteriuria in women who are assessed as being intermediate or high risk of preterm birth (see Appendix E) by sending off a midstream urine (MSU) sample for culture and sensitivity at booking. Following any positive culture and treatment, a repeat MSU to confirm clearance is recommended.
5.12 Asymptomatic women should have access to TVCS to assess the need for further interventions such as cervical cerclage and progesterone supplementation (Appendix E).
5.13 Every provider should have referral pathways to tertiary prevention clinics for the management of women with complex obstetric and medical histories. This should include access to clinicians with the expertise to provide high vaginal (Shirodkar) and transabdominal cerclage. These procedures are performed relatively infrequently and therefore to maintain expertise are best provided on a supra-regional basis.
Perinatal optimisation
5.14 Women identified to be at increased risk of preterm birth should be made aware of the signs / symptoms of preterm labour and encouraged to attend their local maternity unit early if these occur.
5.15 Ensure the neonatal team are involved when a preterm birth is anticipated, so that there is time to meet as a perinatal team to discuss care options with parents prior to birth. This is especially important at earlier gestational ages.
In the case of extreme prematurity where complex decision-making is required (active survival focused care or comfort care), management should be as outlined in the 2019 BAPM Framework for practice regarding perinatal management of extreme preterm birth before 27 weeks’ gestation: “Conversations with parents should be clearly documented and care taken to ensure that the agreed management plan is communicated between perinatal professionals and staff shifts. Decisions and management should be regularly reviewed before and after birth in conjunction with the parents; plans may be reconsidered if the risk for the fetus / baby changes, or if parental wishes change.”
5.16 Women identified to be potentially at increased risk of imminent preterm birth, where active survival focused care is planned, should be made aware of the optimisation interventions that may be offered. Families should also be offered information and support for families from charities such as Bliss.
5.17 Acute tocolysis may be used when short-term delay is desirable – that is, in-utero transfer and probably to ensure adequate antenatal exposure to corticosteroid / magnesium sulphate (that is, no longer than 48 hours). There is no evidence that maintenance tocolysis is beneficial when compared with no tocolysis treatment, oxytocin antagonist and calcium channel blockers appear effective in delaying birth for >48 hours. In the absence of any contraindications nifedipine is the preferred agent for tocolysis.
5.18 Place of birth – babies who are:
a. born at <27 weeks’ gestation
b. <800 g at birth
c. born as a multiple at <28 weeks’ gestation
should be birthed in a maternity service on the same site as a designated neonatal intensive care unit (NICU).
Maternity services must operate in close perinatal collaboration with neonatal networks to ensure that babies predicted to require a higher level of neonatal care than can be provided in the local delivery unit are moved in utero whenever possible. BAPM Antenatal optimisation toolkit
5.19 Antenatal corticosteroids should be offered to women who birth a baby between 22 and 33 weeks’ (22+0 to 33+6) gestation within 1 week prior to delivery. A steroid-to-birth interval of >7 days should be avoided if possible and repeat courses of steroids should be avoided where possible. BAPM Antenatal optimisation toolkit
5.20 Magnesium sulphate to be offered to women who are anticipated to give birth below 30 weeks’ gestation in the 24 hours prior to delivery. This should also be considered for women between 30+0 and 33+6 weeks of pregnancy who are in established labour or are having a planned preterm birth within 24 hours. BAPM Antenatal optimisation toolkit
5.21 Intrapartum antibiotics: All women in preterm labour at <37 weeks’ gestation should be offered intravenous intrapartum antibiotic prophylaxis (benzylpenicillin, where not contraindicated) to prevent early-onset neonatal Group B streptococcal infection irrespective of whether they have ruptured amniotic membranes. A discussion of risk and benefits should be clearly documented. This excludes planned caesarean births without labour. Note: This intervention should be considered up to 36+6 weeks’ gestation.
5.22 Cord management: Babies born at <37 weeks’ gestation should have their umbilical cord clamped at or after 1 minute after birth – this can have benefits for all babies. Perinatal multidisciplinary teams should work together to ensure this can reliably be delivered at all births. BAPM Optimal cord management toolkit
5.23 Normothermia: Babies born at <37 weeks’ gestation should have a first temperature on admission that is both between 36.5 and 37.5°C and measured within 1 hour of birth. Neonatal normothermia can have benefits for all babies. BAPM Normothermia toolkit
5.24 Early maternal breast milk (MBM): Babies born at <34 weeks’ gestation should receive their own mother’s milk, ideally within 6 hours, but aiming always within the first 2 days of life (except in rare situations where there are contraindications to MBM). Perinatal teams should work together to ensure consistent delivery of antenatal advice about MBM, with support (equipment, education, help) for mothers to express within 2 hours of birth. BAPM Maternal breast milk toolkit
5.25 Volume targeted ventilation (VTV): For babies born at <34 weeks’ gestation who need invasive ventilation, VTV should be used in combination with synchronised ventilation as the primary mode of respiratory support. This reduces the chance of death or bronchopulmonary dysplasia (BPD) by 27% and intraventricular haemorrhage (grades 3–4) by 47% compared with pressure-limited ventilation modes.
Note: For preterm babies who do not need invasive ventilation, consider nasal CPAP or nasal high-flow therapy as the primary mode of respiratory support.
- NICE Quality Standard [QS193] Specialist neonatal respiratory care for babies born preterm
- GIRFT Neonatology
- BAPM BPD toolkit
5.26 Caffeine: For babies born at <30 weeks’ gestation, caffeine reduces the chance of death or disability. Caffeine citrate should be started within the first 3 days of life. NICE guideline [NG124] Specialist neonatal respiratory care for babies born preterm
Continuous learning and improvement
5.27 All providers are encouraged to draw on the learning from the 4 BAPM toolkits and a range of resources from other successful regional current programmes (for example, PERIPrem resources, MCQIC).
- BAPM Perinatal optimisation pathway
- NHS England Maternity and Neonatal Safety Improvement Plan
- Healthcare Improvement Scotland Scottish Patient Safety Programme (SPSP) Perinatal Programme
- Health Innovation West England PERIPrem
- NHS Wales PERIPrem Cymru for professionals
5.28 Maternity and neonatal care providers should determine and act on all themes related to preterm birth that are identified from investigation of incidents, perinatal reviews and examples of excellence, focusing particularly on prediction, prevention, preparation and perinatal optimisation, including:
a. risk assessment of women in their first pregnancy for the risk of preterm birth and timely triage to the appropriate care pathway
b. management of women at high risk of preterm birth, including appropriate cervical length surveillance and use of cervical cerclage
c. implementation of optimisation interventions as a whole preterm perinatal optimisation pathway, including measurement and reporting of overall optimisation pathway compliance
5.29 Maternity and neonatal care providers should demonstrate continuing improvement by regular reassessment of the process and outcome indicators below. These data can be accessed through a number of national and network-level data sources including the National Neonatal Audit Programme (NNAP) and neonatal operational delivery network data. Data completeness via electronic maternity and neonatal record systems is vitally important and data quality should be monitored frequently. Trusts should seek to support data quality assurance, including support for data clerk or data manager time.
5.30 Benchmarking: Maternity and neonatal care providers should examine their process and outcome indicators in relation to similar trusts to understand variation and inform potential improvements.
5.31 Sharing learning and improvement: The preterm birth teams (see 5.1) within each maternity and neonatal care provider setting should:
a. review and share their process and outcome indicator data across the perinatal team on a regular basis (at least quarterly) to drive continual improvement
b. share process and outcome indicator data and evidence of improvement with their maternity and neonatal board-level safety champions, LMNS and ICS quality surveillance teams on a quarterly basis
Process indicators
5a. Percentage of singleton infants <27 weeks’ gestation, multiples <28 weeks’ gestation or any gestation with an estimated fetal weight of <800 g, born in a maternity service on the same site as a neonatal intensive care unit (NICU).
5b. Percentage of babies born between 22 and 33 weeks’ gestation (22+0 to 33+6) who receive a full course of antenatal corticosteroids within 1 week of birth.
5c. Percentage of babies born before 30 weeks’ gestation who receive magnesium sulphate within the 24 hours prior to birth.
5d. Percentage of babies born at <34 weeks’ gestation who have their umbilical cord clamped at or after 1 minute after birth.
5e. Percentage of babies born at <34 weeks’ gestation who have a first temperature on admission which is both between 36.5–37.5°C and measured within 1 hour of birth.
5f. Percentage of babies born at <34 weeks’ gestation who receive their own mother’s milk in the first 2 days of life.
Note: To minimise the need for local data collection to support these improvements, the formal collection of process measure data can be restricted to the 6 interventions listed in this section. The use of VTV for preterm babies who require invasive ventilation and the use of early caffeine for babies born at <30 weeks’ gestation are both recommended. While some regions collect data on these interventions, this is not universal and not currently part of the NNAP optimal perinatal care metric. In addition, the gestational limits for some of the indicators and/or the groups studies have been adjusted to align with current nationally collected data (for example, data on babies born only at <34 weeks’ gestation or data on the number of babies receiving antenatal corticosteroids rather than the number of mothers).
Outcome indicators
5g. Mortality to discharge in very preterm babies (NNAP definition): Percentage of babies born below 32 weeks’ gestation who die before discharge home or 44 weeks post-menstrual age (whichever occurs sooner).
5h. Preterm brain injury (NNAP definition): Percentage of babies born at <32 weeks’ gestation with any of the following forms of brain injury:
- intraventricular haemorrhage grade 3 or 4
- cystic periventricular leukomalacia
5i. Percentage of perinatal mortality cases annually (using PMRT for analysis) where the prevention, prediction, preparation or perinatal optimisation of preterm birth was a relevant issue.
5j. Maternity care providers will provide outcome data to the trust board and share this with the LMNS relating to the incidence of women with a singleton pregnancy giving birth (liveborn and stillborn) as a % of all singleton births:
- in the late second trimester (from 16+0 to 23+6 weeks)
- preterm (from 24+0 to 36+6 weeks)
Rationale
Preterm birth, defined as birth at <37+0 weeks’ gestation, is a common complication of pregnancy, comprising around 8% of births in England and Wales. Prematurity is the most significant cause of mortality in children under 5 and is associated with significant morbidity in surviving infants. 69% of infants who die before their 1st birthday are born premature (NCMD data 1 April 2019 to 31 March 2020). Preterm birth is estimated to cost health services in England and Wales £3.4 billion per year.
In Safer Maternity Care: The National Maternity Safety Strategy – progress and next steps the government made it clear that “we will not achieve the national maternity safety ambition (to halve the rates of stillbirths, neonatal and brain injuries that occur during or soon after birth by 2030) unless the rate of preterm births is reduced” and set an additional ambition to reduce the national rate of preterm births from 8% to 6%. The current scope of NICE preterm guidelines is limited principally to acute presentation, and this document specifies those at-risk populations who should be targeted for additional referral and management to meet this ambition. It is anticipated that the rapidly expanding evidence base in this field will contribute to these evolving guidelines, and the open access UK Preterm Clinical Network guidance will be updated periodically.
There are evidence-based perinatal interventions to reduce the risk of preterm mortality or serious brain injury. Perinatal optimisation refers to the process of reliably delivering these evidence-based interventions in the antenatal, intrapartum and neonatal period to improve preterm outcomes.
UK audit data shows that the uptake of available evidence-based interventions to reduce the risk of preterm mortality or serious injury is widely variable between units and networks. The British Association of Perinatal Medicine has released a series of resources to support implementation of the perinatal optimisation pathway.
Implementation
All the elements within SBLCBv3 address iatrogenic preterm and early-term birth, recognising the need to ensure that any decision for birth is based on evidence of maternal and/or fetal compromise. This element focuses on reducing spontaneous preterm birth through prediction, prevention and preparation. This will need to be done in the context of a strong perinatal team including neonatology, obstetrics and midwifery (see BAPM Building successful perinatal optimisation teams).
The preterm birth lead team (see 5.1) should provide leadership and oversight of the implementation of Element 5.
Providers should have provision for care of women at risk of preterm birth, ideally within a preterm birth prevention clinic with midwifery support and access to risk assessment tests, including transvaginal cervix scanning and quantitative fetal fibronectin (qFFN), and potential interventions – for example, cervical cerclage, pessary and progesterone. Where preterm birth prevention clinics are not available, providers should ensure that women can access care that guarantees that they are given evidence-based information, access to risk assessment tests and interventions as appropriate and can actively participate in decisions regarding their management.
Note: NHS England and DHSC have been alerted to a supply discontinuation of hologic FFN cassettes used as a preterm birth marker at point of care. Interim clinical adjustments are in place. Where services are required to provide evidence of local guidelines on the use of qFFN tests, compliance can be achieved if those guidelines acknowledge these clinical adjustments alongside business as usual best practice. Baselines and trajectories for the use of qFFN tests should be agreed with LMNS/ICBs in view of the current supply shortages and the clinical adjustments.
Providers should have access to supra-regional prevention services within their care pathways and networks, including access to high vaginal and transabdominal cerclage.
Further guidance regarding the implementation of this element and the care of women and their babies at risk of preterm birth can be found at:
- BAPM Perinatal optimisation pathway
- NHS England Maternity and Neonatal Safety Improvement Plan
- Healthcare Improvement Scotland Scottish Patient Safety Programme (SPSP) Perinatal Programme
- Health Innovation West England PERIPrem
- NHS Wales PERIPrem Cymru for professionals
- BAPM Perinatal management of extreme preterm birth before 27 weeks of gestation
- NICE guideline [NG25] Preterm labour and birth
- NICE Diagnostics guidance [DG33] Biomarker tests to help diagnose preterm labour in women with intact membranes
- NHS England Clinical adjustments for reduced supply of fetal fibronectin cassettes
- Ockenden review: summary of findings, conclusions and essential actions, 2022
- UK Preterm Clinical Network Reducing preterm birth: Guidelines for commissioners and providers, 2019
Appendix E includes a suggested risk assessment and management algorithm that providers may wish to adopt.
Element 6: Management of pre-existing diabetes in pregnancy
Rationale
Women with Type 1 and Type 2 diabetes have persistently high perinatal mortality with no improvement over the past 5 years. Contemporary annual data from the mandatory National Pregnancy in Diabetes in England and Wales shows that perinatal loss in diabetes is 4–5 times higher than the background population. In women with Type 1 diabetes, stillbirth occurs in 10·4 per 1,000 births, with neonatal death occurring in 7·4 per 1,000 livebirths; In women with Type 2 diabetes it is even higher – stillbirth occurs in 13·5 per 1,000 births and with neonatal death occurring in 11·2 per 1,000 livebirths. The risk of perinatal mortality is highest in women who are the most socioeconomically deprived (increases 2-fold) and those who have suboptimal glucose control in the 3rd trimester (increases 3-fold). As women with diabetes are more likely to be socioeconomically deprived and of South Asian or Black ethnicity than pregnant women without diabetes, there is an urgent need to address these inequalities.
Introducing management of diabetes into the SBLCB allows us to do this in 2 key ways:
- ensuring there are standard pathways of care for multidisciplinary team (MDT) management of these women throughout pregnancy, with increased access to expert and ‘joined-up’ support for their complex care needs
- improving management of glucose control during pregnancy by focusing support on high-risk women who are not achieving safe pregnancy glycaemic targets and by ensuring consistent and high levels of uptake of digital glucose monitoring technology to facilitate this
The final Ockenden report into the Shrewsbury and Telford Hospital NHS Foundation Trust has highlighted the need for continuity of experienced staff within diabetes in pregnancy teams to reduce poor outcomes in women with diabetes.
The 2022 MBRRACE report has highlighted the very high risk of fetal death (stillbirth rate 160 per 1,000 births) associated with diabetic ketoacidosis (DKA).
In version 3.2 of the SBLCB, Element 6 has been updated to reflect NHS England’s 5-year implementation strategy for hybrid closed loop technologies, published in January 2024. Pregnant women with Type 1 diabetes are identified as a priority population group for rollout.
Element description
Providing multidisciplinary care in a joined-up way for women with Type 1 and Type 2 diabetes during pregnancy and offering hybrid closed loop (HCL) technology to women with Type 1 diabetes to reduce maternal complications of diabetes, including perinatal morbidity and mortality.
Interventions
6.1 Women with a diagnosis of pre-existing diabetes in pregnancy should be offered care in a one-stop clinic, providing care to pre-existing diabetes only, which routinely offers multidisciplinary review and has the resource and skill set to address all antenatal care requirements. The multidisciplinary team (MDT) should consist as a minimum of: obstetric consultant, diabetes consultant, diabetes specialist nurse, diabetes dietitian, diabetes midwife.
6.2 Women with Type 1 diabetes should be offered a pregnancy-specific HCL system (see below for definition) and be provided with appropriate education and support to use this.
6.3 Women with Type 2 diabetes should have an objective record of their blood glucose recorded in their hospital records / electronic patient record and be offered alternatives – for example, continuous glucose monitoring (CGM) – if they are not monitoring and/or HbA1c glycaemic targets are not achieved.
6.4 Women with diabetes should have an HbA1c measured at the start of the 3rd trimester. Those with an HbA1c >48 mmol/mmol should be offered increased surveillance including additional diabetes nurse / dietetic support, more frequent face-to-face review and input from their named specialist consultant to plan ongoing care and timing of birth decisions.
| Status | HbA1c Level | Action |
|---|---|---|
| Green | ≤HbA1c 43 mmol/mol | Continue current care |
| Amber | HbA1c 44–48 mmol/mol | Consider additional input to improve glucose management |
| Red | HbA1c >48 mmol/mol | MDT discussion required Offer additional input to improve glucose management including alternative methods of monitoring treatment Offer increased fetal surveillance, and re-discuss increased risk of stillbirth, birth and neonatal complications |
6.5 Providers should agree with their maternal medicine network a pathway for the management of women with diabetes and retinopathy requiring treatment during pregnancy and/or kidney impairment (CKD 2 with significant proteinuria – that is, PCR >30; or CKD 3 or more). For the majority of providers, these cases should be managed in a regional maternal medicine centre, where care can be delivered in a single MDT clinic. In some cases, it may be agreed following an MDT review that sufficient local expertise is in place for local management. However, should they develop worsening renal impairment – rising creatinine or nephrotic range proteinuria (PCR >300), these cases should be referred back for review at the maternal medicine centre.
6.6 Recognising the very high risk of fetal death (stillbirth rate 160 per 1,000 births) associated with diabetic ketoacidosis (DKA), all pregnant women presenting to secondary care with DKA should have ongoing multidisciplinary consultant input and be cared for in line with the jointly agreed trust policy.
Continuous learning
6.7 Maternity care providers involved in the care of women with Type 1 and Type 2 diabetes should examine their outcomes in relation to all themes related to these women. These include risk assessment and management in the antenatal and intrapartum period.
6.8 Maternity care providers that look after women with Type 1 and Type 2 diabetes in pregnancy should submit data to the National Pregnancy in Diabetes (NPID) Audit, review their submissions and develop an action plan to address ongoing challenges.
6.9 Individual trusts should examine their outcomes in relation to other trusts caring for women in pregnancy with Type 1 and Type 2 diabetes and engage with wider regional and national diabetes clinical networks to share examples of good practice and work collaboratively to address challenges.
6.10 Individual trusts should actively gather feedback from service users about their care, and co-produce guidance and proposed care pathways with maternity and neonatal voices partnership (MNVP) members with ‘lived experience’.
6.11 All cases of perinatal death in women with diabetes, or where diabetes is considered to be a possible contributory factor, should be reviewed by a MDT that includes members with expertise in the care of women with diabetes in pregnancy. Learning from these case reviews should be disseminated as appropriate and an action plan developed to reduce the risk of recurrence.
6.12 Any pregnancies where HCL, CGM or HbA1c was not offered in line with the interventions above should be subject to case review to determine service-level issues which could be addressed.
Process indicators
6a. Demonstrate an agreed pathway for women to be managed in a clinic, providing care to women with pre-existing diabetes only, where usual care involves joined-up multidisciplinary review (The core MDT should consist of obstetric consultant, diabetes consultant, diabetes specialist nurse, diabetes dietitian and, diabetes midwife) and holistic pregnancy care planning – this should be a one-stop clinic where possible and include a pathway for the provision / access to additional support (for example, asylum support, psychology, mental health) either within the clinic or within a closely integrated service (with shared documentation, etc).
6b. Demonstrate a pathway for management for women with complex diabetes that has been agreed with the maternal medicine network.
6c. Demonstrate an agreed method of objectively recording blood glucose levels and achievement of glycaemic targets.
6d. Demonstrate appropriate expertise to onboard and manage pregnant women using pregnancy-specific HCL.
6e. Demonstrate an agreed pathway (between maternity services, emergency departments and acute medicine) for the management of women presenting with DKA during pregnancy. This should include a clear escalation pathway for specialist obstetric HDU or ITU input, with the agreed place of care depending on patients gestational age, DKA severity, local facilities and availability of expertise.
Outcome indicators
6f. Percentage of women with Type 1 diabetes who were offered a pregnancy-specific HCL system during pregnancy (aiming for >60% of women in 2025/26 and >95% from 2026/27).
6g. Percentage of women with Type 1 and Type 2 diabetes who have had an HbA1c measured at the start of the 3rd trimester (aiming for >95% of women).
Compliance data for both outcome indicators should be reported by ethnicity and deprivation to ensure focus on at-risk and under-represented groups.
Implementation
Reducing perinatal mortality in pregnancies complicated by diabetes
This element provides a practical approach to reducing perinatal mortality in pregnancy affected by Type 1 or Type 2 diabetes, by implementing MDT pathways and an intensified focus on glucose management within maternity settings in line with the NHS Long Term Plan and NICE guidance. It focuses on demonstrating clear multidisciplinary pathways to provide a dedicated, integrated service for addressing complex needs (and thereby mitigate risk of poor pregnancy outcome).
Furthermore, as glucose is the most significant modifiable risk factor for poor outcome in pregnancies complicated by diabetes, the element includes: clear documentation of assessing glucose control digitally; using HbA1c to risk stratify and provide additional support / surveillance (NPID Audit data); and offering consistent access to an evidence-based pregnancy-specific HCL system to improve glucose control or, if this is declined, CGM (NICE and NHS plan).
What is a pregnancy-specific hybrid closed loop (HCL) system?
An HCL system is a device that combines a continuous glucose monitor, an insulin pump and a computer programme / algorithm (which is provided via access to an app) to automatically adjust insulin delivery for people.
In developing the Technology Appraisal [TA943], NICE reviewed evidence from the AiDAPT trial. The trial results support the importance of the HCL system used in pregnancy having:
- a licence for use in pregnancy
- a glucose target of ≤5 mmol/L
- evidence of a clinically relevant improvement in maternal glucose outcomes (>5% increased time in the pregnancy glucose target range of 3.5–7.8 mmol/L compared to standard care with CGM and standard insulin delivery by multiple daily injections / pump).
Demonstrating appropriate expertise for the use of pregnancy-specific HCL
For the purposes of process measure 6d, this means ensuring:
- there is at least 1 named HCL diabetes consultant and at least 1 named HCL diabetes specialist nurse within the antenatal clinic in each maternity service
- the HCL diabetes consultant and the HCL diabetes specialist nurse are competent (as assessed by the hospital trust) to onboard and manage women using pregnancy-specific HCL
- plans are in place to ensure that all healthcare professionals involved in the onboarding and/or management of women using pregnancy-specific HCL are competent to do so
Funding to support the 5-year implementation strategy for HCL systems
NHS England’s 5-year HCL implementation strategy made a commitment to contribute towards 75% of the incremental (additional) costs ICBs incur in delivering the requirements set out in NICE TA943.
The majority, £13.1 million, will be allocated in year 1 through retrospective activity-based payments, based on the number of new patients meeting the NICE criteria who are onboarded to HCL. The remaining £1 million takes the form of a pregnancy-specific allocation that was transferred to ICBs in September 2024 to cover the costs of switching pregnant people who are already benefitting from insulin pump therapy to a pump that is compatible with a pregnancy-specific HCL.
For years 2–5 of the programme, national per annum funding has been provisionally set, increasing in line with modelled scaling up of provision as well as the impact of distributing funding contribution per patient over 4 years.
Further detail of the reimbursement approach and the funding available in pregnancy for different scenarios is available in the reimbursement guidance for ICBs on the FutureNHS platform.
Appendix A: Acknowledgments
NHS England would like to thank the following contributors to the development of the elements of this Saving Babies’ Lives Care Bundle:
Saving Babies’ Lives Care Bundle Steering Group
- Matthew Jolly – Former National Clinical Director, NHS England and University Hospitals Sussex NHS Foundation Trust
- Tony Kelly – NHS England
- Donald Peebles – University College London and NHS England
- Susanna Crowe – NHS England
- Gordon Smith – University of Cambridge
- Katie Morris – British Maternal and Fetal Medicine Society and University of Birmingham
- Misha Moore – The Royal London Hospital and NHS England
- Jo Locker – Office of Health Improvement and Disparities
- Martyn Willmore – Office of Health Improvement and Disparities
- Julia Robson – Office of Health Improvement and Disparities
- Alex Heazell – University of Manchester
- Tim Draycott – Royal College of Obstetricians
- Nigel Simpson – University of Leeds and Leeds Teaching Hospital NHS Trust
- Sarah Bates – BAPM (British Association Perinatal Medicine) and Great Western Hospital, Swindon
- Dilly OC Anumba – University of Sheffield
- Basky Thilaganathan – Royal College of Obstetricians and Gynaecologists and St George’s, University of London
- Chris Binnie – Service user representative
- Jane Sandall – NHS England
- Charlie Podschies – NHS England
- Karen Thirsk – NHS England
- Sarah Winfield – The Mid-Yorkshire NHS Trust
- Jenny Myers – University of Manchester
- Eleanor Scott – University of Leeds and Leeds Teaching Hospital NHS Trust
- Helen Murphy – University of East Anglia, Norwich, UK
- Rachel Vollans – NHS England
- Alanna Parker – NHS England
Element 1: Reducing smoking in pregnancy
- Jo Locker (Lead) – Office of Health Improvement and Disparities
- Martyn Willmore – Office of Health Improvement and Disparities
- Julia Robson – Office of Health Improvement and Disparities
- Matthew Jolly – Former National Clinical Director, NHS England and University Hospitals Sussex NHS Foundation Trust
- Misha Moore (Lead) – The Royal London Hospital and NHS England
- Paul Cilia La Corte (Lead) – NHS England
- Jayne Coyne – NHS England
- Hannah Ellison – NHS England
- Karen Thirsk – NHS England
Element 2: Risk assessment, prevention, and surveillance of pregnancies at risk of fetal growth restriction
- Ed Johnstone (lead) – University of Manchester and Manchester Academic Health Science Centre
- Matthew Jolly – Former National Clinical Director, NHS England and University Hospitals Sussex NHS Foundation Trust
- Katie Morris – British Maternal and Fetal Medicine Society and University of Birmingham
- Donald Peebles – University College London and NHS England
- Gordon Smith – University of Cambridge
- Jane Sandall – NHS England
- Basky Thilaganathan – Royal College of Obstetricians and Gynaecologists
- Dilly OC Anumba – University of Sheffield
Element 3: Raising awareness of reduced fetal movement
- Alex Heazell (lead) – University of Manchester
- Charlotte Bevan – Sands
- Jane Brewin – Tommy’s
- Anita Dougall – Royal College of Obstetricians and Gynaecologists
- Hannah Hague – Cheshire and Merseyside Strategic Clinical Network
- Elizabeth Hutton – Kicks Count
- Matthew Jolly – Former National Clinical Director, NHS England and University Hospitals Sussex NHS Foundation Trust
- Tony Kelly – NHS England
- Katie Morris – British Society of Maternal and Fetal Medicine
- Jane Munro – Royal College of Midwives
- Donald Peebles – University College London and NHS England
- Devender Roberts – Liverpool Women’s Hospital
- Gordon Smith – University of Cambridge
- Cara Taylor – Central Manchester University NHS Foundation Trust
- Kate Wybrow – King’s College Hospital
- Basky Thilaganathan – Royal College of Obstetricians and Gynaecologists
- Jane Sandall – NHS England
Element 4: Effective fetal monitoring during labour
- Donald Peebles (lead) – University College London and NHS England
- Matthew Jolly – Former National Clinical Director, NHS England and University Hospitals Sussex NHS Foundation Trust
- Tony Kelly – NHS England
- Katie Morris – British Maternal and Fetal Medicine Society
- Gordon Smith – University of Cambridge
- Wendy Randall – West Hertfordshire Hospitals NHS Trust
Element 5: Reducing preterm births
- Anna David – University College London
- Dr Elizabeth Bonney – Leeds Teaching Hospital NHS Trust
- Devender Roberts – Liverpool Women’s Hospital
- Katherine Simpson – Great Western Hospital, Swindon
- Sarah Bates (Lead) – BAPM (British Association Perinatal Medicine) and Great Western Hospital, Swindon
- Andrew Shennan – King’s College London
- Nigel Simpson (Lead) – University of Leeds and Leeds Teaching Hospital NHS Trust
- Matthew Jolly – Former National Clinical Director, NHS England and University Hospitals Sussex NHS Foundation Trust
- Tony Kelly – NHS England
- Katie Morris – British Maternal and Fetal Medicine Society
- Element 6: Management of diabetes in pregnancy
- Sarah Winfield (Lead) – The Mid-Yorkshire NHS Trust
- Jenny Myers (Lead) – University of Manchester
- Eleanor Scott (Lead) – University of Leeds and Leeds Teaching Hospital NHS Trust
- Helen Murphy (Lead) – University of East Anglia, Norwich, UK
- Matthew Jolly – Former National Clinical Director, NHS England and University Hospitals Sussex NHS Foundation Trust
With additional thanks to the Maternity and Neonatal Programme Stakeholder Council and the maternity clinical networks for their comments and feedback.
This document was produced by the NHS England National team, with particular thanks to Karen Thirsk, Rachel Vollans, Charlie Podschies and Alanna Parker.
Appendix B: Medication to reduce the risk of pregnancy complications
All women should take a daily supplement of 400 micrograms (400 µg) folic acid before conception and until the 12th week of pregnancy (some women will require a higher dose as advised by a healthcare professional). Women (and all adults, including breastfeeding women) are also recommended to take 10 µg vitamin D a day.
Elements 2 and 5 of the SBLCB include the assessment of pregnant women for treatment with aspirin. NICE recommends aspirin* to reduce the risk of pregnancy complications related to placental dysfunction, particularly pre-eclampsia. Thus, it is important to take a full history from pregnant women who have had a previous baby with fetal growth restriction (FGR) and/or a preterm birth to determine whether placental dysfunction was a contributory factor.
Aspirin as a preventative medication appears to be safe in pregnancy and therefore there is a substantial net benefit of daily aspirin use to reduce the risk for pre-eclampsia and associated preterm birth. Aspirin is therefore recommended from the first to the 3rd trimester of pregnancy in women, following risk assessment at their pregnancy booking visit.
* Although this use is common in UK clinical practice, at the time of publication, aspirin did not have a UK marketing authorisation for this indication. Community pharmacies cannot legally sell aspirin as a Pharmacy Only Medicine for prevention of pre-eclampsia in pregnancy in England. Aspirin for this indication must be prescribed. The prescriber should see the Summary of Product Characteristics for the manufacturer’s advice on use in pregnancy. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council’s Prescribing guidance: prescribing unlicensed medicines for further information.
Dosage
There is evidence from randomised controlled trials that the dose of aspirin should be 150 mg from 12 weeks’ gestation and may be more effective if taken at night. In some circumstances this may not be appropriate and lower doses (60–75 mg) may be used (for example, in pregnant women with hepatic or renal disease). Predictive algorithms that combine a variety of risk factors to identify pregnant women at risk for pre-eclampsia are available. Providers should use an algorithm such as the one based on the NICE pregnancy hypertension guideline shown in Table 1. Any other algorithm must be agreed with local commissioners (ICBs) following advice from the provider’s clinical network.
Table 1: Clinical risk assessment for pre-eclampsia as indications for aspirin in pregnancy
Risk level: High
Risk factors:
- hypertensive disease during a previous pregnancy
- chronic kidney disease
- autoimmune disease such as systemic lupus erythematosus or antiphospholipid syndrome
- Type 1 or Type 2 diabetes
- chronic hypertension
- placental histology confirming placental dysfunction in a previous pregnancy
Recommendation: Recommend low dosage aspirin if the woman has 1 or more of these high-risk factors
Risk level: Moderate
Risk factors:
- nulliparity
- 40 years or older at booking
- pregnancy interval >10 years
- body mass index (BMI) ≥35 kg/m² at first visit
- family history of pre-eclampsia in a first-degree relative
- multiple pregnancy
Recommendation: Consider aspirin if the woman has 2 or more moderate risk factors
There are a few absolute contraindications to aspirin therapy. Pregnant women with a history of aspirin allergy (for example, urticaria) or hypersensitivity to other salicylates are at risk of anaphylaxis and should not receive aspirin. As there is significant cross-sensitivity between aspirin and other non-steroidal drugs (NSAIDs), aspirin is contraindicated in pregnant women with known hypersensitivity to NSAIDs.
Relative contraindications to aspirin include a history of gastrointestinal bleeding, active peptic ulcer disease, other sources of gastrointestinal or genitourinary bleeding, and severe hepatic dysfunction. The decision to continue aspirin in the presence of obstetric bleeding or risk factors for obstetric bleeding should be considered on a case-by-case basis.
Appendix C: Risk assessment, surveillance pathway and management of fetal growth restriction
This appendix describes a risk assessment and surveillance pathway for pregnant women at increased risk of fetal growth restriction (FGR) and a management pathway when a fetus has been found to be growth restricted, recognising that prior to 34 weeks’ gestation this will require input from fetal medicine services. It has been designed to optimise effectiveness and minimise the scan burden on providers and recognise the potential harm caused by increased intervention in infants at only marginally increased risk of stillbirth. Trusts may wish to follow other pathways, but these should be agreed with their local ICSs and for, some deviations specified in the guidance, the regional maternity team.
Definition of FGR within SBLCBv3
FGR is difficult to diagnose representing those fetuses that have failed to reach their growth potential. A Delphi consensus-based definition has been used in research for both early (defined in the Delphi consensus as <32 weeks) and late-onset FGR, but has not yet been shown to help improve outcomes through intervention. Diagnosing FGR in a current pregnancy and risk assessing whether FGR existed in a previous pregnancy also present different challenge.
The following definitions are suggested to address these challenges and remain practical for most providers. Absent or reversed end-diastolic flow in the umbilical artery is a feature of early-onset FGR but, importantly, even in the absence of this feature (for example, a normal umbilical artery Doppler) after 32 weeks’ gestation does not exclude growth restricted or fetal compromise.
Definition of FGR in a previous pregnancy as a risk factor: defined as any of the following:
- birthweight <3rd centile
- early-onset placental dysfunction necessitating birth at <34 weeks
- birthweight <10th centile with evidence of placental dysfunction as defined below for current pregnancy
Definition of FGR in a current pregnancy: defined as either of the following:
- EFW or abdominal circumference (AC) <3rd centile
- EFW or AC <10th centile with evidence of placental dysfunction (either):
- abnormal uterine artery Doppler (mean pulsatility index >95th centile) earlier in pregnancy (20–24 weeks)
- abnormal umbilical artery Doppler (absent or reversed end diastolic flow or pulsatility index >95th centile)
Suboptimal fetal growth
When assessing fetal growth, a pattern of slowing growth velocity (that is, a downward trend in the percentile) indicates an increased risk of morbidity and stillbirth and should necessitate review. This review should include assessment of all fetal biometry measurements since the anomaly scan to identify potentially erroneous single measurements and also the presence or absence of other risk factors for FGR. Particular attention should be paid to a downward trend in abdominal circumference growth velocity.
FGR is rare >20th centile, so early delivery (<39+0 weeks) should only be considered following senior review, ideally by a dedicated FGR assessment and monitoring service.
Risk assessment and screening
Early-onset FGR is rare (~0.5%).42 Most cases are associated with abnormal uterine artery Doppler indices or already present estimated fetal weight (EFW) <10th centile in the early 3rd trimester. Thus, uterine artery Doppler can be used in the 2nd trimester (18+0 – 24+0 weeks) to help determine the risk of placental dysfunction and of hypertensive disorders or early-onset FGR.
For pregnant women with a normal uterine artery Doppler pulsatility index (PI; mean measurement ≤95th centile), the risk of these disorders is low and thus serial scanning for fetal biometry can be routinely planned from 32 weeks’ gestation.
Pregnant women at moderate risk of FGR do not require uterine artery Doppler assessment, but are still at risk of later-onset FGR so require serial ultrasound assessment of fetal growth from 32 weeks’ gestation.
Ongoing surveillance of fetal growth should be performed at intervals between 21–28 days while fetal growth remains >10th centile. For many pregnancies in the moderate-risk category or those unsuitable for symphysis fundal height (SFH) measurements, an interval of 4 weeks is appropriate. For pregnant women in the high-risk category the scan interval should be confirmed following the first assessment for fetal growth, but routine growth assessment should not occur <14 days.
Trusts are encouraged to invest in training ultrasonographers to perform uterine artery Doppler alongside the fetal anomaly scan with the opportunity to reduce the number of serial scans for growth that a woman would require during the pregnancy.
It should be noted that reference ranges are available for uterine artery Doppler PI throughout pregnancy.43 Thus, while offering uterine artery Doppler at the time of the fetal anomaly scan is appropriate for resource use and convenience, the measurement may be performed at any time during pregnancy.
Figure 1 provides an algorithm for using uterine artery Doppler as a screening tool for risk of early-onset FGR. It defines uterine anomaly as either septate or bicorporeal uteri (class II, III, V Mullerian anomalies). It does not include bicornuate uteri (class IV mullerian anomalies) as these have not been demonstrated to be associated with early-onset FGR. Note: Use of <10th centile EFW calculated at the time of the routine anomaly scan is preferred over <10th centile abdominal circumference (AC).
Figure 1: Algorithm for using uterine artery Doppler as a screening tool for risk of early onset FGR
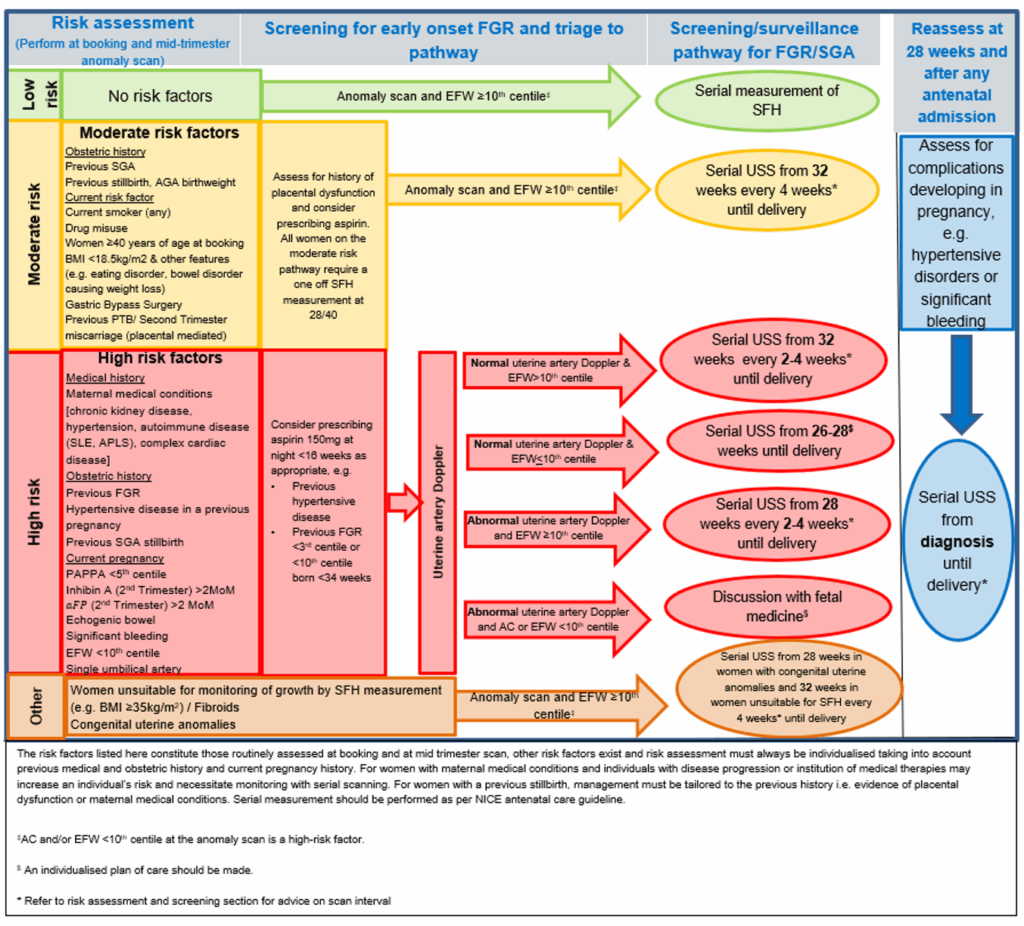
The above flowchart outlines a clinical pathway for fetal growth restriction (FGR) and small for gestational age (SGA) management.
It categorizes patients into low, moderate, high risk, or other based on risk factors, and guides through prevention strategies, identification of early onset FGR, surveillance pathways, and reassessment points.
Moderate and high-risk groups require tailored monitoring including uterine artery Doppler and serial ultrasound scans starting from 28 or 32 weeks.
High-risk pregnancies may need fetal medicine consultations.
The chart emphasizes reassessment at 28 weeks or after antenatal admissions, particularly for complications like hypertensive disorders or bleeding, ensuring individualized and continuous fetal monitoring.
Management of FGR
The RCOG provides detailed recommendations for the monitoring of SGA when EFW is <10th centile. Trusts should either follow this guidance or a similar protocol that has been agreed with local commissioners (ICBs) following advice from the provider’s clinical network as to whether the variation is acceptable.
This appendix describes further recommendations for the management of fetuses with FGR is supported by randomised controlled trial evidence.
The important features for management are:
- absent or reversed end diastolic flow in the umbilical artery is a feature of FGR before 32 weeks’ gestation
- ductus venosus (DV) Doppler is less predictive after 32 weeks’ gestation in the management of the FGR fetus
- a normal umbilical artery Doppler after 32 weeks’ gestation does not mean that the fetus is not growth restricted, nor that there is no evidence of fetal compromise
- after 34 weeks’ gestation, providers with capacity may wish to use assessment of middle cerebral artery (MCA) Doppler pulsatility indices (PI) to help identify and act on potential fetal compromise in later pregnancy
FGR diagnosed before 34 weeks’ gestation
Before 34+0 weeks, management of the FGR fetus requires regional network specialist fetal medicine input to determine the most appropriate monitoring for fetal wellbeing and timing of birth where fetal compromise is demonstrated.
Trusts caring for such pregnant women should have access to personnel who can carry out DV Doppler assessment and computerised CTG (cCTG). If trusts do not have access to DV Doppler or access is intermittent (that is, not 365 days a year), then cCTG must be provided for monitoring and a pre-established referral pathway should be in place to enable assessment of pregnant women by a specialist fetal medicine service within 72 hours.
Pregnant women with early-onset FGR should give birth in a unit with neonatal facilities that can manage the increased risks for FGR preterm infants. Timing should be determined in collaboration with neonatal colleagues, sub-speciality fetal medicine input, steroid administration and magnesium sulphate administration and be guided by current RCOG guidance and findings from the Truffle 2 study.
FGR diagnosed after 34 weeks’ gestation
For fetuses with an EFW <3rd centile diagnosed later in pregnancy, birth should be initiated at 37+0 weeks’ gestation. If other risk factors are present, then involvement of a specialist fetal growth service or fetal medicine service is required to plan birth.
In fetuses with an EFW between the 3rd and <10th centile, other risk factors must be present for birth to be recommended before 39 weeks. These are reduced fetal movements, any umbilical artery or MCA Doppler abnormality, cCTG that does not meet criteria, maternal hypertensive disease, abnormal sFlt1-to-PlGF ratio / free PlGF or reduced liquor volume. If FGR cannot be excluded, then birth after 37 weeks’ gestation should be discussed with the mother and an ongoing personalised management plan.
For all fetuses with an EFW or AC <10th centile, birth or the initiation of induction of labour should be offered at 39+0 weeks after discussion with the mother.
Evidence for the use of MCA Doppler in the management of late-onset FGR is awaited and this is not mandated in the management of FGR in the SBLCB.
For pregnant women who decline induction of labour or birth after 39+0 weeks, counselling must include a discussion regarding evidence that there is no increase in risk for the baby or for the mother from birth/induction at this gestation and that there is no evidence to determine how fetuses with SGA/FGR should be monitored if pregnancy continues.
Appendix D: Risk assessment at the onset of labour
Multidisciplinary training – principles
Include multidisciplinary and scenario-based training – this should involve all medical and midwifery staff who care for pregnant women in birth settings.
All staff to be competent in the use of fetal monitoring equipment.
Teaching about fetal responses to labour including changes in fetal heart rate (FHR) and the impact of antenatal risk factors such as fetal growth restriction and intrapartum risk factors such as maternal pyrexia.
Effective fetal monitoring in low-risk pregnancies using intermittent auscultation (IA), the role of IA in initial assessment and in established labour, and indications for changing from IA to CTG.
Interpretation of CTG including:
- normal FHR parameters
- impact of intrapartum fetal hypoxia on the FHR
- classification of CTG
- holistic interpretation of fetal monitoring in specific clinical circumstances (such as previous caesarean sections, breech and multiple pregnancy)
Channels of communication to follow in response to a deteriorating CTG trace, and escalation.
Application of local fetal monitoring guideline (NICE, FIGO or Physiological).
Multidisciplinary training must integrate the local handover tool (such as SBAR) into teaching programme at all trusts.
Provision of adequate training is a trust priority – as a minimum all staff should receive a full day of multidisciplinary training (including the principles outlined above) each year with reinforcement from regular attendance at fetal monitoring review events.
The training and assessment should be agreed with local commissioners (ICBs) based on the advice of the clinical network.
Competency assessment: all staff will have to pass an annual competency assessment that has been agreed by the local commissioner (ICBs) based on the advice of the clinical network. The assessment should include demonstrating a clear understanding of the areas covered in training (see principles above). Trusts should agree a procedure with their ICB for how to manage staff who fail this assessment.
No member of staff should care for pregnant women in a birth setting without evidence of training and assessment within the last year.
Start of labour risk assessment
All pregnant women should undergo a full clinical assessment when presenting in early or established labour. This should include a review of any risk factors and consideration of whether any complicating factors have arisen that might change recommendations about place of birth. This assessment should be agreed with local commissioners (ICBs) based on the advice of the clinical network and reflect fetal monitoring guidelines. It should be shared with the woman and her birth partner to enable an informed decision about place of birth.
Ongoing labour risk assessments
To include:
- start of labour risk assessment
- intrapartum risk factors
- consideration of FHR parameters when using IA or CTG
- whether the woman and her birth partner have any concerns
This information is used to inform the whole clinical picture, care and escalation if required.
Fresh Eyes
There is no evidence to inform the optimal frequency of a buddy system for IA or CTG and/or its effectiveness. However, the concept was introduced to enable a Fresh Eyes perspective of maternal and fetal wellbeing and has the potential to be beneficial and supportive.
The timeframe for Fresh Eyes should be decided within maternity services and agreed with local commissioners (ICBs) based on the advice of the clinical network.
IA is predominantly used in low-risk labours where the incidence of hypoxia is very low and therefore an hourly Fresh Eyes process can be a distraction from care in labour without conferring benefit. A 4-hourly review may be more beneficial.
CTG is predominantly used in labour where there are risk factors and therefore the risk of sepsis and fetal hypoxia is greater; therefore, Fresh Eyes review at least hourly may be more beneficial.
Fetal monitoring expertise
The dedicated hours for midwifery and obstetric fetal monitoring leads will depend on the size of the maternity unit and should be agreed with local commissioners (ICBs) based on the advice of the clinical network.
Appendix E: Risk assessment, surveillance pathway and management of women at risk of preterm birth
This appendix describes a risk assessment, surveillance and management pathway for pregnant women at risk of preterm birth. It has been designed with reference to NICE guidance and the UK Preterm Clinical Network guidance. It does not address administration of corticosteroids, magnesium sulphate and use of tocolytics for which there is evidence-based guidance.
Prevention
All pregnant women should be assessed at booking for risk factors for preterm birth. This assessment should include modification of population-based risk factors, acknowledging that most preterm births occur in pregnant women who will not have been cared for in a preterm prevention clinic.
Smoking cessation: Smoking doubles the risk of preterm birth and therefore all pregnant women should be asked about smoking and those who smoke should be provided with cessation advice and/or referred. Women who have experienced a previous preterm birth, who stopped smoking early in the pregnancy, modify their risk back to that of a non-smoker. If smoking cessation is delayed until the 3rd trimester this modifiable benefit is lost. The importance of promoting smoking cessation is therefore one of the most important prevention strategies to implement (see Element 1 for more detail).
Maternal age: Young women (<18 years) have an increased risk of preterm birth. Appropriate referral to teenage pregnancy teams should be offered to provide adequate support and advice throughout the pregnancy, which may help prevent preterm birth.
Domestic violence: Women experiencing domestic violence and/or other social pressure should be directly counselled and referred for specific support through local pathways.
Urinary tract infection: As indicated in NICE guidance, a midstream urine sample (MSU) should be taken for all high or intermediate risk pregnant women at booking and sent for culture and sensitivity. Culture-positive samples, even in symptom-free pregnant women (asymptomatic bacteriuria), should be promptly treated. Following any positive culture and treatment, a repeat MSU to confirm clearance is recommended. Those who have a recurrent episode require review in secondary care.
Vaginal infection: Pathogens such as Neisseria gonorrhoeae and Chlamydia trachomatis are associated with preterm birth and screening should be offered to at-risk pregnant women. In particular, healthcare professionals should inform pregnant women under the age of 25 years that the prevalence of chlamydial infection in their age group is high and give them details of their local National Chlamydia Screening Programme.
The role of organisms found in bacterial vaginosis (BV) remains controversial; BV is linked with preterm birth, but the varying methods used to ascertain its presence, and the timing and means of treatment in several studies have meant that no consensus currently exists as to its identification and treatment in at-risk pregnant women. The presence of Group B streptococci in a vaginal swab is not an indication to treat until in labour unless also isolated from a MSU.
Risk assessment
Risk assessment should identify high-risk pregnant women who require management in a preterm birth prevention clinic where further tests may be offered as part of the surveillance pathway. This assessment should take place at the booking appointment with referral by 12 weeks.
Table 1: Risk assessment and management tool for pregnant women at risk of preterm birth
Risk factor: High risk
- previous preterm birth or mid-trimester loss (16–34 weeks’ gestation)
- previous preterm prelabour rupture of membranes <34/40
- previous use of cervical cerclage
- known uterine variant (that is, unicornuate, bicornuate uterus or uterine septum)
- intrauterine adhesions (Ashermann’s syndrome)
- history of trachelectomy (for cervical cancer)
Pathway:
Surveillance
- Referral to local or tertiary preterm prevention clinic by 12 weeks.
- Further risk assessment based on history +/- examination as appropriate in secondary care with identification of pregnant women needing referral to tertiary services.
- All pregnant women to be offered transvaginal cervix scanning every 2–4 weeks between 16 and 24 weeks as a secondary test to more accurately quantify the risk of preterm birth.
- Additional use of quantitative fetal fibronectin (qFFN) in asymptomatic pregnant women may be considered where centres have this expertise.
Management
- Interventions should be offered to pregnant women as appropriate, based on either history or additional risk assessment tests by clinicians able to discuss the relevant risks and benefits based on up-to-date evidence and relevant guidance, for example UK Preterm Clinical Network guidance and NICE guidance.51 These interventions should include cervical cerclage, pessary and progesterone as appropriate.
Risk factor: Intermediate risk
- previous birth by caesarean section at full dilatation
- history of significant cervical excisional event – for example, LLETZ where >15 mm depth removed or >1 LLETZ procedure carried out or cone biopsy (knife or laser, typically carried out under general anaesthetic)
Pathway:
Surveillance
- Refer to preterm birth prevention clinic by 12 weeks.
- Further risk assessment based on history +/- examination as appropriate in secondary care with discussion of option of additional risk assessment tests, including:
- a single transvaginal cervix scan between 18 and 22 weeks as a minimum
- additional use of qFFN in asymptomatic pregnant women can be considered where centres have this expertise
Management
- Interventions should be discussed with pregnant women as appropriate based on either history or additional risk assessment tests. These discussions should be held by clinicians able to discuss the relevant risks and benefits based on up-to-date evidence and relevant guidance. The interventions should include cervical cerclage, pessary and progesterone as appropriate.
- Pregnant women at intermediate risk should be reassessed at 24 weeks for consideration of transfer back to a low-risk pathway.
Pregnant women with any of the additional high-risk factors should be reviewed in a preterm birth prevention clinic where a detailed history should be obtained and an personalised plan made. Additional tests for ascertaining risk should be offered – as a minimum this should include transvaginal cervix scan between 18 and 22 weeks’ gestation. Some providers may wish to schedule this as part of the anomaly scan. Additional cervical length scans should be performed at the discretion of the lead clinician and are likely to be more frequent than the minimum outlined above.
The addition of a second risk assessment tool, quantitative fetal fibronectin (qFFN), is currently being evaluated in symptomatic pregnant women in clinical studies. In asymptomatic pregnant women, this tool may be used from 18 weeks to ascertain risk of second trimester miscarriage or preterm birth, in conjunction with cervical length measurement and support discussions of potential interventions with pregnant women. It can also be used in high-risk pregnant women in late second / early 3rd trimester to determine timing of preparation for preterm birth, for example administration of steroids and magnesium sulphate. In current clinical practice additional risk assessment tools in asymptomatic pregnant women should be used at the discretion of the lead clinician and where there is expertise and clear guidance for use.
The use of other near-patient tests, such as placental alpha macroglobulin-1 (PAMG-1, PartoSure) and insulin-like growth factor binding protein-1 (IGFBP-1, Actim Partus), has been examined by NICE and these are currently not recommended for routine use outside research settings.
Management
After assessment within the preterm birth prevention clinic, based on history and/or additional risk assessment tools pregnant women should be offered treatment to prevent 2nd trimester miscarriage and preterm birth.
Several interventions have been assessed for pregnant women at high risk of preterm birth: cervical cerclage, progesterone and pessaries. Cervical cerclage is an established procedure, progesterone is recommended in certain situations by NICE, and randomised trials suggest benefit from the use of Arabin pessaries in at-risk pregnant women. At present the evidence base cannot determine precisely in which pregnant women, and in what circumstances, each intervention will be most effective. Care should, therefore, always be individualised, taking into account the pregnant women’s wishes and following a discussion with a clinician able to discuss the potential risks and benefits of each intervention.
The following evidence and guidance should be discussed:
History of spontaneous preterm birth or late miscarriage (16–34 weeks):
- offer a history-indicated (planned, prophylactic, elective) cervical cerclage or transvaginal ultrasound surveillance of the cervix within the 2nd trimester
- history-indicated cerclage should be placed by the end of the 1st trimester where possible. However, it may often be prudent to wait until after the dating scan and aneuploidy screening, so that significant fetal malformations can be excluded
- for pregnant women having ultrasound surveillance, discuss intervention when cervix is <25 mm, either cervical cerclage, Arabin pessary or prophylactic progesterone (vaginal or intramuscular)
Previous failed transvaginal suture:
- the circumstances of the failed suture and other clinical factors should be considered before placement, and appropriately experienced clinicians should be involved in the decision-making and surgery. High vaginal or transabdominal cerclage may be considered
- transabdominal placement during pregnancy should be undertaken before 14 weeks. NICE has published guidelines regarding laparoscopic placement
No history of spontaneous preterm birth or mid-trimester loss but transvaginal cervix scan between 16+0 and 26+0 weeks reveals cervix is <25 mm:
- care for these pregnant women should be individualised
- counselling should include the options of continued surveillance or intervention; with clinicians who can discuss the relevant risks and benefits based on to up-to-date evidence and relevant guidance
- interventions should include cervical cerclage, pessary and progesterone as appropriate
Pregnant women with an intervention (cerclage, pessary or progesterone) should remain under the care of the preterm birth prevention clinic until birth.
Those undergoing transvaginal cervix scanning risk assessment should continue this until 24 weeks, which is when this monitoring pathway ends if no intervention is recommended. Pregnant women may then be transferred to routine pathways of care. Midwifery-led care is appropriate if no other additional risk factors are identified.
References
- Marmot M, Goldblatt P, Allen A, et al (2010). Fair society, healthy lives (The Marmot Review). Institute of Health Equity.
- Sheridan E, Wright J, Small N, et al (2013). Risk factors for congenital anomaly in a multiethnic birth cohort: an analysis of the Born in Bradford study. Lancet 382(9901): 1350–1359.
- Gale-Grant O, Fenn-Moltu S, Franca LGS, et al (2022). Effects of gestational age at birth on perinatal structural brain development in healthy term-born babies. Hum Brain Mapp 43(5): 1577–1589. doi: 10.1002/hbm.25743. Epub 2021 Dec 12. PMID: 34897872.
- Alterman N, Johnson S, Carson C, et al (2022). Gestational age at birth and academic attainment in primary and secondary school in England: Evidence from a national cohort study. PLoS One 17(8): e0271952. doi: 10.1371/journal.pone.0271952. PMID: 35976808.
- Grobman WA (2018). A randomized trial of elective induction of labor at 39 weeks compared with expectant management of low-risk nulliparous women. Am J Obstet Gynecol 218: S601.
- Marufu TC, Akankari A, Coleman T, Lewis S (2015). Maternal smoking and the risk of still birth: systematic review and meta-analysis. BMC Public Health 15: Article 239.
- McCowan LME, Dekker GA, Chan E, et al (2009). Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ 338: b1081.
- Yan J, Groothuis PA (2014). Timing of Prenatal Smoking Cessation or Reduction and Infant Birth Weight: Evidence from the United Kingdom Millennium Cohort Study. Mat Child Health J 19: 447–458.
- Osborne JB, Baile BA (2022). Does it matter when I quit? Could I just cut down some? Links between trimester-specific smoking amount, preterm birth, and low birth weight. Birth Defect Res 114(1): 5–12.
- Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A (2013). Maternal 346(January): f108.
- Blencowe H, Cousens S, Jassir FB, et al (2016). National regional and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Global Health 4(2): e98–e108.
- Stock SJ, Ferguson E, Duffy A, Ford I, Chalmers J, Norman JE (2012). Outcomes of elective induction of labour compared with expectant management: population based study. BMJ: 344: e2838.
- Maternal and Child Health Research Consortium (2001). 8th Annual Report. Confidential Enquiry into Stillbirths and Deaths in Infancy. London: Maternal and Child Health Research Consortium.
- Draper ES, Kurinczuk JJ, Kenyon S (eds) on behalf of MBRRACE-UK. MBRRACE-UK Perinatal Confidential Enquiry (2015). Term, singleton, normally formed, antepartum stillbirth. Leicester: The Infant Mortality and Morbidity Studies, Department of Health Sciences, University of Leicester.
- Draper ES, Kurinczuk JJ, Kenyon S (eds) on behalf of MBRRACE-UK. MBRRACE-UK (2017). Perinatal Confidential Enquiry: Term, singleton, intrapartum stillbirth and intrapartum-related neonatal death. Leicester: The Infant Mortality and Morbidity Studies, Department of Health Sciences, University of Leicester.
- Kurinczuk JJ, Smith P, Bevan C, et al (2022). Learning from Standardised Reviews When Babies Die. National Perinatal Review Tool: Fourth Annual Report. Oxford: National Perinatal Epidemiology Unit.
- Scala C, Bhide A, Familiari A, Pagani G, et al (2015). Number of episodes of reduced fetal movement at term: association with adverse perinatal outcome. Am J Obstet Gynecol 213(5): 678 e1-6. doi: 10.1016/j.ajog.2015.07.015.
- Thompson JMD, Wilson J, Bradford BF, et al (2021). A better understanding of the association between maternal perception of foetal movements and late stillbirth-findings from an individual participant data meta-analysis. BMC Med 19(1): 267.
- Warrander LK, Batra G, Bernatavicius G, et al (2012). Maternal perception of reduced fetal movements is associated with altered placental structure and function. PLoS One 7(4): e34851. doi: 10.1371/journal.poneB.0034851.
- Askeland Winje B, Roald B, Petrov Kristensen N, Frøen JF (2012). Placental pathology in pregnancies with maternally perceived decreased fetal movement–a population-based nested case-cohort study PLoS One 7(6): e39259. doi: 10.1371/journal.pone.0039259.
- Levy M, Kovo M, Izaik Y, et al (2020). Reduced fetal movements at term in singleton low risk pregnancies-Is there an association with placental histopathological findings? Acta Obstet Gynecol Scand 99(7): 884-890. doi: 10.1111/aogs.13810.
- Hayes DJL, Dumville JC, Walsh T, et al (2023). Effect of encouraging awareness of reduced fetal movement and subsequent clinical management on pregnancy outcome: a systematic review and meta-analysis. Am J Obstet Gynecol 5(3): 100821.
- Norman J, Heazell AEP, Rodriguez A, Weir CJ, Stock SJE, Calderwood CJ (2018). Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge, cluster-randomised trial. Lancet 392(10158): 1629–1638.
- (MBAM) PMID 34555257 and (Mindfetalness) PMID 31971325.
- Flenady V, Gardener G, Ellwood D, et al (2022). My Baby’s Movements: a stepped-wedge cluster-randomised controlled trial of a fetal movement awareness intervention to reduce stillbirths. Br J Obstet Gynaecol 129(1): 29–41. doi: 10.1111/1471-0528.16944.
- Baker H, Pilarski N, Hodgetts-Morton VA, Morris RK (2021). Comparison of visual and computerised antenatal cardiotocography in the prevention of perinatal morbidity and mortality. A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 263: 33–43. doi: 10.1016/j.ejogrb.2021.05.048.
- Gale C, Statnikov Y, Jawad S, Uthaya SN Modi N, Brain Injuries expert working group (2018). Neonatal brain injuries in England: population-based incidence derived from routinely recorded clinical data held in the National Neonatal Research Database. Arch Dis Child Fetal Neonatal Ed 103(4): F301–F306.
- Alfirevic Z, Devane D, Gyte GML (2013). Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour (Review). The Cochrane Collaboration. John Wiley & Sons Ltd.
- Murphy KW, Johnson P, Moorcraft P, Pattinson R, Russel V, Turnball A (1990). Birth asphyxia and the intrapartum cardiotocograph. Br J Obstet Gynaecol 97: 470–479.
- Ockenden review (2022). Independent report: Final report of the Ockenden review. Department of Health and Social Care.
- Schneeberger C, Geerlings SE, Middleton P, Crowther CA (2015). Interventions for preventing recurrent urinary tract infection during pregnancy. Cochrane Database Syst Rev Issue 7: CD009279. doi: 10.1002/14651858.CD009279.pub3.
- National Institute for Health and Care Excellence (2015). Preterm Labour and birth (NICE Guideline 25).
- National Institute for Health and Care Excellence (2018). Biomarker tests to help diagnose preterm labour in women with intact membranes (Diagnostics guidance 33).
- Simmons D (2021). Adverse pregnancy outcomes in women with type 1 or type 2 diabetes. Lancet 9(3): 129–131.
- Diguisto C, Strachan MWJ, Churchill D, Ayman G, Knight M (2021). A study of diabetic ketoacidosis in the pregnant population in the United Kingdom: Investigating the incidence, aetiology, management and outcomes. Diabetic Med 39(4): e14743. doi: 10.1111/dme.14743.
- National Institute for Health and Care Excellence (2011). Hypertension in pregnancy: diagnosis and management (Clinical guideline 107).
- Rolnik DL, Wright D, Poon LC, et al (2017). Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 377(7): 613–622.
- Ayala DE, Ucieda R, Hermida RC (2012). Chronotherapy with low-dose aspirin for prevention of complications in pregnancy. Chronobiol Int 30: 1–2, 260–279.
- Clinical pharmacology [database online]. Elsevier. Retrieved 20 March 2018.
- Gordijn SJ, Beune, IM, Thilaganathan B, et al (2016). Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 48: 333–339. doi:10.1002/uog.15884.
- Gómez O, Figueras F, Fernández S, Bennasar M, Martínez JM, Puerto B (2008). Reference ranges for uterine artery mean pulsatility index at 11-41 weeks of gestation. Ultrasound Obstet Gynecol 32(2): 128–32.
- Royal College of Obstetricians and Gynaecologists (2024). RCOG Green-Top Guideline 31: Investigation and Care of a Small-for-Gestational-Age Fetus and a Growth Restricted Fetus. London: RCOG.
- National Institute for Health and Care Excellence (2015). Preterm labour and birth (NICE Guideline 25).
- Ashoor G, Syngelaki A, Papastefanou I, Nicholaides KH, Akolekar R (2022). Development and validation of model for prediction of placental dysfunction-related stillbirth from maternal factors, fetal weight and uterine artery Doppler at mid-gestation. Ultrasound Obstet Gynecol 59(1): 61–68.
- Muglu J, Rather H, Arroyo-Manzano D (2019). Risks of stillbirth and neonatal death with advancing gestation at term: A systematic review and meta-analysis of cohort studies of 15 million pregnancies. PLOS Med.
- Roberts D, Brown J, Medley N, Dalziel SR (2017). Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev Issue 3: CD004454. DOI: 10.1002/14651858.CD004454.pub3.
- Crowther CA, McKinlay CJ, Middleton P, Harding JE (2015). Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst Rev Issue 7: CD003935. doi: 10.1002/14651858.CD003935.pub4.
- Chang E (2015). Preterm birth and the role of neuroprotection. BMJ 350: g6661.
- Andres RL, Day MC (2000). Perinatal complications associated with maternal tobacco use. Semin Neonatol 5(3): 231–241.
- UK Preterm Clinical Network (2018). Reducing preterm birth: Guidelines for commissioners and providers. Preterm Clinical Network.
- National Institute for Health and Care Excellence (2018). Biomarker tests to help diagnose preterm labour in women with intact membranes (Diagnostics guidance 33).
- Berghella V, Mackeen AD (2011). Cervical length screening with ultrasound-indicated cerclage compared with history-indicated cerclage for prevention of preterm birth: a meta-analysis. Obstet Gynecol 118(1): 148–155.
- National Institute for Health and Care Excellence (2007). Laparoscopic cerclage for prevention of recurrent pregnancy loss due to cervical incompetence (Interventional procedures guidance 228).
Publication reference: PRN01691

