Section 1: Introduction
Purpose of this document
This document provides additional guidance on the interpretation and verification of the QOF indicators for 2023/24 in England, which are listed in Annex D of the Statement of Financial Entitlements Directions (SFE). It is effective from 1 April 2023 and replaces versions issued in previous years.
This document covers:
- Section 2: the list of QOF indicators as detailed in Annex D of the SFE Directions
- Section 3: specific information about each clinical indicator including the rationale for inclusion and any specific requirements which contractors need to demonstrate to ensure achievement
- Section 4: specific information about each public health indicator including the rationale for inclusion and any specific requirements which contractors need to demonstrate to ensure achievement
- Section 5: detailed information about the requirements of the quality improvement domain
- Section 6: detailed information about Personalised Care Adjustment
- Section 7: a full list of indicators which are no longer in QOF but are subject to ongoing data collection
- Section 8: glossary of acronyms
- Section 9: the process for raising queries in relation to QOF indicators and their interpretation
This guidance should be read in conjunction with the SFE Directions and Business Rules.
Definition of ‘commissioner’
NHS England is the organisation legally responsible for the commissioning of primary care in England. Following the implementation of delegated commissioning references to ‘commissioners’ in this document could refer to NHS England or, since 1 July 2022, integrated care boards (ICBs)
Additional indicator information
Full descriptions of each indicator, its rationale for inclusion and any specific criteria for reporting and verification are detailed in Sections 3, 4 and 5.
Clinical and public health indicators
Clinical and public health indicators are organised by disease or intervention categories. These indicators have been selected as they represent care where:
- The responsibility for ongoing management rests principally with the contractor and the primary care team
- There is good evidence of the health benefits likely to result from improved primary care
Indicator numbering
Indicators are prefixed with an abbreviation of the category to which they belong. For example, coronary heart disease indicator one is identified as CHD001. Indicator IDs are unique to each indicator and are not reused. New indicators will be given the next available unused number. Therefore, this may not flow sequentially from the existing indicator IDs. Similarly, where there has been a change to indicator wording, activity timescales or significant changes to coding or the data extraction logic these indicators will be given a new unique ID. This is to ensure that indicators are not inappropriately compared to those in previous years and to avoid any confusion which could arise from re-using ID numbers.
Where an indicator has been developed through the NICE led process they will also be annotated with their NICE menu ID number (NICE [year] menu ID: NMXX). If a NICE developed indicator has been amended during negotiations this will be annotated with ‘based on NMXX’. References to NICE guidance throughout this document relate to the guidance that has been used to underpin the stated indicators. In some cases, new or updated guidance may have been recently published, or will be published before the end of the QOF year. These guidelines will be reviewed by NICE in due course and any recommendations concerning amending current indicators or development of new indicators will be published in future NICE indicator menus for consideration by relevant parties.
Identifying the target population or disease register
Clinical indicators all have a defined target population. This may be defined within a register indicator or as part of the business rules. This target population will be identified either by the presence of predetermined clinical diagnosis codes in the patient record or by using other attributes of the patient such as age and sex. For example, the target population for cervical screening is constructed using age and sex to determine inclusion in the denominator for each indicator. Where the target population is identified using clinical codes the contractor is responsible for demonstrating that it has systems in place to maintain a high quality, accurate register. This may be verified by the commissioner and contractors may be asked to explain reasons for variation from expected prevalence levels. Contractors are reminded that QOF registers must not be used as the sole input for the purposes of patient care and clinical audit. There may be patients for whom a treatment or activity is clinically appropriate, but they may not meet the criteria as defined by the QOF register. Contractors are asked to hold this in mind when developing call/recall systems.
Patients with co-morbidities will be included in all relevant target populations and registers where they meet the defined criteria. Where a patient is in more than one target population, they are eligible for the interventions outlined in all relevant disease areas.
Some indicators refer to a sub-set of patients in the target population or register. Patients who are not included in an indicator denominator for definitional reasons are classified as ‘exclusions’ and are automatically identified through the business rules and removed from the denominator.
Patients are eligible for the interventions outlined in QOF indicators as soon as they are fully registered with the contractor or a relevant diagnosis is recorded.
Quality improvement indicators
Section 5 provides detailed guidance on the interpretation of the quality improvement indicators and the aims and objectives which their quality improvement plans should be seeking to address.
Reporting, payment calculation and verification
Reporting
Reporting requirements and the rules for the calculation of QOF points and their payment are set out in the SFE. For most indicators anonymised data will be collected automatically from GP clinical systems by the General Practice Extraction Service (GPES) and reported to Calculating Quality Reporting Service (CQRS).
The clinical codes and logical extraction sequence used in this data collection is defined in a series of technical documents – the Business Rules. These are based entirely on SNOMED codes and associated dates, combined with patient characteristics (e.g. age and sex). SNOMED codes are an NHS standard.
Contractors using proprietary coding systems and/or local/practice specific codes will need to be aware that these codes will not be recognised within QOF reporting. The Business Rules are available on the NHS Digital website.
For indicators where achievement is not automatically collected this should be self- declared through the CQRS website. Commissioners may request evidence underpinning this self-declaration as part of their verification processes.
Payment calculation and achievement
CQRS will calculate achievement and payments for QOF as set out in the SFE and report to commissioners and practices. Whilst full details of the achievement calculations are detailed in the SFE, the following key points are useful to note:
- Achievement is measured on the last day of the financial year i.e. 31 March in respect of patients registered with the practice on that Whilst estimates of achievement may be made through the year these may not accurately predict final performance.
- The time period referred to in an indicator is calculated by counting back from the last day of the financial year. Time periods vary between indicators.
- The phrase ‘currently treated’ should be interpreted as a prescription for the specified medication being given in the six months preceding the last day of the financial year i.e. between 1 October and 31 March.
- Some indicators require the intervention to be offered to patients when they reach a defined age or within a specified time before and/or after diagnosis. Care recorded outside of these time periods will not be recognised in the QOF achievement calculation.
There are specific provisions within the SFE which describe the calculations to be made where a contract comes to an end before the last day of the financial year.
Verification
The contractor must ensure that it is able to provide any information that the NHSE or ICB may reasonably request of it to demonstrate that it is entitled to each achievement point to which it says it is entitled. The contractor must make that information available to the commissioner on request. In verifying that an indicator has been achieved and information correctly recorded, the commissioner may choose to inspect the output from a computer search that has been used to provide information on the indicator, or a sample of patient records relevant to the indicator.
Commissioners and practices will be aware of the requirements of access to patient identifiable data, in particular that they should:
- Obtain the minimum necessary information for the specific purpose
- Anonymise data where possible
Where patients have expressed a desire that their information is not shared for this purpose, practices will need to advise the commissioner and make an appropriate note in the record. It is recommended that practices record access to confidential patient data in the relevant patient record, so that an audit trail is in place to fulfil the obligations of the practice towards their patients and that of commissioners to practices.
The terms ‘notes’ and ‘patient record’ are used to indicate either electronic or paper patient records.
Disputes
When a QOF related contractual dispute arises, the commissioner and contractor would be expected to make every reasonable effort to communicate and co-operate with each other with a view to resolving the dispute without the need to refer it for formal determination by NHS Resolution (Primary Care Appeals) (or in certain cases, the courts). Further information is available in the SFE.
Section 2: Summary of all indicators*
* The ‘summary of indicators’ section is an extract from Annex D of the SFE.
Income protected indicators (81 points)
To provide some flexibility, NHS England has agreed to income protect the disease register indicators within QOF for 2023/24. These indicators comprise of 19 indicators, almost a quarter of the total indicators in QOF, with a total points value of 81.
Practices will be expected to continue to maintain these registers and accurately code patient records with up-to-date information on diagnoses, as this activity performs an important role in maintaining clinical quality. There will continue to be a GPES extract of diagnosis which is used to calculate prevalence adjustments for indicators. Failure to maintain the registers will have an impact on prevalence adjustments and therefore will impact on practice income at the end of the financial year.
Practices will be awarded QOF points:
- if they held a disease register last year (2022/23) and therefore earned the max QOF points for the associated register indicator, they will earn max QOF points for that associated disease register indicator this year (2023/24). This is regardless of whether the practice still maintains this disease register for 2023/24 and is applicable across all the register indicators.
- if a practice did not hold a disease register in 2022/23 and so earned no QOF points for that financial year, practices can still obtain the points and payment if they start a register in 2023/24.
Income protection does not mean that the payment amount will be the same in 2023/24 as in 2022/23. QOF earnings will continue to be subject to prevalence adjustments, list size variation and therefore the final payment amount may be different.
- For the majority of practices who have recorded registers, the prevalence and list size adjustments will be based on the 2023/24 calculations, assuming practices maintained their registers in both years.
- For those practices that had a recorded register in 2022/23 but didn’t in 2023/24, the prevalence used will be from 2022/23 and the list size will be from 2023/24. Due to the nature of the CQRS system, this will require a manual adjustment, which is detailed below.
For the small number of practices that had a register in 2022/23 and not in 2023/24, a manual adjustment will be used to make the payment to practices. At the end of the financial year, where this is necessary, CQRS will contact commissioners who in turn will contact practices.
Commissioners will then be required to complete and submit a variation template which will be processed by NHS England to enable the correct payment to be paid to practices.
The Income Protected Points for the indicators set out in the Summary of Indicators in Annex D of the Statement of Financial Entitlements (SFE) are available as follows:
- The points available for an indicator are set out in the second column of the Summary of Indicators in Annex D.
- The income protected indicators are noted clearly in the table in Section 1 in the Summary of Indicators of Annex D;
- The income protected indicators are the disease register indicators, noted clearly in the Summary of Indicators of Annex D.
For the register indicators being income protected for 2023/24, the points available for each of the register indicators will be based on the performance achievements in 2022/23.
| Indicator ID | Indicator | Conditional points |
|---|---|---|
| AF001 | The contractor establishes and maintains a register of patients with atrial fibrillation. | 5 |
| CHD001 | The contractor establishes and maintains a register of patients with coronary heart disease. | 4 |
| HF001 | The contractor establishes and maintains a register of patients with heart failure. | 4 |
| HYP001 | The contractor establishes and maintains a register of patients with established hypertension. | 6 |
| PAD001 | The contractor establishes and maintains a register of patients with peripheral arterial disease. | 2 |
| STIA001 | The contractor establishes and maintains a register of patients with stroke or TIA. | 2 |
| DM017 | The contractor establishes and maintains a register of all patients aged 17 or over with diabetes mellitus, which specifies the type of diabetes where a diagnosis has been confirmed. | 6 |
| AST005 | The contractor establishes and maintains a register of patients with asthma aged 6 years or over, excluding patients with asthma who have been prescribed no asthma related drugs in the preceding 12 months. | 4 |
| COPD015 | The contractor establishes and maintains a register of: Patients with a clinical diagnosis of COPD before 1 April 2023 and Patients with a clinical diagnosis of COPD on or after 1 April 2023 whose diagnosis has been confirmed by a quality assured post bronchodilator spirometry FEV1/FVC ratio below 0.7 between 3 months before and 6 months after diagnosis (or if newly registered at the practice in the preceding 12 months a record of an FEV1/FVC ratio below 0.7 recorded within 6 months of registration); and Patients with a clinical diagnosis of COPD on or after 1 April 2023 who are unable to undertake spirometry. | 8 |
| DEM001 | The contractor establishes and maintains a register of patients diagnosed with dementia. | 5 |
| MH001 | The contractor establishes and maintains a register of patients with schizophrenia, bipolar affective disorder and other psychoses and other patients on lithium therapy. | 4 |
| CAN001 | The contractor establishes and maintains a register of all cancer patients defined as a ‘register of patients with a diagnosis of cancer excluding non-melanotic skin cancers diagnosed on or after 1 April 2003’. | 5 |
| CKD005 | The contractor establishes and maintains a register of patients aged 18 or over with CKD with classification of categories G3a to G5 (previously stage 3 to 5). |
6 |
| EP001 | The contractor establishes and maintains a register of patients aged 18 or over receiving drug treatment for epilepsy. | 1 |
| LD004 | The contractor establishes and maintains a register of patients with learning disabilities. | 4 |
| OST004 | The contractor establishes and maintains a register of patients: Aged 50 or over and who have not attained the age of 75 with a record of a fragility fracture on or after 1 April 2012 and a diagnosis of osteoporosis confirmed on DXA scan, and Aged 75 or over with a record of a fragility fracture on or after 1 April 2014 and a diagnosis of osteoporosis. | 3 |
| RA001 | The contractor establishes and maintains a register of patients aged 16 or over with rheumatoid arthritis. | 1 |
| PC001 | The contractor establishes and maintains a register of all patients in need of palliative care/support irrespective of age. | 3 |
| OB003 | The contractor establishes and maintains a register of patients aged 18 years or over living with obesity, appropriately adjusted for ethnicity in line with NICE guidelines – either with a BMI ≥30 in the preceding 12 months, or a BMI greater than or equal to 27.5 kg/m2 recorded in the preceding 12 months for patients with a South Asian, Chinese, other Asian, Middle Eastern, Black African or African-Caribbean family background. | 8 |
Clinical domain (401 points)
This domain applies to all contractors participating in QOF.
* indicates income protected register indicators.
| Atrial fibrillation (AF) | Points | Thresholds |
| Records AF001*. The contractor establishes and maintains a register of patients with atrial fibrillation | 5 | N/A |
| Ongoing management AF006. The percentage of patients with atrial fibrillation in whom stroke risk has been assessed using the CHA2DS2- VASc score risk stratification scoring system in the preceding 12 months (excluding those patients with a previous CHADS2 or CHA2DS2-VASc score of 2 or more) | 12 | 40-90% |
| AF008. Percentage of patients on the QOF Atrial Fibrillation register and with a CHA2DS2- VASc score of 2 or more, who were prescribed a direct-acting oral anticoagulant (DOAC), or, where a DOAC was declined or clinically unsuitable, a Vitamin K antagonist | 12 | 70-95% |
| Secondary prevention of coronary heart disease (CHD) | Points | Thresholds |
| Records CHD001*. The contractor establishes and maintains a register of patients with coronary heart disease | 4 | N/A |
| Ongoing management CHD005. The percentage of patients with coronary heart disease with a record in the preceding 12 months that aspirin, an alternative anti-platelet therapy, or an anti- coagulant is being taken | 7 | 56–96% |
| CHD015. The percentage of patients aged 79 years or under, with coronary heart disease, in whom the last blood pressure reading (measured in the preceding 12 months) is 140/90 mmHg or less, (or equivalent home blood pressure reading) | 12 | 40-77% |
| CHD016. The percentage of patients aged 80 years or over, with coronary heart disease, in whom the last blood pressure reading (measured in the preceding 12 months) is 150/90 mmHg or less, (or equivalent home blood pressure reading) | 5 | 46-86% |
| Heart failure (HF) | Points | Thresholds |
| Records HF001*. The contractor establishes and maintains a register of patients with heart failure | 4 | N/A |
| Initial diagnosis HF008. The percentage of patients with a diagnosis of heart failure on or after 1 April 2023 which: 1. Has been confirmed by an echocardiogram or by specialist assessment in the 6 months before entering on to the register; or 2. If registered at the practice after diagnosis, with no record of the diagnosis originally being confirmed either by echocardiogram or by specialist assessment, a record of an echocardiogram or a specialist assessment within 6 months of the date of registration. | 6 | 50–90% |
| Ongoing management HF003. In those patients with a diagnosis of heart failure due to left ventricular systolic dysfunction or whose heart failure is due to reduced ejection fraction the percentage of patients who are currently treated with an angiotensin- converting enzyme inhibitor (ACE-I) or Angiotensin II receptor blockers (ARB) | 6 | 60–92% |
| HF006. The percentage of patients with a diagnosis of heart failure due to left ventricular systolic dysfunction or whose heart failure is due to reduced ejection fraction, who are currently treated with a beta-blocker licensed for heart failure. | 6 | 60-92% |
| HF007. The percentage of patients with a diagnosis of heart failure on the register, who have had a review in the preceding 12 months, including an assessment of functional capacity and a review of medication to ensure medicines optimisation at maximal tolerated doses. | 7 | 50-90% |
| Hypertension (HYP) | Points | Thresholds |
| Records HYP001*. The contractor establishes and maintains a register of patients with established hypertension | 6 | N/A |
| Ongoing management HYP008. The percentage of patients aged 79 years or under with hypertension in whom the last blood pressure reading (measured in the preceding 12 months) is 140/90 mmHg or less (or equivalent home blood pressure reading) | 14 | 40-77% |
| HYP009. The percentage of patients aged 80 years or over, with hypertension, in whom the last blood pressure reading (measured in the preceding 12 months) is 150/90 mmHg or less, (or equivalent home blood pressure reading) | 5 | 40-80% |
| Peripheral arterial disease (PAD) | Points | Thresholds |
| Records PAD001*. The contractor establishes and maintains a register of patients with peripheral arterial disease | 2 | N/A |
| Stroke and transient ischaemic attack (STIA) | Points | Thresholds |
| Records STIA001*. The contractor establishes and maintains a register of patients with stroke or TIA | 2 | N/A |
| Ongoing management STIA007. The percentage of patients with a stroke shown to be non-haemorrhagic, or a history of TIA, who have a record in the preceding 12 months that an anti-platelet agent, or an anti-coagulant is being taken | 4 | 57–97% |
| STIA014. The percentage of patients aged 79 years or under, with a history of stroke or TIA, in whom the last blood pressure reading (measured in the preceding 12 months) is 140/90 mmHg or less (or equivalent home blood pressure reading). | 3 | 40-73% |
| STIA015. The percentage of patients aged 80 years or over, with a history of stroke or TIA, in whom the last blood pressure reading (measured in the preceding 12 months) is 150/90 mmHg or less, (or equivalent home blood pressure reading). | 2 | 46-86% |
| Cholesterol control and lipid management (CHOL) | Points | Thresholds |
| Ongoing management CHOL001. Percentage of patients on the QOF Coronary Heart Disease, Peripheral Arterial Disease, Stroke/TIA or Chronic Kidney Disease Register who are currently prescribed a statin, or where a statin is declined or clinically unsuitable, another lipid-lowering therapy | 14 | 70-95% |
| CHOL002. Percentage of patients on the QOF Coronary Heart Disease, Peripheral Arterial Disease, or Stroke/TIA Register, who have a recording of non-HDL cholesterol in the preceding 12 months that is lower than 2.5 mmol/L, or where non-HDL cholesterol is not recorded a recording of LDL cholesterol in the preceding 12 months that is lower than 1.8 mmol/L | 16 | 20-35% |
| Diabetes mellitus (DM) | Points | Thresholds |
| Records DM017*. The contractor establishes and maintains a register of all patients aged 17 or over with diabetes mellitus, which specifies the type of diabetes where a diagnosis has been confirmed | 6 | N/A |
| Ongoing management DM006. The percentage of patients with diabetes, on the register, with a diagnosis of nephropathy (clinical proteinuria) or micro-albuminuria who are currently treated with an ACE-I (or ARBs) | 3 | 57–97% |
| DM012. The percentage of patients with diabetes, on the register, with a record of a foot examination and risk classification: 1) low risk (normal sensation, palpable pulses), 2) increased risk (neuropathy or absent pulses), 3) high risk (neuropathy or absent pulses plus deformity or skin changes in previous ulcer) or 4) ulcerated foot within the preceding 12 months | 4 | 50–90% |
| DM014. The percentage of patients newly diagnosed with diabetes, on the register, in the preceding 1 April to 31 March who have a record of being referred to a structured education programme within 9 months after entry on to the diabetes register | 11 | 40–90% |
| DM033. The percentage of patients with diabetes, on the register, without moderate or severe frailty in whom the last blood pressure reading (measured in the preceding 12 months) is 140/90 mmHg or less (or equivalent home blood pressure reading) | 10 | 38-78% |
| DM020. The percentage of patients with diabetes, on the registers, without moderate or severe frailty in whom the last IFCC-HbA1c is 58 mmol/mol or less in the preceding 12 months | 17 | 35-75% |
| DM021. The percentage of patients with diabetes, on the register, with moderate or severe frailty in whom the last IFCC-HbA1c is 75 mmol/mol or less in the preceding 12 months | 10 | 52-92% |
| DM022. The percentage of patients with diabetes aged 40 years and over, with no history of cardiovascular disease and without moderate or severe frailty, who are currently treated with a statin (excluding patients with type 2 diabetes and a CVD risk score of <10% recorded in the preceding 3 years) | 4 | 50-90% |
| DM023. The percentage of patients with diabetes and a history of cardiovascular disease (excluding haemorrhagic stroke) who are currently treated with a statin | 2 | 50-90% |
| Asthma (AST) | Points | Thresholds |
Records AST005*. The contractor establishes and maintains a register of patients with asthma aged 6 years or over, excluding patients with asthma who have been prescribed no asthma related drugs in the preceding 12 months | 4 | N/A |
Initial diagnosis AST011. The percentage of patients with a diagnosis of asthma on or after 1 April 2023 with either:1. A record of quality assured spirometry and one other objective test (FeNO or, bronchodilator reversibility or peak flow variability) between 3 months before or 6 months after diagnosis; or 2. If newly registered in the preceding 12 months with a diagnosis of asthma recorded on or after 1 April 2023 but no record of objective tests being performed at the date of the registration, with a quality assured spirometry and one other objective test (FeNO or bronchodilator reversibility or peak flow variability) recorded within 6 months of the registration. | 15 | 45–80% |
Ongoing management AST007. The percentage of patients with asthma on the register, who have had an asthma review in the preceding 12 months that includes an assessment of asthma control using a validated asthma control questionnaire, a recording of the number of exacerbations, an assessment of inhaler technique and a written personalised action plan. | 20 | 45–70% |
| AST008. The percentage of patients with asthma on the register aged 19 or under, in whom there is a record of either personal smoking status or exposure to second-hand smoke in the preceding 12 months. | 6 | 45–80% |
| Chronic obstructive pulmonary disease (COPD) | Points | Thresholds |
| Records COPD015*. The contractor establishes and maintains a register of: 1. Patients with a clinical diagnosis of COPD before 1 April 2023; and 2. Patients with a clinical diagnosis of COPD on or after 1 April 2023 whose diagnosis has been confirmed by a quality assured post-bronchodilator spirometry FEV1/FVC ratio below 0.7 between 3 months before or 6 months after diagnosis (or if newly registered at the practice in the preceding 12 months without a record of spirometry having been performed, a record of an FEV1/FVC ratio below 0.7 recorded within 6 months of registration); and Patients with a clinical diagnosis of COPD on or after 1 April 2023 who are unable to undertake spirometry | 8 | N/A |
| Ongoing management COPD010. The percentage of patients with COPD on the register, who have had a review in the preceding 12 months, including a record of the number of exacerbations and an assessment of breathlessness using the Medical Research Council dyspnoea scale | 9 | 50–90% |
| COPD014. The percentage of patients with COPD and Medical Research Council (MRC) dyspnoea scale ≥3 at any time in the preceding 12 months, with a subsequent record of referral to a pulmonary rehabilitation programme (excluding those who have previously attended a pulmonary rehabilitation programme) | 2 | 40-90% |
| Dementia (DEM) | Points | Thresholds |
| Records DEM001*. The contractor establishes and maintains a register of patients diagnosed with dementia | 5 | N/A |
| Ongoing management DEM004. The percentage of patients diagnosed with dementia whose care plan has been reviewed in the preceding 12 months | 14 | 35–70% |
| Depression (DEP) | Points | Thresholds |
| Initial management DEP004. The percentage of patients aged 18 or over with a new diagnosis of depression in the preceding 1 April to 31 March, who have been reviewed not earlier than 10 days after and not later than 56 days after the date of diagnosis | 10 | 45–80% |
| Mental health (MH) | Points | Thresholds |
| Records MH001*. The contractor establishes and maintains a register of patients with schizophrenia, bipolar affective disorder and other psychoses and other patients on lithium therapy | 4 | N/A |
| Ongoing management MH002. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a comprehensive care plan documented in the record, in the preceding 12 months, agreed between individuals, their family and/or carers as appropriate | 5 | 40–90% |
| MH003. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of blood pressure in the preceding 12 months | 3 | 50–90% |
| MH006. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of BMI in the preceding 12 months | 3 | 50-90% |
| MH007. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of alcohol consumption in the preceding 12 months | 3 | 50-90% |
| MH011. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of a lipid profile in the preceding 12 months (in those patients currently prescribed antipsychotics, and/or have pre-existing cardiovascular conditions, and/or smoke, and/or are overweight (BMI of ≥23 kg/m2 or ≥25 kg/m2 if ethnicity is recorded as White)) or preceding 24 months for all other patients | 7 | 50-90% |
| MH012. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of blood glucose or HbA1c in the preceding 12 months | 7 | 50-90% |
| MH021: Percentage of patients with schizophrenia, bipolar affective disorder and other psychoses and those on lithium therapy, who received all six elements of the Physical Health Check for people with Severe Mental Illness | 6 | 50-80% |
| Cancer (CAN) | Points | Thresholds |
| Records CAN001*. The contractor establishes and maintains a register of all cancer patients defined as a ‘register of patients with a diagnosis of cancer excluding non-melanotic skin cancers diagnosed on or after 1 April 2003’ | 5 | N/A |
| Ongoing management CAN004. The percentage of patients with cancer, diagnosed within the preceding 24 months, who have a patient Caner Care Review using a structured template recorded as occurring within 12 months of the date of diagnosis | 6 | 50–90% |
| CAN005. The percentage of patients with cancer, diagnosed within the preceding 12 months, who have had the opportunity for a discussion and informed of the support available from primary care, within 3 months of diagnosis | 2 | 70-90% |
| Chronic kidney disease (CKD) | Points | Thresholds |
| Records CKD005*. The contractor establishes and maintains a register of patients aged 18 or over with CKD with classification of categories G3a to G5 (previously stage 3 to 5) | 6 | N/A |
| Epilepsy (EP) | Points | Thresholds |
| Records EP001*. The contractor establishes and maintains a register of patients aged 18 or over receiving drug treatment for epilepsy | 1 | N/A |
| Learning disability (LD) | Points | Thresholds |
| Records LD004*. The contractor establishes and maintains a register of patients with learning disabilities | 4 | N/A |
| Osteoporosis: secondary prevention of fragility fractures (OST) | Points | Thresholds |
| Records OST004*. The contractor establishes and maintains a register of patients: 1. Aged 50 or over and who have not attained the age of 75 with a record of a fragility fracture on or after 1 April 2012 and a diagnosis of osteoporosis confirmed on DXA scan, and 2. Aged 75 or over with a record of a fragility fracture on or after 1 April 2014 and a diagnosis of osteoporosis. | 3 | N/A |
| Rheumatoid arthritis (RA) | Points | Thresholds |
| Records RA001*. The contractor establishes and maintains a register of patients aged 16 or over with rheumatoid arthritis | 1 | N/A |
| Palliative care (PC) | Points | Thresholds |
| Records PC001*. The contractor establishes and maintains a register of all patients in need of palliative care/support irrespective of age. | 3 | N/A |
| Non diabetic hyperglycaemia (NDH) | Points | Thresholds |
| Records NDH002. The percentage of patients with non-diabetic hyperglycaemia who have had an HbA1c or fasting blood glucose performed in the preceding 12 months. | 18 | 50–90% |
Public health domain (160 points)
Public health domain: This domain applies to all contractors participating in QOF, with the exception of the additional services sub-domain (discussed below).
| Blood pressure (BP) | Points | Thresholds |
| BP002. The percentage of patients aged 45 or over who have a record of blood pressure in the preceding 5 years | 15 | 50–90% |
| Obesity (OB) | Points | Thresholds |
| Records OB003*. The contractor establishes and maintains a register of patients aged 18 years or over living with obesity, appropriately adjusted for ethnicity in line with NICE guidelines – either with a BMI greater than or equal to 30 kg/m2 recorded in the preceding 12 months, or a BMI greater than or equal to 27.5 kg/m2 recorded in the preceding 12 months for patients with a South Asian, Chinese, other Asian, Middle Eastern, Black African or African-Caribbean family background | 8 | N/A |
| Smoking (SMOK) | Points | Thresholds |
| Records SMOK002. The percentage of patients with any or any combination of the following conditions: CHD, PAD, stroke or TIA, hypertension, diabetes, COPD, CKD, asthma, schizophrenia, bipolar affective disorder or other psychoses whose notes record smoking status in the preceding 12 months | 25 | 50–90% |
| Ongoing management SMOK004. The percentage of patients aged 15 or over who are recorded as current smokers who have a record of an offer of support and treatment within the preceding 24 months | 12 | 40–90% |
| SMOK005. The percentage of patients with any or any combination of the following conditions: CHD, PAD, stroke or TIA, hypertension, diabetes, COPD, CKD, asthma, schizophrenia, bipolar affective disorder or other psychoses who are recorded as current smokers who have a record of an offer of support and treatment within the preceding 12 months | 25 | 56–96% |
| Vaccination and Immunisations (VI) | Points | Thresholds |
| VI001. The percentage of babies who reached 8 months old in the preceding 12 months, who have received at least 3 doses of a diphtheria, tetanus and pertussis containing vaccine before the age of 8 months | 18 | 89-96% |
| VI002. The percentage of children who reached 18 months old in the preceding 12 months, who have received at least 1 dose of MMR between the ages of 12 and 18 months | 18 | 86-96% |
| VI003. The percentage of children who reached 5 years old in the preceding 12 months, who have received a reinforcing dose of DTaP/IPV and at least 2 doses of MMR between the ages of 1 and 5 years | 18 | 81-96% |
| VI004. The percentage of patients who reached 80 years old in the preceding 12 months, who have received a shingles vaccine between the ages of 70 and 79 years | 10 | 50-60% |
Public health domain – additional services sub-domain
The additional services sub-domain applies to contractors who provide additional services under the terms of the GMS contract and participate in QOF.
| Cervical screening (CS) | Points | Thresholds |
| CS005. The proportion of women eligible for screening and aged 25-49 years at the end of period reported whose notes record that an adequate cervical screening test has been performed in the previous 3 years and 6 months | 7 | 45-80% |
| CS006. The proportion of women eligible for screening and aged 50-64 years at the end of period reported whose notes record that an adequate cervical screening test has been performed in the previous 5 years and 6 months | 4 | 45-80% |
Quality improvement domain (74 points)
This domain applies to all contractors participating in QOF.
| Workforce and Wellbeing | Points | Thresholds |
| QI013. The contractor can demonstrate continuous quality improvement activity focused upon workforce and wellbeing as specified in the QOF guidance. | 27 | N/A |
| QI014. The contractor has participated in network activity to regularly share and discuss learning from quality improvement activity focused on workforce and wellbeing as specified in current QOF guidance. This would entail attending two primary care network meetings, at the start and towards the end of QI activity. If a practice is not within a PCN, the expectation is that two meetings would be held locally with other practices. | 10 | N/A |
| Optimising demand and capacity in general practice | Points | Thresholds |
| Part 1: Optimise use of staff capacity QI016. The contractor can demonstrate that it has in place a recognised and validated approach to understanding demand/activity, capacity and appointment data and has made improvements to data quality to better reflect practice work. | 10 | N/A |
| QI017. The contractor can demonstrate that it has utilised demand and capacity data to inform operational decisions and plan for demand and capacity matching | 6 | N/A |
| QI018. The contractor has participated in network activity to review the smart cards of all staff employed under the Additional Roles Reimbursement Scheme (ARRS) | 6 | N/A |
| Part 2: Reducing avoidable appointments QI019. The contractor can demonstrate improvement in reducing avoidable appointments. A suggested approach is outlined below: 1. Using BI tools, if available and practice collected data where not, to understand the practice activity including variations over the days of the week, time of day and time of year. 2. Developing an understanding of the telephone queue either by extracting data from their cloud- based telephony system or asking staff to collect data over a period. 3. Using that data to understand their peaks of activity and better matching their capacity to their demand by, for instance, reviewing rotas. 4. Using improvement techniques described in the Primary Care Transformation Teams webinar series on Demand and Capacity which provides practical advice and guidance. 5. Referencing the Royal College of General Practitioner’s 6 steps to start to improve delivering continuity of care from their Continuity Toolkit for those who need it and adapting to suit the needs of the practice. | 15 | N/A |
Section 3: Clinical domain
Atrial fibrillation (AF)
| Indicator | Points | Thresholds |
| Records AF001. The contractor establishes and maintains a register of patients with atrial fibrillation | 5 | N/A |
| Ongoing management AF006. The percentage of patients with atrial fibrillation in whom stroke risk has been assessed using the CHA2DS2- VASc score risk stratification scoring system in the preceding 12 months (excluding those patients with a previous CHADS2 or CHA2DS2-VASc score of 2 or more) | 12 | 40-90% |
| AF008. Percentage of patients on the QOF Atrial Fibrillation register and with a CHA2DS2- VASc score of 2 or more, who were prescribed a direct-acting oral anticoagulant (DOAC), or, where a DOAC was declined or clinically unsuitable, a Vitamin K antagonist | 12 | 70-95% |
AF – rationale for inclusion of indicator set
AF is the most common heart rhythm disorder, affecting approximately 2% of the adult population, and estimates suggest its prevalence is increasing. AF causes palpitations and breathlessness in many people, but it may also be asymptomatic and therefore go undetected. Left untreated, AF is a significant risk factor for stroke: it is estimated that it is responsible for approximately 20% of all strokes and is associated with increased mortality and significant morbidity. Men are more commonly affected than women. AF prevalence increases with age and in association with heart disease, diabetes, obesity and hypertension (NICE NG196 atrial fibrillation).
AF001 Rationale
The register includes all patients with an initial event; paroxysmal; persistent and permanent AF.
AF001 Reporting and verification
See indicator wording for requirement criteria.
Where a patient has been diagnosed with AF and been subsequently successfully treated, if there is an ‘AF resolved code’ present in their record after the latest AF recording, they will be removed from the register.
AF may resolve in some specific and limited situations. Contractors should also note that patients who have been recorded with AF resolved, continue to be at an increased risk of stroke compared to patients who have never had an episode of AF (Adderley et al, 2018). Contractors should consider the implications of this for individual patients before using the AF resolved code.
AF006 (NICE 2014 menu ID: NM81)
AF006 Rationale
The NICE guideline on atrial fibrillation recommends that people with symptomatic or asymptomatic paroxysmal, persistent or permanent AF, atrial flutter or a continuing risk of arrhythmia recurrence after cardioversion back to sinus rhythm should have an assessment of their stroke risk using the CHA2DS2-VASc risk assessment tool.
The CHA2DS2-VASc system scores one point, up to a maximum of nine, for each of the following risk factors (except previous stroke or TIA, or age ≥75 which scores double, hence the ‘2’):
- C: congestive HF (one point)
- H: hypertension (one point)
- A2: age 75 or over (two points)
- D: diabetes mellitus (one point)
- S2: previous stroke or TIA or thromboembolism (two points)
- V: vascular disease (e.g. PAD, MI, aortic plaque) (one point)
- A: age 65-74 years (one point)
- Sc: sex category (i.e. female sex) (one point)
AF006 Reporting and verification
See indicator wording for requirement criteria.
Stroke risk assessment should be repeated on an annual basis unless the patient has previously scored 2 or more using either CHA2DS2-VASc at any time, or CHADS2 prior to 1 April 2015.
AF008 (NICE 2022 menu ID:NM231)
AF008 Rationale
This indicator aims to support people with AF who are at increased risk of stroke so that they may be offered anti-coagulation drug therapy.
The risk of stroke is five times higher for patients with AF than for the general population, and 20–30% of all strokes are attributed to this arrhythmia. The Stroke Association estimate that if AF were adequately treated, around 7,000 strokes would be prevented and over 2,000 lives saved every year in England alone.
The NHS Long Term Plan commits to reducing stroke in England in three ways:
- Diagnosing more patients with undiagnosed AF (the “detect” gap)
- Ensuring patients diagnosed with AF are offered anticoagulation where appropriate (the “protect” gap)
- Optimising the anticoagulant pathway to ensure patient outcomes are optimised (the “perfect” gap)
This indicator has been developed to support LTP ambitions on the “protect” gap and complement QOF indicator AF007, which rewards practices for ensuring that up to 70% of patients on their AF register are anticoagulated. It has two objectives:
- To increase the overall percentage of AF patients who are prescribed an anticoagulant
- To increase the use of DOACs as a proportion of anticoagulants prescribed
Anticoagulation therapy can prevent around two thirds of strokes caused by AF. However, 16% of patients with AF are not on any form of anticoagulant.
Likewise, 14% of patients currently receiving anticoagulation therapy are prescribed Warfarin. NICE guidance was updated in 2021 (NG196) to recommend that clinicians prescribe DOACs, rather than Warfarin as first-line treatment for patients with AF. Warfarin is associated with a more significant risk of serious bleeding (particularly intracranial haemorrhage) than DOACs. DOACs also do not require as much monitoring, freeing up capacity in primary care and improving quality of life for patients. Other benefits of DOACs over Warfarin include:
- Fixed dosing with predictable pharmacokinetics and pharmacodynamics
- Low drug–drug and food interactions, and no dietary restrictions
- Rapid onset and offset and shorter half-life
- Predictable effects on clotting, so routine monitoring of clotting factors is not needed
- Wide therapeutic window
In line with NG196, practices may achieve against this indicator by working to switch patients who are currently prescribed Warfarin or by prescribing patients who are newly diagnosed with AF a DOAC. However, it is important that switching patients who are currently prescribed Warfarin is done in a clinically appropriate way and as the result of a shared decision-making conversation. Recognising the importance of this, the indicator has been designed to accommodate patients who are unsuitable for a switch to DOACs or who declined to do so after a conversation with their clinician. PCNs will not be penalised for continuing to prescribe Warfarin where a patient has declined a DOAC or where a DOAC is clinically unsuitable. In these circumstances, the prescription of Warfarin will count as a “success”. Please consult above and business rules for more information.
AF008 Reporting and verification
See indicator wording for requirement criteria.
Secondary prevention of coronary heart disease (CHD)
| Indicator | Points | Thresholds |
| Records CHD001. The contractor establishes and maintains a register of patients with coronary heart disease | 4 | N/A |
| Ongoing management CHD005. The percentage of patients with coronary heart disease with a record in the preceding 12 months that aspirin, an alternative anti-platelet therapy, or an anti- coagulant is being taken | 7 | 56–96% |
| CHD015. The percentage of patients aged 79 years or under, with coronary heart disease, in whom the last blood pressure reading (measured in the preceding 12 months) is 140/90 mmHg or less, (or equivalent home blood pressure reading) | 12 | 40-77% |
| CHD016. The percentage of patients aged 80 years or over, with coronary heart disease, in whom the last blood pressure reading (measured in the preceding 12 months) is 150/90 mmHg or less, (or equivalent home blood pressure reading) | 5 | 46-86% |
CHD – rationale for inclusion of indicator set
CHD is the single most common cause of premature death in the UK (British Heart Foundation, 2024). The research evidence relating to the management of CHD is well established and if implemented can reduce the risk of death from CHD and improve the quality of life for patients.
This indicator set focuses on the management of patients with established CHD.
CHD001 Rationale
The register includes all patients who have had coronary artery revascularisation procedures, such as coronary artery bypass grafting (CABG). Patients with Cardiac Syndrome X are not included on the CHD register.
Contractors should record those with a history of myocardial infarction (MI) as well as those with a history of CHD.
CHD001 Reporting and verification
See indicator wording for requirement criteria.
CHD005 (NICE 2015 menu ID: NM88)
CHD005 Rationale
NICE guidance recommends all people who have had an MI should be offered aspirin (or clopidogrel if aspirin is contraindicated). Antiplatelet therapy with clopidogrel is equivalent to aspirin in preventing further cardiovascular events in people with coronary heart disease or ischaemic stroke.
CHD005 Reporting and verification
See indicator wording for requirement criteria.
CHD015 (NICE 2022 menu ID: NM225)
CHD015 Rationale
This indicator measures the intermediate outcome of a blood pressure of 140/90 mmHg or less in people aged 79 years or under with CHD. The aim is to promote secondary prevention of cardiovascular disease through satisfactory blood pressure control. This may be achieved through lifestyle advice or drug therapy.
This indicator has been updated for 2023/24 to align with updated NICE guidance which recommends differential blood pressure control targets for readings taken with Home Blood Pressure Monitoring (HBPM) and readings taken in a clinical setting. A target for stage 1 hypertension of 140/90mmHg taken in a clinic corresponds to an HBPM target of 135/85 mmHg.
CHD015 Reporting and verification
See indicator wording for requirement criteria
CHD016 (NICE 2022 menu ID: NM226)
CHD016 Rationale
This indicator measures the intermediate outcome of a blood pressure of 150/90 mmHg or less in people aged 80 years and over with coronary heart disease as recommended by the NICE clinical guideline for hypertension.
This indicator has been updated for 2023/24 to align with updated NICE guidance which recommends differential blood pressure control targets for readings taken with Home Blood Pressure Monitoring (HBPM) and readings taken in a clinical setting. A target of 150/90mmHg taken in a clinic corresponds to an HBPM target of 145/85 mmHg.
CHD0016 Reporting and verification
See indicator wording for requirement criteria.
Cholesterol Control and Lipid Management (CHOL)
| Indicator | Points | Thresholds |
| Ongoing management CHOL001. Percentage of patients on the QOF Coronary Heart Disease, Peripheral Arterial Disease, Stroke/TIA or Chronic Kidney Disease Register who are currently prescribed a statin, or where a statin is declined or clinically unsuitable, another lipid-lowering therapy | 14 | 70-95% |
| CHOL002. Percentage of patients on the QOF Coronary Heart Disease, Peripheral Arterial Disease, or Stroke/TIA Register, who have a recording of non-HDL cholesterol in the preceding 12 months that is lower than 2.5 mmol/L, or where non-HDL cholesterol is not recorded a recording of LDL cholesterol in the preceding 12 months that is lower than 1.8 mmol/L | 16 | 20-35% |
CHOL – rationale for inclusion of indicator set
High cholesterol is one of the most significant risk factors for CVD. Globally, a third of ischaemic heart disease is attributable to high cholesterol. It is estimated to account for 7.1% of deaths and 3.7% of disability-adjusted life years (DALYS) in England.
CHOL001 (Based on NICE 2022 menu ID: NM212)
CHOL001 Rationale
The aim of this indicator is to ensure that all patients with established cardiovascular disease, defined as Coronary Heart Disease, Peripheral Arterial Disease, Stroke/TIA or Chronic Kidney Disease receive treatment to reduce cholesterol in line with NICE guidelines, read a summary here.
Treatment with a statin is recommended as first line therapy for the secondary prevention of CVD (NICE 2014). Options recommended by NICE when a statin is declined or clinically unsuitable due to contraindications or intolerance include:
- Ezetimibe (NICE TA385, 2022), with the addition of bempedoic acid (NICE TA694, 2021) if a sufficient fall in cholesterol is not achieved with ezetimibe monotherapy.
- PCSK9 inhibitors (NICE TA393, 2016. NICE TA394, 2016) for people with an LDL persistently above 5 or 4.0 mmol/L depending on their CVD risk profile
- Inclisiran (NICE TA733, 2021) for people with an LDL persistently 6mmol/L or above.
Where a statin is declined or clinically unsuitable due to contraindications or intolerance, these treatments will be included as a ‘success’.
CHOL001 Reporting and verification
See indicator wording for requirement criteria.
CHOL002
CHOL002 Rationale
The purpose of the indicator is to introduce an interim outcome measure for use of lipid lowering treatments outlined in CHOL001 above for patients with established cardiovascular disease. This aims to ensure that all patients with established cardiovascular disease, defined as Coronary Heart Disease, Peripheral Arterial Disease, or Stroke/TIA are considered for intensification of therapy where there is an insufficient reduction in cholesterol with first line therapy, usually a statin.
The aim of managing non-HDL cholesterol to less than 2.5 mmol/L is aligned with the Joint British Societies recommendations that can be found here: Joint British Societies publish new guidelines for CVD prevention: JBS3 – IPCCS.
Where a non-HDL cholesterol target has not been achieved, treatment should be intensified in line with NICE guidance, read a summary here.
Patients may be considered for the addition of ezetimibe or injectable therapies in line with the NICE inclusion criteria for the individual agents – for example, for inclisiran, patients must have an LDL≥ 2.6mmol/L and for the use of PCSK9i(mabs), an LDL cholesterol > 3.5 or 4mmol/L depending on their risk profile. Where statin intolerance exists and ezetimibe monotherapy is ineffective, the addition of bempedoic acid may be considered in line with the statin intolerance pathway.
CHOL002 Reporting and verification
See indicator wording for requirement criteria.
Heart failure (HF)
| Indicator | Points | Thresholds |
| Records HF001. The contractor establishes and maintains a register of patients with heart failure | 4 | N/A |
| Initial diagnosis HF008. The percentage of patients with a diagnosis of heart failure on or after 1 April 2023 which: 1. Has been confirmed by an echocardiogram or by specialist assessment in the 6 months before entering on to the register; or 2. If registered at the practice after diagnosis, with no record of the diagnosis originally being confirmed either by echocardiogram or by specialist assessment, a record of an echocardiogram or a specialist assessment within 6 months of the date of registration. | 6 | 50–90% |
| Ongoing management HF003. In those patients with a diagnosis of heart failure due to left ventricular systolic dysfunction or whose heart failure is due to reduced ejection fraction the percentage of patients who are currently treated with an angiotensin- converting enzyme inhibitor (ACE-I) or Angiotensin II receptor blockers (ARB). | 6 | 60–92% |
| HF006. The percentage of patients with a diagnosis of heart failure due to left ventricular systolic dysfunction or whose heart failure is due to reduced ejection fraction, who are currently treated with a beta-blocker licensed for heart failure. | 6 | 60-92% |
| HF007. The percentage of patients with a diagnosis of heart failure on the register, who have had a review in the preceding 12 months, including an assessment of functional capacity and a review of medication to ensure medicines optimisation at maximal tolerated doses | 7 | 50-90% |
HF – rationale for inclusion of indicator set
HF represents the only major cardiovascular disease with increasing prevalence and carries a poor prognosis for patients. This indicator set refers to all patients with HF unless specified otherwise.
HF001
HF001 Rationale
All patients with a diagnosis of HF, are included on the register.
HF001 Reporting and verification
See indicator wording for requirement criteria.
There are two disease registers used for the HF indicators for the purpose of calculating APDF (practice prevalence):
- Register 1: patients with HF is used to calculate APDF for HF001, HF008, and
- Register 2: patients with HF due to left ventricular systolic dysfunction (LVSD) is used to calculate APDF for HF003 and HF006.
Register 1 is defined in indicator HF001. Register 2 is a sub-set of register 1 and is composed of patients with a diagnostic code for LVSD or a reduced ejection fraction of <40% as well as for HF.
HF008 (based on NM171)
HF008 Rationale
The aim of this indicator is to encourage practices to confirm diagnoses of heart failure and establish the underlying causes.
Symptoms and signs suggestive of heart failure are not sufficient to make a definitive diagnosis and further investigation is required to confirm cardiac dysfunction and to identify causes. The NICE guideline for chronic heart failure recommends that the results of NT-proBNP tests should be used to determine whether people with suspected heart failure should be referred onwards. People with raised NT-proBNP should have echocardiography and specialist assessment within 6 weeks, but for those with very high levels this should be done more urgently, within 2 weeks. The NICE guideline for acute heart failure recommends that people with new suspected acute heart failure who have raised natriuretic peptides should have echocardiography within 48 hours of admission to hospital.
HF008 Reporting and verification
See indicator wording for requirement criteria. For measurement purposes, three months before the date of diagnosis is defined as 93 days.
HF003 (NICE 2019 menu ID: NM172)
HF003 Rationale
There is strong clinical and cost-effectiveness evidence to support the use of ACE-I in all patients with HF with LVSD. ACE-I improve symptoms, reduce the hospitalisation rate and improve the survival rate. This is applicable in all age groups.
It is possible to have a diagnosis of LVSD without HF, for example, asymptomatic people who might be identified coincidently but who are at high risk of developing subsequent HF. In such cases, ACE-I’s delay the onset of symptomatic HF, reduce cardiovascular events and improve long-term survival. This indicator only applies to patients with HF and therefore excludes this other group of patients who are nevertheless to be considered for treatment with ACE-I.
NICE NG106 recommends ACE-I is used as first-line therapy in all patients with HF with reduced ejection fraction usually defined as LVSD and that ARBs are used only in patients who are intolerant of ACE-I.
The denominator includes patients whose heart failure is due to reduced ejection fraction, and the wording for HF003 has been changed for 2023/24 to make this clearer.
HF003 Reporting and verification
See indicator wording for requirement criteria.
HF006 (NICE 2019 menu ID: NM173)
HF006 Rationale
The NICE guideline for chronic heart failure recommends that beta-blockers licensed for HF are used as first-line therapy in all patients with HF with reduced ejection fraction usually defined as LVSD. It also recommends that treatment with beta-blockers is not withheld solely because of age or the presence of peripheral vascular disease (PVD), erectile dysfunction (ED), DM, interstitial pulmonary disease and COPD without reversibility. The only co-morbidities with a clear contra-indication to beta-blocker use are those with asthma and reversible airways obstruction (these groups were excluded from clinical trials).
The British National Formulary (BNF) states that “the beta-blockers bisoprolol and carvedilol are of value in any grade of stable HF and LVSD; nebivolol is licensed for stable mild to moderate HF in patients aged over 70, beta-blocker treatment should be initiated at a very low dose and titrated very slowly over a period of weeks or months by those experienced in the management of HF. Symptoms may deteriorate initially, calling for adjustment of concomitant therapy.”
Contractors are advised that patients already prescribed an unlicensed beta-blocker prior to diagnosis of HF due to LVSD do not have their drug therapy changed to meet the criteria of this indicator. Those patients already prescribed an unlicensed beta-blocker will be excluded from the indicator denominator.
The denominator includes patients whose heart failure is due to reduced ejection fraction, the wording for HF006 has been changed for 2023/24 to make this clearer.
HF006 Reporting and verification
See indicator wording for requirement criteria.
Patients prescribed a beta-blocker unlicensed for heart failure before being given a diagnosis of heart failure will be excluded from this indicator.
HF007 (based on NM174) HF007 Rationale
Regular review is associated with improvement in quality of life and a reduction in the need for urgent hospitalisation. NICE guideline NG106 recommends short monitoring intervals (days to 2 weeks) if the clinical condition or medication has changed and 6-monthly for people with stable heart failure.
HF007 Reporting and verification
See indicator wording for requirement criteria.
Hypertension (HYP)
| Indicator | Points | Thresholds |
| Records HYP001. The contractor establishes and maintains a register of patients with established hypertension | 6 | N/A |
| Ongoing management HYP008. The percentage of patients aged 79 years or under with hypertension in whom the last blood pressure reading (measured in the preceding 12 months) is 140/90 mmHg or less (or equivalent home blood pressure reading) | 14 | 40-77% |
| HYP009. The percentage of patients aged 80 years or over with hypertension in whom the last blood pressure reading (measured in the preceding 12 months) is 150/90 mmHg or less (or equivalent home blood pressure reading) | 5 | 40-80% |
HYP001
The contractor establishes and maintains a register of patients with established hypertension
HYP001 Rationale
Effective treatment of hypertension aims to reduce the risk of cardiovascular problems such as heart attacks and strokes.
Patients who have had one-off high blood pressure readings and women who have been hypertensive in pregnancy should not be included in the register.
NICE NG13621 uses the following definitions:
Stage 1 hypertension
Clinic blood pressure ranging from 140/90 mmHg to 159/99 mmHg and subsequent ambulatory blood pressure monitoring (ABPM) daytime average or home blood pressure monitoring (HBPM) average blood pressure ranging from 135/85 mmHg to 149/94 mmHg
Stage 2 hypertension
Clinic blood pressure of 160/100 mmHg or higher but less than 180/120 mmHg and subsequent ABPM daytime average or HBPM average blood pressure of 150/95 mmHg or higher.
Stage 3 or severe hypertension
Clinic systolic blood pressure of 180 mmHg or higher or clinic diastolic blood pressure of 120 mmHg or higher.
If clinic blood pressure reading is between 140/90 mmHg and 180/120 mmHg the NICE guideline for hypertension recommends offering ABPM to confirm a diagnosis of hypertension. If ABPM is unsuitable or the person is unable to tolerate it HBPM is a suitable alternative to confirm a diagnosis of hypertension.
For patients aged 39 or under, NICE recommend that practitioners consider seeking specialist evaluation of secondary causes of hypertension.
HYP001 Reporting and verification
See indicator wording for requirement criteria.
The contractor may be required by commissioners to discuss their plans for ensuring that new diagnoses are confirmed using ABPM or HBPM as appropriate.
HYP008 (NICE 2022 menu ID: NM223)
HYP008 Rationale
This indicator measures the intermediate outcome of a blood pressure of 140/90 mmHg or less in people aged 79 years or under with hypertension. Its intent is to promote the primary and secondary prevention of cardiovascular disease through satisfactory blood pressure control. The intermediate outcome can be achieved through lifestyle advice or the use of drug therapy.
This indicator has been updated for 2023/24 to align with updated NICE guidance which recommends differential blood pressure control targets for readings taken with Home Blood Pressure Monitoring (HBPM) and readings taken in a clinical setting. A target for stage 1 hypertension of 140/90mmHg taken in a clinic corresponds to an HBPM target of 135/85 mmHg.
HYP008 Reporting and verification
See indicator wording for requirement criteria.
HYP009 (NICE 2022 menu ID: NM224)
HYP009 Rationale
The NICE guideline for hypertension recommends that patients aged 80 years and over with hypertension should be treated to a target blood pressure below 150/90 mmHg. It also recommends that this group of patients should be offered the same antihypertensive drug treatment as people aged 55-80 years, taking into account any co-morbidities.
Where people have had a lower treatment target before the age of 80 years their treatment should continue and not be adjusted or down titrated. There is an important distinction between continuing long term and well tolerated treatment in people aged 80 years and older, and starting blood pressure lowering therapy at this age.
This indicator has been updated for 2023/24 to align with updated NICE guidance which recommends differential blood pressure control targets for readings taken with Home Blood Pressure Monitoring (HBPM) and readings taken in a clinical setting. A target of 150/90mmHg taken in a clinic corresponds to an HBPM target of 145/85 mmHg.
HYP009 Reporting and verification
See indicator wording for requirement criteria.
Peripheral arterial disease (PAD)
| Indicator | Points | Thresholds |
| Records PAD001. The contractor establishes and maintains a register of patients with peripheral arterial disease | 2 | N/A |
PAD – rationale for inclusion of indicator set
PAD is one of the three main categories of CVD and patients with PAD, including those who are asymptomatic, have an increased risk of mortality from CVD due to MI and stroke. The relative risks of all-cause mortality are two to three times that of age and sex matched to groups without PAD.
Treatment of PAD focuses on cardiovascular risk factor management. Smoking is a very important risk factor for PAD and management of PAD includes smoking cessation (see smoking indicator set). Other established risk factors are high blood pressure and diabetes. This would mean that patients with PAD and high blood pressure would also be included in the hypertension indicator set and patients with diabetes and PAD would also be included in the diabetes indicator set.
Further information:
PAD001 (NICE 2011 menu ID: NM32)
PAD001 Rationale
Patients with PAD may have symptoms but can also be asymptomatic. About 20 per cent of patients aged 60 or over have PAD, although only a quarter of these patients have symptoms. Symptoms become severe and progressive in approximately 20 per cent of patients with symptomatic PAD.
Reduced ankle brachial pressure index is an independent predictor of cardiac and cerebrovascular morbidity and mortality and may help to identify patients who would benefit from secondary prevention.
PAD001 Reporting and verification
See indicator wording for requirement criteria.
Stroke and TIA (STIA)
| Indicator | Points | Thresholds |
| Records STIA001. The contractor establishes and maintains a register of patients with stroke or TIA | 2 | N/A |
| Ongoing management STIA007. The percentage of patients with a stroke shown to be non-haemorrhagic, or a history of TIA, who have a record in the preceding 12 months that an anti-platelet agent, or an anti-coagulant is being taken | 4 | 57–97% |
| STIA014. The percentage of patients aged 79 years or under, with a history of stroke or TIA, in whom the last blood pressure reading (measured in the preceding 12 months) is 140/90 mmHg or less (or equivalent home blood pressure reading). | 3 | 40-73% |
| STIA015. The percentage of patients aged 80 years or over, with a history of stroke or TIA, in whom the last blood pressure reading (measured in the preceding 12 months) is 150/90 mmHg or less, (or equivalent home blood pressure reading). | 2 | 46-86% |
STIA – rationale for inclusion of indicator set
Stroke is the third most common cause of death in the developed world. One quarter of stroke deaths occur under the age of 65. There is evidence that appropriate diagnosis and management can improve outcomes (NICE NG128, 2019).
STIA001
STIA001 Rationale
For patients diagnosed prior to 1 April 2003 it is accepted that various diagnostic criteria may have been used. For this reason, the presence of the diagnosis of stroke or TIA in the record will be acceptable. Generally, patients with a diagnosis of transient global amnesia or vertebra-basilar insufficiency are not included in the retrospective register. However, contractors may wish to review patients previously diagnosed and if appropriate attempt to confirm the diagnosis.
It is up to the contractor to decide, on clinical grounds, when to include a patient on the register e.g. when a ‘dizzy spell’ becomes a TIA. Patient records coded with ‘Amaurosis fugax’, but without a code for TIA are excluded from the register.
STIA001 Reporting and verification
See indicator wording for requirement criteria.
STIA007 (NICE 2015 menu ID: NM94)
STIA007 Rationale
Long-term anti-platelet therapy reduces the risk of serious vascular events following a stroke by about a quarter. It is advised that anti-platelet therapy is prescribed for the secondary prevention of recurrent stroke and other vascular events in patients who have sustained an ischaemic cerebrovascular event.
The British National Formulary (BNF) makes the following recommendations:
“Patients should receive long-term treatment following a transient ischaemic attack or an ischaemic stroke to reduce the risk of further cardiovascular events.
Following a transient ischaemic attack or an ischaemic stroke (not associated with AF), long-term treatment with clopidogrel [unlicensed in transient ischaemic attack] is recommended. If clopidogrel is contra-indicated or not tolerated, patients can receive modified-release dipyridamole in combination with aspirin; if both aspirin and clopidogrel are contra-indicated or not tolerated, then modified-release dipyridamole alone is recommended; if both modified-release dipyridamole and clopidogrel are contra-indicated or not tolerated, then aspirin alone is recommended.
Patients with stroke associated with AF should be reviewed for long-term treatment with warfarin sodium or an alternative anti-coagulant (see initial management under ischaemic stroke).”
Further information – NICE TA210 (201) Clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events.
STIA007 Reporting and verification
See indicator wording for requirement criteria.
STIA014 (NICE 2022 menu ID: NM227)
STIA014 Rationale
This indicator measures the intermediate outcome of a blood pressure of 140/90 mmHg or less in people aged 79 years and under who have experienced a stroke or TIA. It aims to promote the secondary prevention of cardiovascular disease through satisfactory blood pressure control. The intermediate outcome can be achieved through lifestyle advice or drug therapy subject to the caveat below.
The NICE guideline on hypertension recommends drug therapy in people aged 79 years and under with stage 1 hypertension and cardiovascular disease.
Antihypertensive drug treatment is recommended for people of any age with stage 2 hypertension.
This indicator has been updated for 2023/24 to align with updated NICE guidance which recommends differential blood pressure control targets for readings taken with Home Blood Pressure Monitoring (HBPM) and readings taken in a clinical setting. A target for stage 1 hypertension of 140/90mmHg taken in a clinic corresponds to an HBPM target of 135/85 mmHg.
STIA014 Reporting and verification
See indicator wording for requirement criteria.
STIA015 (NICE 2022 menu ID: NM228
STIA015 Rationale
This indicator measures the intermediate outcome of a blood pressure of 150/90 mmHg or less in people aged 80 years and over with a history of stroke or TIA. The aim of treating people to this target is to promote secondary prevention of vascular events through satisfactory blood pressure control.
This indicator has been updated for 2023/24 to align with updated NICE guidance which recommends differential blood pressure control targets for readings taken with Home Blood Pressure Monitoring (HBPM) and readings taken in a clinical setting. A target of 150/90mmHg taken in a clinic corresponds to an HBPM target of 145/85 mmHg.
STIA015 Reporting and verification
See indicator wording for requirement criteria.
Diabetes mellitus (DM)
| Indicator | Points | Thresholds |
| Records DM017. The contractor establishes and maintains a register of all patients aged 17 or over with diabetes mellitus, which specifies the type of diabetes where a diagnosis has been confirmed | 6 | N/A |
| Ongoing management DM006. The percentage of patients with diabetes, on the register, with a diagnosis of nephropathy (clinical proteinuria) or micro-albuminuria who are currently treated with an ACE-I (or ARBs) | 3 | 57–97% |
| DM012. The percentage of patients with diabetes, on the register, with a record of a foot examination and risk classification: 1) low risk (normal sensation, palpable pulses), 2) increased risk (neuropathy or absent pulses), 3) high risk (neuropathy or absent pulses plus deformity or skin changes in previous ulcer) or 4) ulcerated foot within the preceding 12 months | 4 | 50–90% |
| DM014. The percentage of patients newly diagnosed with diabetes, on the register, in the preceding 1 April to 31 March who have a record of being referred to a structured education programme within 9 months after entry on to the diabetes register | 11 | 40–90% |
| DM033. The percentage of patients with diabetes, on the register, without moderate or severe frailty in whom the last blood pressure reading (measured in the preceding 12 months) is 140/90 mmHg or less (or equivalent home blood pressure reading) | 10 | 38-78% |
| DM020. The percentage of patients with diabetes, on the register, without moderate or severe frailty in whom the last IFCC-HbA1c is 58 mmol/mol or less in the preceding 12 months | 17 | 35-75% |
| DM021. The percentage of patients with diabetes, on the register, with moderate or severe frailty in whom the last IFCC-HbA1c is 75 mmol/mol or less in the preceding 12 months | 10 | 52-92% |
| DM022. The percentage of patients with diabetes aged 40 years and over, with no history of cardiovascular disease and without moderate or severe frailty, who are currently treated with a statin (excluding patients with type 2 diabetes and a CVD risk score of <10% recorded in the preceding 3 years) | 4 | 50-90% |
| DM023. The percentage of patients with diabetes and a history of cardiovascular disease (excluding haemorrhagic stroke) who are currently treated with a statin | 2 | 50-90% |
DM – rationale for inclusion of indicator set
Diabetes mellitus (DM) is one of the common endocrine diseases affecting all age groups with approximately 3.5 million people in the England having the condition. Effective control and monitoring can reduce mortality and morbidity. Much of the management and monitoring of diabetes, particularly type 2 diabetes, is undertaken by the GP and members of the primary care team.
Further information:
- NICE NG28 (2015, updated 2021) – Type 2 diabetes in adults
- NICE NG19 (2015, updated 2019) – Diabetic foot
- NICE NG18 (2015, updated 2020) – Diabetes (type 1 and type 2) in children and young people
- NICE NG17 (2015, updated 2021) – Type 1 diabetes in adults
The indicators for diabetes are generally those which would be expected to be done, or checked, in an annual review. There is no requirement for the contractor to carry out all these items, but it is the contractor’s responsibility to ensure that they have been done.
DM017 (NICE 2011 menu ID: NM41)
DM017 Rationale
A greater understanding and knowledge of the complexities of diabetes has led to increasing difficulty in accurately diagnosing or classifying the type of diabetes. In March 2011, a report by the Royal College of General Practitioners (RCGP) and NHS Diabetes was published which examined the issue of coding, classification, and diagnosis of diabetes in primary care in England (RCGP and NHS Diabetes, 2011). The summary findings of the report included an algorithm to provide guidance to healthcare professionals on making a new diagnosis of diabetes. In line with this report, the diabetes register indicator includes all types of diabetes within the proposed algorithm. Women with gestational diabetes are excluded from this indicator set.
If it is too early in the clinical course to diagnose the specific type of diabetes, or if the specific diagnosis is uncertain, contractors are asked to use the parent term ‘diabetes mellitus’. Contractors are expected to update these patients’ records when their specific type of diabetes is confirmed. This is advised to be within six to 12 months of the initial diagnosis of diabetes mellitus.
This indicator does not specify how the diagnosis is made and a record of the diagnosis will, for the purposes of the QOF, be regarded as sufficient evidence of diabetes. However, there are a substantial number of patients with diabetes who remain undiagnosed and, a number of patients receiving treatment with an incorrect diagnosis of diabetes. Contractors are therefore encouraged to adopt a systematic approach to the diagnosis of diabetes.
The World Health Organisation (WHO) 2006 states that fasting plasma glucose ≥7.0 mmol/l (126 mg/dl) or 2-h plasma glucose ≥11.1 mmol/l (200 mg/dl) is used as criteria for diagnosing diabetes.
In 2011 an addendum to the 2006 WHO diagnostic criteria was published to allow the use of glycated haemoglobin (HbA1c) in diagnosing DM. The addendum does not invalidate the 2006 recommendations on the use of plasma glucose measurements to diagnose diabetes. The WHO recommend that HbA1c can be used as a diagnostic test for diabetes, provided that stringent quality assurance tests are in place and assays are standardised to criteria aligned to the international reference values and there are no conditions present that preclude its accurate measurement. An HbA1c of 48 mmol/mol (6.5 per cent) is recommended as the cut- off point for diagnosing diabetes. A value less than 48 mmol/mol (6.5 per cent) does not exclude diabetes diagnosed using glucose tests.
The use of HbA1c for diagnosing diabetes can avoid the problem of day-to-day variability of glucose values and importantly it avoids the need for the patient to make preceding dietary preparations (such as fasting or consuming a glucose drink).
The WHO also recommends that the diagnosis of diabetes in an asymptomatic patient is not made on the basis of a single abnormal plasma glucose or HbA1c value. At least one additional HbA1c or plasma glucose test result with a value in the diabetic range is required, either fasting, from a random (casual) sample, or from an oral glucose tolerance test (OGTT).
The Business Rules include a clinical code for “diabetes in remission”. This refers to maintenance of non-diabetic glycaemic levels off all glucose-lowering medication. For type 2 diabetes, this may be achieved through lifestyle interventions or bariatric surgery. However, people with remission of diabetes may still experience the macrovascular and microvascular complications of diabetes and therefore need continued monitoring.
Practices should review their patient records and re-code patients previously coded as “diabetes resolved” as “diabetes in remission” if they still require monitoring for the reasons outlined above.
DM017 Reporting and verification
See indicator wording for requirement criteria.
Verification – Commissioners may require randomly selecting a number of patient records of patients coded with the parent term ‘diabetes mellitus’ and requesting information about how long the specific diagnosis has been unknown.
Commissioners may require contractors to demonstrate that they have processes in place to ensure that patient records are updated once a specific diagnosis has been made. Good practice is that this occurs within six to 12 months of the initial diagnosis.
DM006 (NICE 2015 menu ID: NM95)
DM006 Rationale
NICE guidelines (NICE NG17, NICE NG28) recommend the use of ACE-I (or ARBs) to slow the progression of renal disease in patients with diabetes with urine albumin: creatinine ratio (ACR) ≥3 mg/mmol. Trial evidence suggests that these are most effective when given in the maximum dose quoted in the BNF.
It is recommended that patients with a diagnosis of micro-albuminuria or proteinuria are commenced on an ACE-I or considered for treatment with ARBs.
DM006 Reporting and verification
See indicator wording for requirement criteria.
DM012 (NICE 2010 menu ID: NM13)
DM012 Rationale
Patients with diabetes are at high risk of foot complications that could lead to ulcer, amputation or death. Evaluation and risk classification on an annual basis are important for the detection of feet at most risk.
The NICE guideline on diabetic foot problems outlines foot risk classification and recommends at least annual reassessment.
For the purposes of QOF the clinical codes for ‘moderate risk’ are used to record the concept of ‘increased risk’.
DM012 Reporting and verification
See indicator wording for requirement criteria.
DM014 (NICE 2011 menu ID: NM27)
DM014 Rationale
Diabetes is a progressive long-term medical condition that is predominantly managed by the person with the diabetes and/or their carer as part of their daily life. Accordingly, understanding of diabetes, informed choice of management options and the acquisition of relevant skills for successful self-management play an important role in achieving optimal outcomes. These needs are not always fulfilled by conventional clinical consultations. Structured educational (SE) programmes have been designed not only to improve people’s knowledge and skills, but also to help motivate and sustain people with both type 1 and type 2 diabetes in taking control of their condition and in delivering effective self-management. Structured education programmes are supported by NICE guidance (NICE NG17, NICE NG28).
The indicator requires that SE is offered to every person with diabetes and/or their carer from the time of diagnosis. An alternative education programme of equal standard may be offered to people unable or unwilling to participate in group education sessions.
There are several accredited digital education programmes including nationally commissioned services which are available to all GPs to refer in to. Healthy Living is a programme for people living with type 2 diabetes and their carers, and My Type 1 is for people living with type 1 diabetes. Referral to these programmes will also meet the criteria for this indicator. These programmes are also available to people who have been diagnosed within any timeframe, supporting annual reinforcement.
This indicator suggests referral to a programme within nine months of entry onto the diabetes register to be appropriate for people with type 1 or type 2 diabetes. A timeframe of nine months for this indicator has been set to take into account the differing expectations for referral into SE programmes from diagnosis for people with type 1 and type 2 diabetes.
DM014 Reporting and verification
- NICE NG17 (2015, updated 2021) – Type 1 diabetes in adults
- NICE NG18 (2015, updated 2020) – Diabetes (type 1 and type 2) in children and young people
See indicator wording for requirement criteria. For measurement purposes, nine months is defined as 279 days.
DM033 (NICE 2022 menu ID: NM218)
DM033 Rationale
Lowering blood pressure in people with diabetes reduces the risk of developing micro and macrovascular complications.
Applying universal BP targets to all people with diabetes may inadvertently lead to the potential for undertreatment in those with less complex need and overtreatment in those with complex needs and co-morbidity (Kearney et al, 2019). This indicator focuses upon blood pressure management in people with diabetes without moderate or severe frailty and thus aims to reduce potential undertreatment and support better control of biomedical targets in people with the greatest capacity to benefit.
Contractors should note that the BP target in this indicator is higher than that recommended for patients with Type 1 diabetes in NG17, where they should be aiming for 135/85mmHg or 130/80mmHg if the person has albuminuria or two or more features of metabolic syndrome. Contractors should use their clinical judgement when setting individual blood pressure targets, particularly for people with advanced age, living with frailty or multimorbidity.
This indicator has been updated for 2023/24 to align with updated NICE guidance which recommends differential blood pressure control targets for readings taken with Home Blood Pressure Monitoring (HBPM) and readings taken in a clinical setting. A target for stage 1 hypertension of 140/90mmHg taken in a clinic corresponds to an HBPM target of 135/85 mmHg.
DM033 Reporting and verification
See indicator wording for requirement criteria.
DM020 (NICE 2018 menu ID: NM157)
DM020 Rationale
Glycated haemoglobin (HbA1c) is commonly used to monitor glucose control as it provides a measure of average glycaemia over the preceding 8-12 weeks. Rising levels of HbA1c increase the risk of mortality and developing macrovascular and microvascular complications. However, applying universal target levels regardless of comorbidities may inadvertently lead to over-treatment, especially in older people with type 2 diabetes and people living with frailty (Strain et al, 2018). This indicator allows for an individualised management approach that adjusts care according to an individual’s frailty status. It aims to enable patients without moderate or severe frailty to benefit from tighter glycaemic control. Whilst the target in this indicator is higher than those presented in NICE guidelines (NICE NG17, NICE NG28), this has been pragmatically selected as it represents the point at which people with type 2 diabetes should be considered for treatment intensification.
DM020 Reporting and verification
See indicator wording for requirement criteria.
DM021 (NICE 2018 menu ID: NM158)
DM021 Rationale
This indicator allows for an individualised management approach that adjusts care according to an individual’s frailty status. It aims to reduce complications and improve quality of life for people with moderate or severe frailty. NICE guidelines recommend that individualised HbA1c targets should be agreed with people with both type 1 and type 2 diabetes which consider factors such as their daily activities, aspirations, likelihood of complications, comorbidities, and occupation. Individual targets, even for people with moderate or severe frailty, should be lower than the level specified in this indicator. The target in this indicator has been pragmatically selected as a level that HbA1c should not go beyond to avoid people becoming symptomatic of hyperglycaemia.
DM021 Reporting and verification
See indicator wording for requirement criteria.
DM022 (NICE 2018 menu ID: NM162)
DM022 Rationale
Cardiovascular risk is elevated in people with type 1 and type 2 diabetes. The NICE guideline for cardiovascular disease risk assessment and lipid modification recommends that people with type 1 diabetes are offered statin treatment for primary prevention when they are older than 40 years, or they have had diabetes for more than 10 years, or they have established nephropathy or other CVD risk factors. It also recommends that people with type 2 diabetes should be offered statin therapy if they have a 10% or greater 10-year risk of developing CVD, estimated using the QRISK2 assessment tool. The Business Rules for this indicator include clinical codes for QRISK, QRISK2, QRISK3, Framingham and Joint British Societies risk score.
In September 2016, the NICE guideline for cardiovascular risk assessment and lipid modification was amended and reinforced its recommendation of high-intensity statin treatment, for primary prevention (atorvastatin 20mg) and secondary prevention (atorvastatin 80mg).
DM022 Reporting and verification
See indicator wording for requirement criteria.
People with type 2 diabetes who have a less than 10% 10-year risk of developing CVD recorded in the preceding 3 years will be excluded from the denominator for this indicator.
DM023 (NICE 2018 menu ID: NM163)
DM023 Rationale
The NICE guideline for cardiovascular disease risk assessment and lipid modification recommends that high intensity statin therapy be considered for the secondary prevention of CVD. Statin therapy helps to lower levels of low-density lipoprotein (LDL) cholesterol and is associated with a reduction in MI, coronary heart disease and stroke. Treatment should start with atorvastatin 80mg, however there are situations in which a lower dose or alternative high intensity statin should be used. This indicator wording allows for the selection of an appropriate and individualised dosage.
DM023 Reporting and verification
See indicator wording for requirement criteria.
Asthma (AST)
| Indicator | Points | Thresholds |
| Records AST005. The contractor establishes and maintains a register of patients with asthma aged 6 years or over, excluding patients with asthma who have been prescribed no asthma related drugs in the preceding 12 months | 4 | N/A |
| Initial diagnosis AST011. The percentage of patients with a diagnosis of asthma on or after 1 April 2023 with either: 1. A record of quality assured spirometry and one other objective test (FeNO or, bronchodilator reversibility or peak flow variability) between 3 months before or 6 months after diagnosis; or 2. If newly registered in the preceding 12 months with a diagnosis of asthma recorded on or after 1 April 2023 but no record of objective tests being performed at the date of the registration, with a quality assured spirometry and one other objective test (FeNO or bronchodilator reversibility or peak flow variability) recorded within 6 months of the registration. | 15 | 45–80% |
| Ongoing management AST007. The percentage of patients with asthma on the register, who have had an asthma review in the preceding 12 months that includes an assessment of asthma control using a validated asthma control questionnaire, a recording of the number of exacerbations, an assessment of inhaler technique and a written personalised action plan | 20 | 45–70% |
| AST008. The percentage of patients with asthma on the register aged 19 or under, in whom there is a record of either personal smoking status or exposure to second-hand smoke in the preceding 12 months | 6 | 45–80% |
AST – rationale for inclusion of indicator set
Asthma is a common condition which responds well to appropriate management and is principally managed in primary care.
AST005 (based on NM165)
AST005 Rationale
The diagnosis of asthma is a clinical one; there is no single confirmatory diagnostic blood test, radiological investigation or histopathological investigation. In most patients, the diagnosis can be corroborated by suggestive changes in lung function tests and measurement of airways inflammation.
One of the main difficulties in asthma is the variable and intermittent nature of asthma. Some of the symptoms of asthma are shared with diseases of other systems. Features of an airway disorder in adults such as cough, wheeze and breathlessness should be corroborated where possible by measurement of airflow inflammation, limitation and reversibility. Obstructive airways disease produces a decrease in peak expiratory flow (PEF) and forced expiratory volume in one second (FEV1) but which may resolve after bronchodilators have been administered. One or
both of these should be measured, but may be normal if the measurement is made between episodes of bronchospasm. Asthmatic inflammation of the airways produces higher levels of the fraction of exhaled nitric oxide (FeNO), which can be measured using a point of care test. If lung function and FeNO are repeatedly normal in the presence of symptoms, then the diagnosis of asthma is in doubt.
Children
A definitive diagnosis of asthma can be difficult to obtain in young children. Asthma is to be suspected in any child with wheezing, ideally heard by a health professional on auscultation and distinguished from upper airway noises.
In school children, bronchodilator responsiveness, PEF variability tests of airways inflammation or bronchial hyperactivity may be used to confirm the diagnosis, with the same reservations as above.
Focus the initial assessment in children suspected of having asthma on the:
- Presence of key features in the history and examination
- Careful consideration of alternative
It is well recognised that asthma is a variable condition and many patients will have periods when they have minimal symptoms. It is inappropriate to attempt to monitor symptom-free patients on no therapy or very occasional therapy.
This produces a significant challenge for the QOF. It is important that resources in primary care are targeted to patients with the greatest need – in this instance, patients who will benefit from asthma review rather than insistence that all patients with a diagnostic label of asthma are reviewed on a regular basis.
It is for this reason that the asthma register is constructed annually by searching for patients with a history of asthma, excluding those who have had no prescription for asthma-related drugs in the preceding 12 months.
Further information – SIGN guideline 158. SIGN and BTS. British guideline on the management of asthma. 2016. NICE guideline NG80: Asthma: diagnosis, monitoring and chronic asthma management.
AST005 Reporting and verification
See indicator wording for requirement criteria.
Part of the register criteria for asthma is based on appropriate prescribing of therapies. From October 2014, the Business Rules were updated to exclude drug therapies licensed only for use in patients with a diagnosis of COPD as they are not licensed as a treatment for asthma.
Patients with asthma whose sole asthma medication is one associated with COPD will no longer appear on the QOF asthma register. Patients receiving additional, appropriate asthma treatment such as short-acting bronchodilators or steroid inhalers will remain on the register. Practices may wish to review the records of any patients affected by this change to review their asthma treatment however, a change in prescribing should only be done where clinically appropriate.
AST011 (based on NM166)
AST011 Rationale
This indicator has been updated from AST006 to allow re-baselining of the start date to reflect changed requirements and the difficulty undertaking spirometry during the COVID-19 pandemic.
The aim of this indicator is to encourage use of objective tests to confirm asthma diagnosis, and subsequently improve accuracy of diagnosis and reduce incidences of patients receiving inappropriate care. This will mean that some patients may require referral for the necessary diagnostic tests to be completed following locally commissioned pathways. Results of testing should inform subsequent treatment for people with asthma and lead to improved health and wellbeing.
Spirometry is the key investigation for distinguishing obstructive and restrictive respiratory conditions and will determine subsequent investigations. It is crucial that diagnostic spirometry is performed to published quality standards (Levy ML et al, 2009. Association for respiratory technology and physiology) and therefore referral to a specialist service may be required.
Adults (aged 17 and over) should be diagnosed, if they have symptoms suggestive of asthma and:
- A FeNO level of 40 parts per billion (ppb) or more with either positive bronchodilator reversibility or positive peak flow variability or bronchial hyperreactivity, or
- A FeNO level between 25 and 39 ppb and a positive bronchial challenge test, or
- Positive bronchodilator reversibility and positive peak flow variability irrespective of FeNO level (NICE NG80, 2017).
Children (aged 5 to 16) should be diagnosed if they have symptoms suggestive of asthma and:
- A FeNO level of 35 ppb or more and a positive peak flow variability, or
- Obstructive spirometry and positive bronchodilator reversibility (NICE NG80, 2017).
Referral may be required for FeNO testing and for challenge testing to measure bronchial hyperreactivity, which is a hallmark of asthma. The bronchial challenge test involves breathing in gradually increasing doses of a medication, such as methacholine or mannitol, whilst measuring the FEV1.
If an adult, young person or child with symptoms suggestive of asthma cannot perform a particular test, try to perform at least 2 other objective tests. Diagnose suspected asthma based on symptoms and any positive objective test results. PCAs are available where people cannot perform objective testing.
It is recognised that the current structure of diagnostic services in primary care means delivery of objective testing in children and adults with equity of access is a significant challenge. A PCA will be available in 2023/24 allowing anyone who does not have access to objective testing to support their asthma diagnosis to not be included in the denominator. Ensuring availability of quality assured spirometry and FeNO measurement to support an accurate diagnosis in all adults and children aged 5 years and over remains a clinical priority and this will allow time for local areas to set up services and appropriately train staff.
Where objective testing is not available, clinical guidance should be followed (NICE NG80, 2017. BTS/SIGN clinical guideline 158).
More specialist assessment may be required in those in whom the diagnosis is still unclear, which may include assessment of airway inflammation (e.g. nitric oxide measurement), bronchial hyper-responsiveness testing and consideration of alternative diagnoses. It is recommended that children with combined food allergy and asthma and any patient with late onset asthma where there is a suspicion of an occupational cause are referred for specialist assessment.
If another diagnosis is more likely
If an alternative diagnosis is suspected, investigation and management are to follow guidelines for that condition.
Co-morbidity: asthma and COPD
A proportion of patients with asthma will have both asthma and COPD e.g. they have airway obstruction that does not reverse to normal but also have substantial reversibility (NICE NG115, 2018).
AST011 Reporting and verification
See indicator wording for requirement criteria. For measurement purposes, three months prior to diagnosis is defined as 93 days.
AST007 (based on NM167)
AST007 Rationale
This indicator aims to encourage the use of validated asthma questionnaires, recording of the number of exacerbations, and written action plans in annual asthma reviews. These reviews can help identify people at increased risk of poor outcomes and allow them to use information from their review to self-manage their asthma and maximise their future health.
The BTS/SIGN clinical guideline proposes a structured system for recording inhaler technique, morbidity, PEF levels, current treatment and asthma action plans.
QOF explicitly requires an assessment of asthma control using a validated asthma control questionnaire using the Asthma Control Questionnaire or Asthma Control Test, a recording of the number of exacerbations, a face-to-face assessment of inhaler technique (or by video where that’s not possible) and a written personalised action plan.
If the asthma appears to be uncontrolled, take in account the possible reasons below before adjusting medicines:
- Alternative diagnoses
- Smoking (active or passive)
- Poor inhaler technique
- Lack of adherence
- Occupation exposures
- Psychosocial factors
- Seasonal or environmental
For more information on asthma management and recommendations made to prevent deaths from asthma in the future, see the National Review of Asthma Deaths (NRAD).
AST007 Reporting and verification
See indicator wording for requirement criteria.
The Business Rules require that contractors code the review and the assessment of asthma control using the Asthma Control Questionnaire or the Asthma Control Test, the number of exacerbations in the month before the asthma review and the provision of a written personalised asthma plan recorded on the same day as the asthma review in order to meet the requirements of this indicator.
AST008 (based on NM168)
AST008 Rationale
There are very few studies that have considered the question of whether smoking affects asthma severity (Polosa et al, 2013). One controlled cohort study suggested that exposure to passive smoke at home delayed the recovery from an acute attack. There is also epidemiological evidence that smoking is associated with poor asthma control (Price et al, 2005) NICE guidance recommends taking smoking status (active or passive) into account before starting or adjusting medicines for asthma (NICE NG80, 2017).
This indicator aims to encourage general practice to ask children and young people aged 6 to 19 years with asthma about their exposure to tobacco and second-hand smoke. Support can then be offered to patients and the people they live with to understand the risks of smoking and exposure to second-hand smoke for those with asthma, and how to access smoking cessation services.
AST008 Reporting and verification
See indicator wording for requirement criteria.
Chronic obstructive pulmonary disease (COPD)
| Indicator | Points | Thresholds |
Records COPD015. The contractor establishes and maintains a register of: 1. Patients with a clinical diagnosis of COPD before 1 April 2023; and 2. Patients with a clinical diagnosis of COPD on or after 1 April 2023 whose diagnosis has been confirmed by a quality assured post-bronchodilator spirometry FEV1/FVC ratio below 0.7 between 3 months before or 6 months after diagnosis (or if newly registered at the practice in the preceding 12 months without a record of spirometry having been performed, a record of an FEV1/FVC ratio below 0.7 recorded within 6 months of registration); and 3. Patients with a clinical diagnosis of COPD on or after 1 April 2023 who are unable to undertake spirometry | 8 | N/A |
| Ongoing management COPD010. The percentage of patients with COPD on the register, who have had a review in the preceding 12 months, including a record of the number of exacerbations and an assessment of breathlessness using the Medical Research Council dyspnoea scale | 9 | 50–90% |
| COPD014. The percentage of patients with COPD and Medical Research Council (MRC) dyspnoea scale ≥3 at any time in the preceding 12 months, with a subsequent record of referral to a pulmonary rehabilitation programme (excluding those who have previously attended a pulmonary rehabilitation programme) | 2 | 40-90% |
COPD – rationale for inclusion of indicator set
Chronic obstructive pulmonary disease (COPD) describes a group of lung conditions that cause obstructive airways disease and includes chronic bronchitis and emphysema. COPD is a common disabling condition responsible for significant unscheduled healthcare utilisation. When applicable, the most effective intervention is smoking cessation. Pulmonary rehabilitation has been shown to produce an improvement in quality of life and decrease exacerbations. Inhaled bronchodilators and, in some cases, inhaled corticosteroids can be of benefit.
Pulmonary rehabilitation has been shown to produce an improvement in quality of life.
The majority of patients with COPD are managed by GPs and members of the primary care team with onward referral to secondary care when required. This indicator set focuses on the diagnosis and management of patients with symptomatic COPD.
COPD015 (based on NM169)
COPD015 Rationale
The aim of this indicator is to encourage practices to maintain a register of patients with a diagnosis of COPD and to use that register of patients to inform the care they deliver, including objective testing to support diagnosis of COPD as recommended in NICE guidance NG115: Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Linking diagnosis and objective testing to entry onto the QOF COPD disease register aims to contribute towards a reduction in both misdiagnosis and the risk of overtreatment in people with COPD. Referral to a specialist service may be appropriate for objective testing and to make an accurate diagnosis.
The wording of this indicator has been updated for 2023/24 to re-baseline the start date to reflect difficulties undertaking spirometry during the COVID-19 pandemic.
COPD015 Reporting and verification
See indicator wording for requirement criteria. Patients with clinical diagnoses of COPD and no record of objective tests will not be excluded from the register but the expectation is that, over time, the proportion of patients with spirometry confirming fixed airflow obstruction will increase relative to those without spirometry recorded.
Where patients have co-existing COPD and asthma they will be included on both disease registers.
COPD0010 (NICE 2019 menu ID: NM170)
COPD0010 Rationale
This indicator aims to encourage the use of recording of number of exacerbations and assessments of breathlessness in annual COPD reviews and is supported by NICE guidance. Understanding the frequency of exacerbations can help when creating personalized management plans, identifying triggers and avoiding future exacerbations.
In making assessments of the patient’s condition as part of an annual review and when considering management changes, it is essential that health care professionals record:
- Number of exacerbations
- The degree of breathlessness (Medical Research Council [MRC] dyspnoea scale).
A tool such as the COPD Assessment Test (CAT) could be used to assess current health status.
Additionally, there is evidence that inhaled therapies can improve the quality of life in some patients with COPD. However, there is evidence that patients require training in inhaler technique and that such training requires reinforcement. Where a patient is prescribed an inhaled therapy, their technique is to be assessed face to face (or by video where that’s not possible) during any review.
The MRC dyspnoea scale gives a measure of breathlessness and is recommended as part of the regular review. It is available in the NICE guideline on COPD, section 1.1, diagnosing COPD table one.
COPD0010 Reporting and verification
See indicator wording for requirement criteria.
COPD014 (NICE 2012 menu ID: NM47)
Pulmonary rehabilitation is a multidisciplinary programme of care which aims to reduce disability and improve quality of life in patients with a chronic respiratory impairment. It is individually tailored and designed to optimise each patient’s physical and social performance and independence.
The NICE guideline for COPD recommends that pulmonary rehabilitation should be offered to all patients who consider themselves to be functionally disabled due to their COPD (usually MRC dyspnoea scale score of ≥3). Whilst most patients are likely to benefit, a rehabilitation programme is not suitable for patients who are unable to walk, have unstable angina or who have recently had a myocardial infarction.
Medical management should be optimised before referral.
The wording of this indicator has been updated to measure referrals made, not offers of referral.
COPD014 Reporting and verification
See indicator wording for requirement criteria.
Patients who have previously attended a pulmonary rehabilitation programme will be excluded from the denominator for this indicator.
Where practices do not have locally commissioned pulmonary rehabilitation programmes they may exclude patients from the denominator using the specific service unavailable codes.
Dementia (DEM)
| Indicator | Points | Thresholds |
| Records DEM001. The contractor establishes and maintains a register of patients diagnosed with dementia | 5 | N/A |
| Ongoing management DEM004. The percentage of patients diagnosed with dementia whose care plan has been reviewed in the preceding 12 months | 14 | 35–70% |
DEM – rationale for inclusion of indicator set
Dementia is a syndrome characterised by an insidious but ultimately catastrophic progressive global deterioration in intellectual function and is a main cause of late-life disability. The prevalence of dementia increases with age and is estimated to be approximately seven per cent in those over 65. Alzheimer’s disease accounts for around 50 to 75 per cent of cases of dementia with vascular dementia accounting for up to 20 per cent (Alzheimer’s Society, 2020).
The annual incidence of dementia of the Alzheimer’s type rises to 34.3/100 person years at risk in the 90 year age group; the prevalence is higher in women than in men due to the longer lifespan of women. Other types of dementia such as Lewy Body dementia and frontotemporal dementia are relatively rare but can be very distressing.
DEM001
DEM001 Rationale
It is expected that the diagnosis will largely be recorded following patients being referred to secondary care with suspected dementia or as an additional diagnosis when a patient is seen in secondary care. However, it is also important to include patients where it is inappropriate or not possible to refer to a secondary care provider for a diagnosis and where the GP has made a diagnosis based on their clinical judgement and knowledge of the patient.
DEM001 Reporting and verification
See indicator wording for requirement criteria.
DEM004 (NICE 2015 menu ID: NM107)
DEM004 Rationale
The NICE guideline for dementia recommends agreeing care plans with health and social services for people who have dementia, and having formal reviews at agreed frequencies.
Where a patient does not already have a care plan or an advanced care plan in place, it is expected that the practice will develop a care plan.
The care plan or advanced care plan review should be conducted face to face or remotely in line with personal choice and should focus on supporting the needs of the patient and their carer. Regular review can help ensure that any changes in need can be addressed. In particular the review should address the following key issues (in line with the D.E.M.E.N.T.I.A framework set out in NHS England’s Dementia: Good personalised care and support planning guide):
- An appropriate physical, mental health and social review,
- A medication review, with particular attention to antipsychotic medication in consideration of:
- Side effects such as risk of diabetes and dyslipidaemia; and
- Anticholinergic effects in line with NICE guidance [NG97],
- A record of the patients’ wishes for the future,
- Communication and co-ordination arrangements with secondary care (if applicable),
- Identification of the patients’ carer(s); and
- Obtain appropriate permissions to authorise the practice to speak directly to the nominated carer(s) and provide details of support services available to the patient and their family, if applicable, the carer’s needs for information commensurate with the stage of the illness and his or her and the patient’s health and social care needs,
- As appropriate, the carer should be included in the care plan or advanced care plan discussions,
- If applicable, the impact of caring on the care-giver,
- Offer the carer a health check (where the carer is registered at a different practice, the patients practice should inform the patient’s carer that they can seek advice from their own practice) to address any physical and mental health impacts, including signposting to any other relevant services to support their health and wellbeing.
The practice will agree with the patient and their carer, what is to be covered in the review and the duration of the consultation – where appropriate, extended consultations may take up to 30 minutes (the practice should agree with the patient the most suitable length of this for this appointment, this could be provided as two 15 minute appointments if this is more appropriate for the patient). Ideally the first such appointment would be within six months of diagnosis.
A series of well-designed cohort and case control studies have demonstrated that patients with Alzheimer-type dementia do not complain of common physical symptoms but experience them to the same degree as the general population.
Patient assessments therefore include the assessment of any behavioural changes caused by:
- Concurrent physical conditions (e.g. joint pain or inter-current infections)
- New appearance of features intrinsic to the disorder (e.g. wandering) and delusions or hallucinations due to the dementia or as a result of caring behaviour (e.g. being dressed by a carer).
Depression could also be considered as it is more common in patients with dementia than those without (Alzheimer’s society, 2017).
Patients and carers are to be given relevant information about the diagnosis and sources of help and support (bearing in mind issues of confidentiality). Evidence suggests that healthcare professionals can improve satisfaction for carers by acknowledging and dealing with their distress and providing more information on dementia (Eccles et al, 1998). As the illness progresses, needs may change, and the review may focus more on issues such as respite care.
There is good evidence from well-designed cohort studies and case control studies of the benefit of healthcare professionals asking about the impact of caring for a person with dementia and the effect this has on the caregiver. It is important to remember that male carers are less likely to complain spontaneously and that the impact of caring is dependent not on the severity of the cognitive impairment but on the presentation of the dementia, for example, on factors such as behaviour and affect. If the carer is not registered at the practice, but the GP is concerned about issues raised in the consultation, then with appropriate permissions they can contact the carer’s own GP for further support and treatment.
As the illness progresses and more agencies are involved, the review could additionally focus on assessing the communication between health and social care and non-statutory sectors as appropriate, to ensure that potentially complex needs are addressed. Communication and referral issues highlighted in the review need to be followed up as part of the review process.
Further information
- NICE NG97 (2018)
- NICE QS184 (2019)
- Forget me not dementia
- GOV.UK National service framework for older people (2001)
- NICE PH16 (2008) Mental wellbeing in over 65s: occupational therapy and physical activity interventions.
- NHS Looking after someone with dementia (2021).
DEM004 Reporting and verification
See indicator wording for requirement criteria.
Verification – Commissioners may require randomly selecting a number of patient records of patients in which the review has been recorded as taking place to confirm that the four key issues are recorded as having been addressed, if applicable.
Depression (DEP)
| Indicator | Points | Thresholds |
| Initial management DEP004. The percentage of patients aged 18 or over with a new diagnosis of depression in the preceding 1 April to 31 March, who have been reviewed not earlier than 10 days after and not later than 56 days after the date of diagnosis | 10 | 45–80% |
DEP – rationale for inclusion of the indicator set
Depression is common and disabling.
The Adult Psychiatric Morbidity Survey, 2014 estimated prevalence for a depressive episode among people aged 16 in England was 3.3 per cent. If the broader and less specific category of mixed depression and anxiety (‘common mental health disorder – not otherwise stated’) is included, these figures increase dramatically to 7.8 per cent. It contributes 12 per cent of the total burden of non-fatal global disease and by 2020, looks set to be second after CVD in terms of the world’s disabling diseases (Murray CJ, 2012). Major depressive disorder is increasingly seen as chronic and relapsing, resulting in high levels of personal disability, lost quality of life for patients, their family and carers, multiple morbidity, suicide, higher levels of service use and many associated economic costs. In 2007, the total cost of depression in England was reported to be £7.5 billion of which health service costs comprised £1.7 billion and lost earnings £5.8 billion. When the cost of informal care, lower productivity and other public sector costs are included this figure is estimated at between £20.2-23.8 billion a year (GOV.UK 2011).
DEP004 (based on NICE 2012 menu ID: NM50)
DEP004 Rationale
The NICE guideline on depression in adults states that patients with mild depression or sub-threshold symptoms be reviewed and re-assessed after initial presentation, normally within two weeks.
It recommends that patients with mild or moderate depression who start antidepressants are reviewed after one week if they are considered to present an increased risk of suicide or after two weeks if they are not considered at increased risk of suicide. Patients are then re-assessed at regular intervals determined by their response to treatment and whether or not they are considered to be at an increased risk of suicide.
This indicator promotes a single depression review between ten and 56 days inclusive after the date of diagnosis. For some patients this may not be their first review as they will have been reviewed initially within a week of the diagnosis.
Unless a patient’s symptoms have resolved, further reviews may be required.
When assessing a person who may have depression, conduct a comprehensive assessment that does not rely simply on a symptom count. Take into account both the degree of functional impairment and/or disability associated with the possible depression and the duration of the episode.
Only face-to-face or telephone contact with a clinician is acceptable to meet the requirements for this indicator.
DEP004 Reporting and verification
See indicator wording for requirement criteria.
Those patients whose on-going care is being provided by specialist mental health services may have a personalised care adjustment applied.
It is recommended that where the diagnosis is made by specialist mental health services and the patient has been discharged for follow-up by the primary care team, the contractor should find out the diagnosis date in order to record this and invite the patient for a review within the timeframe specified.
Suspected depression seen in secondary care may not always be referred to specialist mental health services for further assessment and management. It may be in the form of a discharge letter from an acute medical or surgical ward, A&E or from an outpatient appointment. It may be reasonable in these circumstances for a contractor to contact the patient to ask them to attend for an assessment to assess if they have a clinical diagnosis of depression.
The register (DEPCC01), for the purpose of calculating the APDF, is defined as ‘number of patients aged 18 or over with a new diagnosis of depression in the preceding 1 April to 31 March or with a new diagnosis of depression in the last 3 months of the previous QOF year and did not achieve in the previous QOF year.
Verification – Commissioners may ask contractors about the percentage of telephone reviews conducted and who they were delivered by.
Mental health (MH)
| Indicator | Points | Thresholds |
| Records MH001. The contractor establishes and maintains a register of patients with schizophrenia, bipolar affective disorder and other psychoses and other patients on lithium therapy | 4 | N/A |
| Ongoing management MH002. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a comprehensive care plan documented in the record, in the preceding 12 months, agreed between individuals, their family and/or carers as appropriate | 5 | 40–90% |
| MH003. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of blood pressure in the preceding 12 months | 3 | 50–90% |
| MH006. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of BMI in the preceding 12 months | 3 | 50-90% |
| MH007. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of alcohol consumption in the preceding 12 months | 3 | 50-90% |
| MH011. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of a lipid profile in the preceding 12 months (in those patients currently prescribed antipsychotics, and/or have pre-existing cardiovascular conditions, and/or smoke, and/or are overweight (BMI of ≥23 kg/m2 or ≥25 kg/m2 if ethnicity is recorded as White) or preceding 24 months for all other patients | 7 | 50-90% |
| MH012. The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of blood glucose or HbA1c in the preceding 12 months | 7 | 50-90% |
| MH021: Percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who received all six elements of the Physical Health Check for people with Severe Mental Illness | 6 | 50-80% |
MH – rationale for inclusion of indicator set
This indicator set reflects the complexity of mental health problems, and the complex mix of physical, psychological and social issues that present to GPs.
For many patients with mental health problems, the most important aspects of care quality relate to the interpersonal skills of the doctor, the time given in consultations and the opportunity to discuss a range of management options.
This indicator set focuses on patients with severe mental illness (SMI). There are separate indicator sets that focus on patients with depression and dementia.
NICE CG178 recommends primary care utilise registers to monitor the physical health of patients with psychosis or schizophrenia.
NICE CG185 recommends that patients with bipolar affective disorder have a physical health review, normally in primary care, performed at least annually, including the following health checks:
- Weight or BMI, diet, nutritional status and level of physical activity
- Cardiovascular status, including pulse and blood pressure
- Metabolic status, including glycosylated haemoglobin (HbA1c) and blood lipid profile
- Liver function
- Renal and thyroid function, and calcium levels, for people taking long-term
QOF rewards practices for delivering all six elements of the comprehensive annual physical health check for patients with schizophrenia, bipolar affective disorder and other psychoses as defined in the NHS Long Term Plan.
In addition to smoking, poor diet and lack of exercise, antipsychotic drugs vary in their liability for metabolic side effects such as weight gain, lipid abnormalities and disturbance of glucose regulation. Specifically, they increase the risk of the metabolic syndrome, a recognised cluster of features (hypertension, central obesity, glucose intolerance or insulin resistance or dyslipidaemia) which is a predictor of type 2 diabetes and CHD (Mackin et al, 2007).
Due to the combination of lifestyle factors and side effects of anti-psychotic medication, there is a high incidence of cardiovascular disease (CVD) causing premature death in people with SMI (15 years for bipolar disorder and 25 years for schizophrenia). The aim of the comprehensive annual physical health check is to identify and address risk factors for CVD.
Further information
- NICE CG178 (2014) Psychosis and schizophrenia in adults
- Practices may wish to use the Lester tool; a mental health physical review template
MH001
MH001 Rationale
The register includes all patients with a diagnosis of schizophrenia, bipolar affective disorder and other psychoses and other patients on lithium therapy.
Patients on lithium therapy are defined as patients with a prescription for lithium within the preceding six months.
Remission from severe mental illness
Historically, patients have been added to the mental health disease register for schizophrenia, bipolar affective disorder and other psychoses, but over time it has become apparent that it would be appropriate to exclude some of them from the associated indicators because their illness is in remission.
Making an accurate diagnosis of remission for a patient with a diagnosis of severe mental illness can be challenging and the evidence base to support when to use the ‘remission code’ is largely based on clinical judgement. A longitudinal international study of recovery from psychotic illnesses found that as many as 56 per cent of patients recovered from psychotic illnesses to some extent, although only 16 per cent recover if a more stringent concept of recovery is used.
In the absence of strong evidence of what constitutes ‘remission’ from severe mental illness, it is advised that clinicians should only consider using the remission codes if the patient has been in remission for at least five years, that is where there is:
- No record of antipsychotic medication,
- No mental health in-patient episodes; and
- No secondary or community care mental health follow-up for at least five
Where a patient is recorded as being ‘in remission’, they remain on the register (in case their condition relapses at a later date) but they are excluded from the denominator for subsequent indicators (i.e. they are excluded from the denominator for MH002, MH003, MH006, MH007, MH011, MH012 and SMOK002).
The accuracy of this diagnosis and the coding should be reviewed on an annual basis by a GP. If a patient who has been coded as ‘in remission’ experiences a relapse then this should be recorded as such in their patient record.
In the event that a patient experiences a relapse and is coded as such, they will once again be included in all the associated indicators for schizophrenia, bipolar affective disorder and other psychoses.
MH001 Reporting and verification
See indicator wording for requirement criteria.
Verification – Commissioners may require randomly selecting a number of patient records in which a ‘remission code’ has been recorded and request evidence as to why it was appropriate for that patient to be considered ‘in remission’ and to confirm the ongoing accuracy of this coding.
Contractors may be expected to demonstrate they have a protocol to guide their clinicians as to how this would work and who would be suitable to make the decision. It would not be appropriate for non-clinical members of the practice to make the decision as to when to enter this code.
MH002 (NICE 2015 menu ID: NM108)
MH002 Rationale
This indicator reflects good professional practice and is supported by NICE CG178 and CG185.
Patients on the mental health disease register should have a documented primary care consultation that acknowledges, especially in the event of a relapse, a plan for care. This consultation may include the views of their relative(s) or carer(s) where appropriate.
Up to half of patients who have a severe mental illness are seen only in a primary care setting. For these patients, it is important that the primary care team takes responsibility for discussing and documenting a care plan in their primary care record.
When constructing the primary care record, research supports the inclusion of the following information:
- Patient’s current health status and social care needs including how needs are to be met, by whom and the patient’s expectations
- Co-ordination arrangements with secondary care and/or mental health services and a summary of what services are actually being received
- Occupational status – for people being supported by secondary mental health services in England, there is a 65% employment gap compared with the general population (NHS England, 2019). Studies show a clear interest in work and employment activities among users of mental health services with up to 90 per cent wishing to go into or back to work (Grove et al, 1999).
- ‘Early warning signs’ from the patient’s perspective that may indicate a possible relapse (Birchwood et al, 2001). Many patients may already be aware of their early warning signs (or relapse signature) but it is important for the primary care team to also be aware of noticeable changes in thoughts, perceptions, feelings and behaviours leading up to their most recent episode of illness as well as any events the patient thinks may have acted as triggers.
- The patient’s preferred course of action (discussed when well) in the event of a clinical relapse, including who to contact and wishes around medication.
If a patient is treated within community mental health services and has a documented care plan this is acceptable for the purposes of QOF provided the practice has evidence of a review having taken place (NHS England, 2021).
Where a patient has relapsed after being recorded as being in remission their care plan should be updated subsequent to the relapse. Care plans dated prior to the date of the relapse will not be acceptable for QOF purposes.
MH002 Reporting and verification
See indicator wording for requirement criteria.
Verification – Commissioners may require contractors to randomly select a number of care plans to ensure that they are being maintained annually.
MH003 (based on NM17)
MH003 Rationale
NICE guidance (NICE CG178, NICE CG185) recommends annual monitoring of blood pressure for people with bipolar disorder, psychosis or schizophrenia. Patients with schizophrenia have mortality between two and three times that of the general population and most of the excess deaths are from diseases that are the major causes of death in the general population. A prospective record linkage study of the mortality of a community cohort of 370 patients with schizophrenia found that the increased mortality risk is probably life-long and it suggested that cardiovascular mortality of schizophrenia has increased over the past 25 years relative to the general population (Brown et al, 2010). The NICE guideline on bipolar disorder also states that the standardised mortality ratio for cardiovascular death may be twice that of the general population but appears to be reduced if patients adhere to long-term medication.
Hypertension in people with schizophrenia is estimated at 19 per cent compared with 15 per cent in the general population (Hennekens C et al, 2005). A cross-sectional study of 4310 patients diagnosed with bipolar disorder in 2001 receiving care at veterans’ administration facilities found a prevalence of hypertension of 35 per cent (Kilbourne et al, 2004).
There is evidence to suggest that physical conditions such as cardiovascular disorders go unrecognised in psychiatric patients. A direct comparison of cardiovascular screening (blood pressure, lipid levels and smoking status) of patients with asthma, patients with schizophrenia and other attendees indicated that general practice were less likely to screen patients with schizophrenia for cardiovascular risk compared with the other two groups (Roberts et al, 2007).
Recording (and treating) cardiovascular risk factors are therefore very important for patients with a serious mental illness.
MH003 Reporting and verification
See indicator wording for requirement criteria.
MH006 (based on NM16)
MH006 Rationale
As noted above, people with serious mental illness are at increased risk of premature and preventable cardiovascular mortality and morbidity when compared to the general population. Obesity is a key risk factor linked to this. when compared to the general population people with psychosis lead more sedentary lives, eat less fruit and vegetables, are more likely to be obese and to smoke. In addition to these lifestyle factors, antipsychotic drugs vary in their liability for metabolic side effects such as weight gain, lipid abnormalities and disturbance of glucose regulation.
Specifically, they increase the risk of metabolic syndrome, a recognised cluster of features (hypertension, central obesity, glucose intolerance or insulin resistance and dyslipidaemia), which is a predictor of type 2 diabetes and coronary heart disease.
About 40% of people with schizophrenia are living with obesity which is also common in people with bipolar disorders. NICE Guidelines CG178 and CG185 recommend annual monitoring of weight or BMI in these patient groups.
MH006 Reporting and verification
See indicator wording for requirement criteria.
MH007 (based on NM15)
MH007 Rationale
Alcohol and other substance misuse by people with schizophrenia is increasingly recognised as a major problem, both in terms of its prevalence and its clinical and social effects (Banerjee et al, 2001). The National Psychiatric Morbidity Survey in England found that 16 per cent of people with schizophrenia were drinking above the lower risk consumption levels (14 units) of alcohol (Meltzer et al, 1996. Farrell et al, 1998). Bipolar affective disorder is also highly co-morbid with alcohol and other substance abuse.
MH007 Reporting and verification
See indicator wording for requirement criteria.
MH011 (based on NM129)
MH011 Rationale
NICE guidance (NICE CG178, NICE CG185) recommends annual blood lipid profiles for people with bipolar disorder, psychosis or schizophrenia. A 2014 literature review that explored obesity and serious mental ill health, concluded that the use of antipsychotic medication may interfere with the normal processes which regulate food intake and metabolism (Bradshaw et al, 2014).
Individuals with severe mental illness have five times the risk of dyslipidaemia than the general population (NHS England, 2016).
MH011 Reporting and verification
Within the Business Rules currently being prescribed an antipsychotic medication is defined as a prescription in the preceding 6 months; pre-existing cardiovascular conditions are defined as CHD, diabetes, stroke, peripheral arterial disease and chronic kidney disease; being a current smoker is defined as a patient whose notes record smoking status in the preceding 12 months and being overweight is defined as latest BMI of ≥ 23 kg/m2 or ≥25 kg/m2 if ethnicity is recorded as White.
MH012 (based on NM130)
MH012 Rationale
NICE guidance (NICE CG178, NICE CG185) recommends annual monitoring of blood glucose or HbA1c for people with bipolar disorder, psychosis or schizophrenia. Diabetes is 2–3 times more common among people with SMI than the general population (Vinogradova et al, 2010). Effects of severe mental illness on survival of people w) and antipsychotic medication can be diabetogenic (Smith et al, 2008). The National Diabetes Audit confirms previous studies that type 2 diabetes is twice as common among people with SMI than in the general population. The rates of type 1 diabetes are about the same as the general population, although the overall numbers are small. People with SMI are more likely to develop type 2 diabetes earlier than the general population, frequently in the fourth and fifth decades. People with an SMI are more likely to develop type 1 diabetes later than those without a SMI, as late as the third and fourth decades of life (Cohen et al, 2018).
MH012 Reporting and verification
See indicator wording for requirement criteria.
Patients who have a diagnosis of diabetes will be excluded from this indicator.
MH021 (based on NICE NM232)
MH021 Rationale
See rationale for MH002, MH006, MH007, MH011, MH012 and SMOK002 and the following NICE guidance referenced (CG17830 and CG18531).
Good clinical practice in this area consists of a single coordinated physical health review of the patient made up of six components, supporting a ‘Making Every Contact Count’ approach, which provides better service user experience by reducing the need for multiple appointments.
MH021 Reporting and verification
See indicator wording for requirement criteria.
Cancer (CAN)
| Indicator | Points | Thresholds |
| Records CAN001. The contractor establishes and maintains a register of all cancer patients defined as a ‘register of patients with a diagnosis of cancer excluding non-melanotic skin cancers diagnosed on or after 1 April 2003’ | 5 | N/A |
| Ongoing management CAN004. The percentage of patients with cancer, diagnosed within the preceding 24 months, who have a patient Cancer Care Review using a structured template recorded as occurring within 12 months of the date of diagnosis | 6 | 50–90% |
| CAN005. The percentage of patients with cancer, diagnosed within the preceding 12 months, who have had the opportunity for a discussion and informed of the support available from primary care, within 3 months of diagnosis | 2 | 70-90% |
CAN – rationale for inclusion of indicator set
It is recognised that the principal active management of cancers occurs in the secondary care setting. However, general practice has a key role in the referral and/or subsequent support of these patients and in ensuring that care is appropriately co-ordinated. This indicator set is not evidence-based but does represent good professional practice.
These indicators for cancer aim to increase the personalisation of cancer care and the timing of the cancer care review.
CAN001
CAN001 Rationale
The register can be developed prospectively as the intention is to ensure appropriate care and follow-up for patients with a diagnosis of cancer. For the purposes of the register all cancers are included except non-melanomatous skin lesions.
CAN001 Reporting and verification
See indicator wording for requirement criteria.
CAN005 (based on NM204)
CAN005 Rationale
Most practices will see patients with a new cancer diagnosis following assessment and management in a secondary or tertiary care setting. This indicator aims to encourage GP practices to proactively provide patients with the opportunity for a discussion to make them aware of the support available from their GP and wider practice team. The intention is to facilitate early and supportive conversations and ensure patients are aware of what help is available.
This indicator supports recommendations 1.1.1, 1.3.4 and 1.3.5 from NICE guideline CG138 Patient experience in adult NHS services.
CAN005 Reporting and verification
See indicator wording for requirement criteria.
This indicator will only apply to patients who have received their diagnosis on or after 1 April 2021.
For the purposes of this indicator, the twelve-month timeframe starts from the date of diagnosis irrespective of whether or not the diagnosis was made in primary care.
CAN004 (NICE menu 2020 ID: NM205)
CAN004 Rationale
A GP will have an average of eight or nine new cancer diagnoses per year and will be looking after 20 to 30 patients with cancer. The increasing number of cancer survivors has led to an increase in the number of people requiring follow-up care, monitoring and management, therefore primary care has an important role in supporting people to live well with and beyond cancer. This review represents an opportunity to address patients’ needs for individual assessment, care planning and on-going support and information requirements.
The Cancer Care Review should be a holistic conversation that covers clinical, practical, emotional, psychological and financial (where appropriate) aspects of the person’s cancer care. The clinical element of the review should be conducted by a GP, General Practice Nurse, or Allied Health Professional. The use of non-clinical supporting roles should be underpinned by robust links to clinical teams and appropriate training and supervision should be provided. Consideration of the co- ordination of care between sectors is recommended. Practices should use Macmillan’s national, integrated electronic CCR template within your Primary Care IT system to support a well-structured review. Further information on how to access Macmillan’s CCR templates on all major GP IT systems can be found on the Macmillan website.
This template can be used as an aide memoire when carrying out a CCR. It also includes supporting information which can be shared with the patient as well as providing a helpful coded record of topics discussed.
Macmillan also provides Top Tips on cancer care reviews which encourages a fuller discussion of the diagnosis and recording of cancer therapy, an offer of relevant information, medication review, benefits counselling and recording of a carer’s details. Top Tips on Late Effects, Fatigue, Anxiety, Nutrition and other common problems are also available. Further information on care following a cancer diagnosis and the potential role for primary care can be found on the Macmillan website.
CAN004 Reporting and verification
See indicator wording for requirement criteria.
For the purposes of this indicator, the twelve-month timeframe starts from the date of diagnosis irrespective of whether or not the diagnosis was made in primary care.
This indicator will not include patients whose latest unresolved cancer diagnosis was earlier than 1 January 2022 as these patients should have already been reviewed.
Verification – Commissioners may wish to review records where a review is claimed to confirm that the review has been completed using a structured template within twelve months of diagnosis.
Chronic kidney disease (CKD)
| Indicator | Points | Thresholds |
| Records CKD005. The contractor establishes and maintains a register of patients aged 18 or over with CKD with classification of categories G3a to G5 (previously stage 3 to 5) | 6 | N/A |
CKD – rationale for inclusion of indicator set
NICE guidance recommends classifying CKD using a combination of glomerular filtration rate (GFR) and albumin creatinine ratio (ACR), see description in table 1.
The Health Survey for England (2016) found that 13% of adults (16 years and over) had any CKD (stages 1 to 5). The prevalence of stages 3 to 5 was 5% for all adults, rising to 34% in people aged 75 and over. At the end of 2018 there were 826 children and young people and 66,612 adults receiving renal replacement therapy in the UK according to the UK Renal Registry annual report.
This indicator applies to patients with category G3a, G3b, G4 and G5 CKD (GFR<60 ml/min/1.73 m² on at least 2 occasions separated by a period of at least 90 days).
Late presentation of patients with kidney failure increases morbidity, mortality and healthcare associated with costs. The total cost of CKD in England in 2009/10 was estimated as being circa £1.4 billion.
Early identification of CKD is therefore important to not only allow appropriate measures to be taken to slow or prevent the progression to more serious CKD, but also to highlight and manage the key associated risks related to patient safety and avoidable harm.
Table 1. Classification of CKD using GFR and ACR categories
GFR category | ACR category A1: normal to mildly increased (less than 3 mg/mmol) | ACR category A2: moderately increased (3 to 30 mg/mmol) | ACR category A3: severely increased (over 30 mg/mmol) |
| GFR category G1: normal and high (90 ml/min/1.73 m2 or over) | Low risk No CKD if there are no other markers of kidney damage | Moderate risk | High risk |
| GFR category G2: mild reduction related to normal range for a young adult (60 to 89 ml/min/1.73 m2) | Low risk No CKD if there are no other markers of kidney damage | Moderate risk | High risk |
| GFR category G3a: mild to moderate reduction (45 to 59 ml/min/1.73 m2) | Moderate risk | High risk | Very high risk |
| GFR category G3b: moderate to severe reduction (30 to 44 ml/min/1.73 m2) | High risk | Very high risk | Very high risk |
| GFR category G4: severe reduction (15 to 29 ml/min/1.73 m2) | Very high risk | Very high risk | Very high risk |
CKD005 (NICE 2014 menu ID: NM83)
CKD005 Rationale
This indicator aims to establish a register of people with CKD categories G3a to G5 to enable appropriate advice, treatment and support to be provided for people with moderate to severe CKD and so help preserve kidney function and reduce the risk of developing co-morbidity.
Eating a meal containing protein can elevate creatinine, therefore it is recommended that patients do not eat meat in the 12 hours before their creatinine is measured and eGFR estimated.
CKD005 Reporting and verification
See indicator wording for requirement criteria.
Epilepsy (EP)
| Indicator | Points | Thresholds |
| Records EP001. The contractor establishes and maintains a register of patients aged 18 or over receiving drug treatment for epilepsy. | 1 | N/A |
EP – rationale for inclusion of indicator set
Epilepsy is the most common serious neurological condition, affecting about five to ten per 1000 of the population at any one time. Few epilepsies are preventable, but appropriate clinical management can enable most patients with epilepsy to lead a full and productive life. For the purposes of the QOF, epilepsy is defined as ‘recurrent unprovoked seizures’.
EP001
EP001 Rationale
The disease register includes patients aged 18 or over, as care for younger patients is generally undertaken outside of primary care.
The phrase ‘receiving treatment’ has been included in order to exclude the large number of patients who may have had epilepsy in the past, may have not received treatment and been fit-free for many years. Some patients may still be coded as ‘epilepsy’ or ‘history of epilepsy’ and will be picked up on computer searches.
Patients with a history of epilepsy who are not on drug therapy are excluded from the register. Drugs on repeat prescription will be picked up on a search.
EP001 Reporting and verification
See indicator wording for requirement criteria.
Verification – Commissioners may require a comparison of the expected prevalence with the reported prevalence recognising that reported prevalence will be reduced as the register is limited to those patients receiving drug treatment.
Learning disabilities (LD)
| Indicator | Points | Thresholds |
| Records LD004. The contractor establishes and maintains a register of patients with learning disabilities | 4 | N/A |
LD – rationale for inclusion of indicator set
People with learning disabilities are among the most vulnerable and socially excluded in our society. It is estimated that there are approximately 20/1,000 people with mild learning disabilities and 3-4/1,000 with severe and profound learning disabilities in the UK. Over the past three decades, almost all the long-stay NHS beds for people with learning disabilities have closed and virtually all people with learning disabilities are now living in the community and depend on general practice for their primary care needs.
The health inequalities gap for people with a learning disability has increased, particularly during Covid-19. For some with a learning disability, primary care may be their only contact with health care services and so the only opportunity to support and maintain their health and wellbeing. 6 out of 10 people with a learning disability died before they were 65. This compares to around 1 in 10 of the general population. 49% of the deaths of people with a learning disability were preventable.
LD004 (NICE 2015 menu ID: NM73)
LD004 Rationale
This register indicator includes people of any age with a learning disability. This is because without a complete register of people with learning disabilities, practices may not be aware of the reasonable adjustments that may be needed for a child or young person with learning disabilities and their family, and of the help and support that may be useful to them. In order to ensure accurate recording, primary care will need to liaise with the wider health education and social care network to identify and include onto the register a child or young person, with the aim to improve health outcomes through identification and prevention, utilising the guidance available where a diagnosis is not provided.
Evidence suggests there are an increasing number of children with learning disabilities now surviving childhood, some of whom will have profound and multiple disabilities as they grow up (Emerson, 2009). It also suggests that health services are often unprepared for these children and young people and the complexity of their problems (Betz, 2004).
A full register of patients with learning disabilities and their ethnicity will provide primary care practitioners with the first important building block in providing better quality and more appropriate services for this patient population.
Learning disabilities are heterogeneous conditions, but are defined by three core criteria:
- Lower intellectual ability (usually defined as an Intelligence Quotient [IQ] of less than 70) or a significantly reduced ability to understand new or complex information;
- Significant impairment of social or adaptive functioning; and
- Onset in childhood
An IQ below 70 should not be used on its own to determine whether someone has a learning disability. The definition encompasses people with a broad range of disabilities. It includes adults with autism who also have learning disabilities, but not people with a higher level autistic spectrum disorder who may be of average or above average intelligence. The definition does not include all those people who have a “learning difficulty”, e.g. specific difficulties with learning, such as dyslexia.
NHS England has published guidance aimed at improving the identification of people with a learning disability. Practices should review this guidance and update their registers at least annually to ensure that they are accurate. Practices should refer to the table in appendix four ‘learning disability identification check-list’ of this guidance, to assist them in identifying a person with a learning disability.
It is a statutory requirement under the Equality Act 2010 that public sector agencies make ‘reasonable adjustments’ to their practice that will make them as accessible and effective as they would be for people without disabilities. Reasonable adjustments include removing physical barriers to accessing health services, but importantly also include making whatever alterations are necessary to information, policies, procedures, staff training, communication interventions and service delivery to ensure that they work equally well for people with learning disabilities (Public Health England, 2013).
LD004 Reporting and verification
See indicator wording for requirement criteria.
There was a significant revision of the clinical codes used to create this register in 2019. Full details are available in the Business Rules and coding guidance published in 2019. Where practices are cautious to add a code where there is no confirmed diagnosis, they are encouraged to use the code ‘on the LD register’.
Osteoporosis: secondary prevention of fragility fractures (OST)
| Indicator | Points | Thresholds |
| Records OST004. The contractor establishes and maintains a register of patients: 1. Aged 50 or over and who have not attained the age of 75 with a record of a fragility fracture on or after 1 April 2012 and a diagnosis of osteoporosis confirmed on DXA scan, and 2. Aged 75 or over with a record of a fragility fracture on or after 1 April 2014 and a diagnosis of osteoporosis | 3 | N/A |
OST – rationale for inclusion of indicator set
Osteoporotic fragility fractures can cause substantial pain and severe disability and are associated with decreased life expectancy. Osteoporotic fragility fractures occur most commonly in the spine (vertebrae), hip (proximal femur) and wrist (distal radius). They also occur in the arm (humerus), pelvis, ribs and other bones.
Fractures of the hands and feet (for example metacarpal and metatarsal fractures) are not generally regarded as osteoporotic fragility fractures.
Interventions for secondary prevention of fractures in patients who have had an osteoporotic fragility fracture include pharmacological intervention.
OST004 (NICE 2011 menu ID: NM29)
OST004 Rationale
Fragility fractures are fractures that result from low-level trauma, which means mechanical forces that would not ordinarily cause fracture. The WHO has described this as a force equivalent to a fall from a standing height or less. Reduced bone density is a major risk factor for fragility fractures (WHO, 2018, NICE CG146 (2012, updated 2017).
Osteoporosis is a disease characterised by low bone mass and structural deterioration of bone tissue. The WHO defines osteoporosis as a bone mineral density of 2.5 or more standard deviations below that of a normal young adult (T- score of -2.5 or less) measured by a central dual-energy X-ray absorptiometry (DXA) scan. Bone mineral density is the major criterion used to diagnose and monitor osteoporosis.
NICE guidance on osteoporosis fragility fractures recommends that a diagnosis of osteoporosis may be assumed in women aged 75 or over with a fragility fracture if the responsible clinician considers a DXA scan to be clinically inappropriate or unfeasible. The SIGN guideline on the management of osteoporosis recommends that in frail elderly women (aged 80 or over) a DXA scan would be a prerequisite to establish that bone mass density (BMD) is sufficiently low before starting treatment with bone-sparing agents (bisphosphonates), unless the patient has suffered multiple vertebral fractures.
Osteoporotic fragility fractures can cause substantial pain and severe disability and are associated with decreased life expectancy. Osteoporotic fragility fractures occur most commonly in the spine (vertebrae), hip (proximal femur) and wrist (distal radius). They also occur in the arm (humerus), pelvis, ribs and other bones.
Fractures of the hands and feet (for example, metacarpal and metatarsal fractures) are not generally regarded as osteoporotic fragility fractures.
In women, the prevalence of osteoporosis increases markedly with age after menopause, from approximately two per cent at 50 years, rising to more than 25 per cent at 80 years. The NICE cost impact report for technology appraisal TA161 uses a prevalence of 11 per cent of post-menopausal women aged 50 or over with osteoporosis and a clinically apparent osteoporotic fragility fracture, rising to 19 per cent for ages 65 or over. There are an estimated 180,000 new fragility fractures in postmenopausal women in the UK each year; three quarters in women aged 65 or over.
Postmenopausal women with an initial fracture are at substantially greater risk of subsequent fractures. Half of patients with a hip fracture have previously had a fragility fracture of another bone.
Hip fractures are associated with increased mortality; estimates of the relative mortality risk vary from two to greater than ten in the 12 months following hip fracture. However, it is unclear to what extend this can be attributed to fracture alone, as opposed to pre-existing co-morbidity.
The SIGN guideline recommends that patients who have suffered one or more fragility fractures are priority targets for investigation and treatment of osteoporosis.
This indicator promotes structured case finding for osteoporosis in patients who have had a fragility fracture. Its aim is to promote the secondary prevention of fragility fracture in patients with osteoporosis.
OST004 Reporting and verification
The Business Rules for the two-part register will look for the following criteria: In patients aged 50 or over and who have not attained the age of 75:
- The earliest DXA scan with a positive result of osteoporosis
- The earliest diagnosis of osteoporosis
- A fragility fracture at any point on or after the implementation date (1 April 2012).
In patients aged 75 or over:
- In patients aged 75 or over:
- The earliest diagnosis of osteoporosis
- A fragility fracture at any point on or after the implementation date (1 April 2014).
Patients aged 50 or over and under the age of 75 in whom a diagnosis of osteoporosis has not been confirmed with DXA scanning will not be included in the register.
For patients aged 75 or over the diagnosis of osteoporosis can be either confirmed with DXA scanning or clinically assumed (if DXA scan is considered to be clinically inappropriate or unfeasible).
Patients with fragility fractures sustained in the last three months of the year will be excepted from this indicator.
Although this indicator defines two separate registers, the disease register for calculating the APDF is defined as the sum of the number of patients on both registers.
Rheumatoid arthritis (RA)
| Indicator | Points | Thresholds |
| Records RA001. The contractor establishes and maintains a register of patients aged 16 or over with rheumatoid arthritis | 1 | N/A |
RA – rationale for inclusion of indicator set
Rheumatoid arthritis (RA) is a chronic, disabling auto-immune disease characterised by inflammation in the peripheral joints, which causes swelling, stiffness, pain and progressive joint destruction. For a small proportion of people with RA, inflammatory disease outside the joints (i.e. eye and lung disease, vasculitis) can pose a significant problem. RA affects around one per cent of the population; of these people, approximately 15 per cent have severe RA.
Although the confirmation of diagnosis and initiation of treatment may take place in secondary care, primary care has an important role to play in the management of RA. This may include checking cardiovascular risk and blood pressure, checking the person’s risk for osteoporosis and assessing for signs of low mood or depression. An annual face-to-face review in primary care is an opportunity to assess the effect of the disease upon the person’s life, for example side effects to medication and whether they would benefit from any referrals to the MDT.
RA001 (NICE 2012 menu ID: NM55)
RA001 Rationale
The RA register includes patients aged 16 or over with established and recent-onset disease and in whom there is a definite diagnosis of RA, irrespective of evidence of positive serology and current disease activity status.
The register is restricted to patients aged 16 or over, to conform to international standards for differentiating RA from juvenile idiopathic arthritis.
The register also includes patients with inactive RA. There are three potential groups of patients whose disease may be referred to as inactive:
- Patients who are being treated and whose disease is in remission
- Patients who are not receiving treatment for RA but have evidence of past disease, e. joint deformities. This type of RA is sometimes known as ‘burnt out’ RA. These patients are on the register as they remain at risk of the systemic effects of RA
- Patients who are not receiving treatment for RA who have no evidence of past disease but there is doubt about their diagnosis. The contractor may wish to request (ESR) or plasma viscosity, C-reactive protein (CRP), rheumatoid factor and hand X-ray to determine the accuracy of the Inaccurate diagnoses can be removed from the patient’s patient record which would also remove them from the register.
Recognition of synovitis in primary care and prompt referral for specialist advice is key to the early identification and treatment of RA. Synovitis is inflammation of the membrane that lines the inside of synovial joints (most of the joints in the body).
Symptoms of inflammation include pain, swelling, heat and loss of function of an affected joint.
Identifying recent-onset RA can be challenging in primary care because of the variety of ways in which synovitis can present itself and the small number of patients who have RA compared with the number of patients with musculoskeletal symptoms. NICE guideline NG100 recommends that patients with persistent synovitis are referred for specialist opinion. Urgent referral is needed when any of the following are present:
- The small joints of the hands or feet are affected
- More than one joint is affected
- There has been a delay of three months or longer between the onset of symptoms and seeking medical advice.
Early identification of recent-onset RA is important because long-term outcomes are improved if disease modifying anti-rheumatic drugs (DMARDs) treatment is started within three months of the onset of symptoms.
RA001 Reporting and verification
See indicator wording for requirement criteria.
Verification – Commissioners may wish to discuss with contractors the process they use to identify patients with RA, and the number of patients with inactive disease whose diagnoses have been reviewed and the outcomes of this review.
Palliative care (PC)
| Indicator | Points | Thresholds |
| Records PC001. The contractor establishes and maintains a register of all patients in need of palliative care/support irrespective of age | 3 | N/A |
PC – rationale for inclusion of indicator set
Palliative or end of life care is the active total care of patients with life-limiting disease and their families by a multi-professional team. The first National End of Life Care (EoLC) Strategy was published in July 2008 followed by:
- The National Palliative and EoLC Ambitions for palliative and EoLC: A national framework for local action 2021-2026
- Our commitment to you for EoLC: The Government response to the review of choice in EoLC (2016)
- NHS Long Term Plan (2019) commitment to personalised palliative and end of life care planning.
There is also a commitment to improve access to palliative and end of life care for children and young people. Timely identification of people in need of this support will be key to making these quality improvements.
PC001
PC001 Rationale
About one per cent of the population in the UK die each year (over half a million), with an average of 20 deaths per GP per year. A quarter of all deaths are due to cancer, a third from organ failure, a third from frailty or dementia and only one twelfth of patients have a sudden death. It is estimated that three quarters of all deaths may be anticipated. Considerable benefits of identifying these patients include providing the best health and social care to both patients and families and reducing crises, by prioritising them, anticipating need, initiating conversations and enabling patients to be able to make informed decisions about the care and support they need.
Identifying patients in need of palliative care, assessing their needs and preferences, offering conversations about advance care planning and proactively planning and addressing their care needs, are the key steps in the provision of high- quality palliative and end of life care in general practice. This indicator is focused on identifying these patients – a critical first step in addressing the key elements of good medical practice identified by the General Medical Council.
A patient is included on the register if any of the following apply:
- Their death in the next 12 months can be reasonably predicted (rather than trying to predict, clinicians often find it easier to ask ‘the ‘surprise question’ – ‘Would I be surprised if this patient were still alive in 12 months?’)
- They have advanced or irreversible disease and clinical indicators of progressive deterioration and thereby a need for palliative care e.g. they have one or more core/general and one disease specific indicator in accordance with the gold standard framework (GSF) prognostic indicators guidance or the Supportive and Palliative Care Indicators Tool (SPICT)
- They are entitled to a DS 1500 form (the DS 1500 form is designed to speed up the payment of financial benefits and can be issued when a patient is considered to be approaching the terminal stage of their illness. For these purposes, a patient is considered as eligible for these Special Rules if they are suffering from a progressive disease and are not expected to live longer than six months).
The register applies to all patients fulfilling the criteria regardless of age or diagnosis. The creation of a register will not in itself improve care but it should facilitate proactive MDT discussion, encourage important conversations with the individual and those important to them, and enable the wider practice team to provide more appropriate and personalised palliative and end of life care.
PC001 Reporting and verification
See indicator wording for requirement criteria.
There is no APDF calculation in respect of the palliative care indicators. In the rare case of a nil register at year end, if a contractor can demonstrate that it established and maintained a register during the financial year then they will be eligible for payment for PC001.
Non diabetic hyperglycaemia (NDH)
| Indicator | Points | Thresholds |
| Records NDH002. The percentage of patients with non-diabetic hyperglycaemia who have had an HbA1c or fasting blood glucose performed in the preceding 12 months | 18 | 50–90% |
NDH – rationale for inclusion of indicator set
NDH is defined as an HbA1c of 42-47mmol/mol or a fasting plasma glucose (FPG) of 5.5-6.9mmol/l. There were 3 million people with NDH in England in March 2021.
The NHS has invested heavily in behavioural interventions for those with NDH in order to prevent and delay the onset of Type 2 diabetes. The Healthier You: NHS Diabetes Prevention Programme (NHS DPP) is the largest undertaking of its kind in the world and over 230,000 people have already benefited since its introduction in 2016. It has proven effective at causing weight loss and reducing HbA1c (Diabetologia, 2019).
The programme is available across the whole of England, and GPs can refer patients aged 18 who have been diagnosed as having NDH in the 12 months prior to referral. Individuals with a previous history of Gestational Diabetes Mellitus (GDM) and ‘normoglycaemia’ within the 12 months prior to date of referral are also eligible.
Individuals must not be pregnant, have a previous diagnosis of Type 2 Diabetes, or be recorded as living with moderate/severe frailty.
NDH002 (NICE 2017 menu ID: NM150)
NDH002 Rationale
NICE Guidance (PH38121) recommends that everyone with NDH is offered an annual blood test to check for progression to Type 2 diabetes. Despite this there is wide variation in the monitoring of people with NDH.
The aim of this indicator is to promote early identification of when people cross the threshold into the Type 2 diabetes category, as it is associated with reduced CVD event rate and lower mortality in the individuals identified. Criteria for diagnosing diabetes are discussed in the diabetes section of this guidance.
NDH002 Reporting and verification
See indicator wording for requirement criteria.
The register for the purpose of calculating the APDF is defined as all patients aged 18 or over with a record of non-diabetic hyperglycaemia or pre-diabetes, which has not been superseded by a diagnosis of diabetes recorded prior to the beginning of the financial year.
Section 4: Public Health domain
Blood pressure (BP)
| Indicator | Points | Thresholds |
| BP002. The percentage of patients aged 45 or over who have a record of blood pressure in the preceding 5 years | 15 | 50–90% |
BP002 (based on NM61)
BP002 Rationale
Detecting elevated blood pressure and, where indicated, treating it, is known to be an effective health intervention. Raised blood pressure is common if it is measured on a single occasion but with repeated measurement blood pressure tends to drop. NICE guideline recommendations for the diagnosis and treatment of hypertension are to be followed by practitioners when deciding on whether to treat raised blood pressure.
The age limit of aged 45 or over, has been chosen as the vast majority of patients develop hypertension after this age. The age range 45 or over, coupled with a five- year reference period is in line with the NHS Health Checks Scheme, which starts at 40 years old. It is also to align the indicator more closely with the vascular checks programme and the cost-effectiveness modelling undertaken to support that programme.
It is anticipated that contractors will opportunistically check blood pressures in all adult patients.
BP002 Reporting and verification
See indicator wording for requirement criteria.
Generally, personalised care adjustment criteria (see Section 6) do not apply to this indicator. However, practices are able to remove patients from the denominator where the patient declines to accept offered care.
Obesity (OB)
| Indicator | Points | Thresholds |
| Records OB003. The contractor establishes and maintains a register of patients aged 18 years or over living with obesity, appropriately adjusted for ethnicity in line with NICE guidelines – either with a BMI greater than or equal to 30 kg/m2 recorded in the preceding 12 months, or a BMI greater than or equal to 27.5 kg/m2 recorded in the preceding 12 months for patients with a South Asian, Chinese, other Asian, Middle Eastern, Black African or African-Caribbean family background. | 8 | N/A |
OB – rationale for inclusion of indicator set
The Global Burden of Disease study identifies obesity as one of the top five risk factors contributing to premature death in England along with smoking, poor diet, high blood pressure and drug and alcohol use (Steel et al, 2018). Nearly two-thirds of adults in England are living with overweight or obesity, some of the worst figures in Europe (OECD Obesity update 2017). As noted in the NHS Long Term Plan obesity is linked with type 2 diabetes, high blood pressure, high cholesterol, increased rates of respiratory, musculoskeletal and liver disease and certain types of cancer.
The NHS Long Term Plan commits to a targeted offer of support and access to weight management services in primary care for people with a diagnosis of hypertension or type 2 diabetes with a BMI >30, (adjusted appropriately for ethnicity) amongst other actions to reduce obesity.
Further information
- NICE has produced multiple guidelines on clinical and public health approaches to tacking obesity, they can be accessed via the NICE website.
OB003
OB003 Rationale
The register includes all patients whose BMI has been recorded by the practice as part of routine care. It is expected that this data will inform public health planning and support onward referral to weight management services.
NICE guideline CG189 recommends using BMI as a practical estimate of adiposity in adults. Identifying people with a BMI ≥25 includes a preventative aspect of care in managing obesity and supports interventions for people at risk of obesity i.e. those who are overweight but not yet obese. People with a South Asian, Chinese, other Asian, Middle Eastern, Black, African or African-Caribbean family background are prone to central adiposity and their cardiometabolic risk occurs at lower body mass index (BMI). NICE guidance therefore, recommends a differential BMI threshold for identification of obesity in relation to these patient groups. Therefore, the wording of this indicator has been updated for 2023/24.
OB003 Reporting and verification
See indicator wording for requirement criteria.
Smoking (SMOK)
| Indicator | Points | Thresholds |
| Records SMOK002. The percentage of patients with any or any combination of the following conditions: CHD, PAD, stroke or TIA, hypertension, diabetes, COPD, CKD, asthma, schizophrenia, bipolar affective disorder or other psychoses whose notes record smoking status in the preceding 12 months | 25 | 50–90% |
| Ongoing management SMOK004. The percentage of patients aged 15 or over who are recorded as current smokers who have a record of an offer of support and treatment within the preceding 24 months | 12 | 40–90% |
| SMOK005. The percentage of patients with any or any combination of the following conditions: CHD, PAD, stroke or TIA, hypertension, diabetes, COPD, CKD, asthma, schizophrenia, bipolar affective disorder or other psychoses who are recorded as current smokers who have a record of an offer of support and treatment within the preceding 12 months | 25 | 56–96% |
SMOK – rationale for inclusion of indicator set
Smoking has been identified as the top modifiable risk factor for neurological diseases and premature death in England (The Lancet, 2021). In England, 10% of pregnant women were known to be smokers at the time of delivery (NHS Digital, 2020). Smoking is linked to a wide range of disease and conditions including cancers, respiratory disease, cardiovascular disease, stomach and duodenal ulcers, erectile dysfunction and infertility, osteoporosis, cataracts, age related macular degeneration and periodontitis (US DH and Human Services 2004). Smoking during pregnancy can cause serious pregnancy related health problems, these include: complications during labour and an increased risk of miscarriage, premature birth, still birth, low birth-weight and sudden unexpected death in infancy. Smoking during pregnancy also increases the risk of infant mortality by an estimated 40 per cent (Department of Health, 2007).
The aim of this domain is to increase the proportion of successful smoking quit attempts by providing the best available treatment. There is good evidence to suggest that offering support and treatment is sufficient to motivate some smokers to attempt to stop who would not have done so with brief advice to quit alone.
‘An offer of treatment’ means offering a referral to a local NHS Stop Smoking Service adviser (who might be a member of the practice team) plus pharmacotherapy.
Where such treatment is not acceptable to the patient, an alternative form of brief support, such as follow-up appointments with a GP or practice nurse trained in smoking cessation, may be offered.
The NICE guidance on tobacco identifies the evidence-based interventions for adults who smoke:
- Behavioural support (individual and group)
- Very brief advice
- Bupropion
- Nicotine replacement therapy (NRT) – short and long acting
- Varenicline
- Nicotine-containing e-cigarettes
For people who smoke and who are using, or are interested in using, a nicotine- containing e-cigarette on general sale to quit smoking, NICE recommend you explain that:
- Although these products are not licensed medicines, they are regulated by the Tobacco and Related Products Regulations 2016
- There is not enough evidence to know whether there are long-term harms from e-cigarette use
- Use of e cigarettes is likely to be substantially less harmful than smoking
- Any smoking is harmful, so people using e cigarettes should stop smoking tobacco completely.
Due to the potential for ex-smokers to resume smoking within three years of cessation, it is good clinical practice to ask patients with a history of smoking their current smoking status and offer treatment and advice where necessary. It is also good practice to ask and record the smoking status of newly registered patients and to offer support and treatment where necessary.
SMOK002 (NICE menu 2011 ID: NM38)
SMOK002 Rationale
See rationale above.
SMOK002 Reporting and verification
See indicator wording for requirement criteria. The contractor should report smoking status using the following guidance:
Smokers
For patients who smoke, smoking status should be recorded in the preceding 12 months.
Non-smokers
It is recognised that life-long non-smokers are very unlikely to start smoking and repeatedly asking smoking status can be unnecessary. Smoking status for this group of patients should be recorded in the preceding 12 months for until the end of the financial year in which the patient reaches the age of 25.
Once a patient is over the age of 25 years (e.g. in the financial year in which they reach the age of 26 or in any year following that financial year) to be classified as a non-smoker they should be recorded as:
- Never smoked which is both after their 25th birthday and after the earliest diagnosis date for the disease which led to the patient’s inclusion on the SMOK002 register (e.g. one of the conditions listed on the SMOK002 register).
Ex-smokers
Ex-smokers can be recorded as such in the preceding 12 months for SMOK002. Practices may choose to record ex-smoking status on an annual basis for three consecutive financial years and after that smoking status need only be recorded if there is a change. This is to recognise that once a patient has been an ex-smoker for more than three years they are unlikely to restart.
For the purposes of QOF users of electronic cigarettes who have never smoked or given up smoking should be classified as non-smokers or ex-smokers respectively.
The disease register for the purpose of calculating APDF for SMOK002 and SMOK005 is defined as the sum of the number of patients on the disease registers for each of the conditions listed in the indicator wording. Patients with one or more co-morbidities e.g. diabetes and CHD are only counted once.
SMOK004 (based on NM40)
SMOK004 Rationale
See rationale above.
SMOK004 Reporting and verification
See indicator wording for requirement criteria. There is no APDF calculation for SMOK004.
SMOK005 (NICE 2011 menu ID: NM39)
SMOK005 Rationale
See rationale above for guidance on ‘support and treatment’ and smoking cessation.
This indicator relates to patients who are on the disease registers for CHD, PAD, stroke or TIA, hypertension, diabetes, COPD, CKD, asthma and mental health who are recorded as current smokers.
SMOK005 Reporting and verification
See indicator wording for requirement criteria.
The disease register for the purpose of calculating APDF for SMOK002 and SMOK005 is defined as the sum of the number of patients on the disease registers for each of the conditions listed in the indicator wording. Patients with one or more co-morbidities e.g. diabetes and CHD are only counted once.
Vaccination and Immunisations (VI)
| Indicator | Points | Thresholds |
| VI001. The percentage of babies who reached 8 months old in the preceding 12 months, who have received at least 3 doses of a diphtheria, tetanus and pertussis containing vaccine before the age of 8 months | 18 | 89-96% |
| VI002. The percentage of children who reached 18 months old in the preceding 12 months, who have received at least 1 dose of MMR between the ages of 12 and 18 months | 18 | 86-96% |
| VI003. The percentage of children who reached 5 years old in the preceding 12 months, who have received a reinforcing dose of DTaP/IPV and at least 2 doses of MMR between the ages of 1 and 5 years | 18 | 81-96% |
| VI004. The percentage of patients who reached 80 years old in the preceding 12 months, who have received a shingles vaccine between the ages of 70 and 79 years | 10 | 50-60% |
VI – rationale for inclusion of indicator set
Vaccination currently prevents 2-3 million deaths worldwide every year (WHO, 2022). Recently, the World Health Organization (WHO) listed vaccine hesitancy as one of their top 10 biggest threats to global health. Health workers, especially those in communities, remain the most trusted advisors and influencers of vaccination decisions, and play a key role in providing patients with trusted, credible information on vaccines (WHO, 2019).
Note on vaccinations delivered overseas
Where a patient has been vaccinated overseas in accordance with the UK National Vaccination Schedule – i.e. the schedule of the overseas country conforms to the UK schedule – practices can record delivery of the vaccination in their clinical system to ensure that the vaccination counts towards QOF achievement. For avoidance of doubt, if a patient has been vaccinated overseas in accordance with the UK national schedule and appropriate evidence has been provided of this vaccination event, the patient should count as a success in respect of any relevant QOF indicator – it should not simply trigger a Personalised Care Adjustment.
When a patient or their representative reports that a vaccination has been delivered overseas or in another setting, individual clinicians should exercise their judgement to determine that a vaccination has been delivered and to record it in the patient record. The Green Book states, “If children and adults coming to the UK do not have a documented or reliable verbal history of immunisation, they should be assumed to be unimmunised and a full course of required immunisations should be planned.” Patients arriving from overseas with a “documented or reliable verbal history of immunisation” can be assumed to be immunised and recorded as such in the GP patient record – though in the case of reliable verbal histories, it may not be possible to record the batch number or exact vaccination date.
Where a patient has been vaccinated overseas in accordance with the UK national schedule, the practice can ensure that the vaccination counts towards QOF achievement but does not attract an item of service payment by coding the vaccination event in the following way:
- Backdate the event date of the vaccination SNOMED code to accurately reflect when the vaccination was delivered.
- Set the GMS flag to ‘No’ (for EMIS and Cegedim practices) or the ‘Event done’ flag to ‘No’ (for TPP practices)*
- If the vaccination is for MMR or Shingles, use the “MMR vaccination given by other healthcare provider” or “Shingles vaccination given by other healthcare provider” SNOMED code.**
- Add free text associated with the vaccination SNOMED code to note the date the vaccine was given and where.
* The purpose of the GMS flag is to denote when an activity was delivered in fulfilment of the practice’s GMS (inclusive of PMS and APMS) contract (GMS=True), or either delivered by the practice outside the GMS contract or delivered by another healthcare provider (GMS=False). TPP has not implemented a GMS flag, but offers analogous functionality in the form of an ‘Event done’ flag which, if set to false, denotes that the practice did not deliver the activity.
** Vaccination given by other healthcare provider’ SNOMED codes exist for a limited number of vaccines. MMR and Shingles are the only vaccinations in QOF with a ‘vaccination given by other healthcare provider’ code available.
Note on new Personalised Care Adjustment (PCA) in 2023/24
As part of the changes to the GP Contract 2023/24 published on 6 March 2023, a new PCA was introduced for VI001, VI002 and VI003 to take into account patients who registered at the practice too late (either too late in age, or too late in the financial year) to be vaccinated in accordance with the UK national schedule (or, where they differ, the requirements of the relevant QOF indicator).
From April 2023, this new automated PCA has been built into the business rule logic underpinning the QOF V&I GPES extracts and applies in circumstances where a child is registered with a practice and:
- there is insufficient time to provide any incomplete vaccinations either within the required timeframes to meet the indicator requirements, or
- where a child has an incomplete vaccination status and is now older than the cut-off age required by the indicator.
The PCA cannot be applied manually and will be automatically applied by the indicator logic. The PCA will be superseded in the extract logic by success i.e. the relevant vaccinations being given before the relevant cut-off age required by the indicators. The PCA applies once the individuals are registered with the practice and the relevant logic parameters are met. Where the PCA is applied, it will remove the child from both the denominator and numerator thus not impacting on achievement of the relevant indicator.
In the event a child is registered with a practice and has already reached the relevant indicator’s cut off age – where the cut off age is 8 months for VI001, 18 months for VI002 and 5 years for VI003 – and had incomplete vaccinations, then the automatic PCA will be applied. This is because it is by no fault of the practice that this child was not vaccinated.
However, for a child that is registered with a practice at an age younger than the cut off age for the relevant indicator, then the PCA is flexibly applied depending on both the time remaining prior to the child reaching the cut off age, and the number of outstanding doses. A timeframe of 31 days per outstanding dose from registration date to meeting the cut off age for the indicator is applied. Further information can be found in the business rules – Quality and Outcomes Framework (QOF) business rules v48.0 2023-2024 – NHS Digital.
Practices may want to check whether this PCA is active on the system by using the various system reporting tools such as ‘How am I driving?’ before the end of the financial year.
Some examples of how the new PCA applies to the three V&I indicators are provided below:
For VI001:
- If a child is registered with a practice on or after 7 months of age and has two or fewer doses of diphtheria, tetanus and pertussis containing vaccine prior to registering then the PCA would automatically be applied as there would be insufficient time to offer and administer the required doses.
- If a child is registered with a practice on or after 6 months of age and has one or no doses of diphtheria, tetanus and pertussis containing vaccine prior to registering then the PCA would automatically be applied as there would be insufficient time. However, if a child registered with the practice at 6 months of age and had already had two doses of diphtheria, tetanus and pertussis containing vaccine prior to registering and the third dose was not given by the practice before the child turns 8 months, then the practice would not achieve the indicator for this specific child – this is because the practice would have had sufficient time to give the remaining dose.
- If a child registered with a practice on or after 5 months of age and had no doses of diphtheria, tetanus and pertussis containing vaccine prior to registering then the PCA would automatically be applied as there would be insufficient time. However, if a child registered with the practice at 5 months of age and had already had one or two doses of diphtheria, tetanus and pertussis containing vaccine prior to registering and the third dose was, or second and third doses were, not given by the practice before the child turns 8 months, then the practice would not achieve the indicator for this specific child – this is because the practice would have had sufficient time to give the remaining one or two dose(s).
- If a child registered with a practice between 1-4 months of age and had no doses of diphtheria, tetanus and pertussis containing vaccine prior to registering and the practice does not give all three doses before the child turns 8 months old, then the practice would not achieve the indicator for this specific child. The automated PCA would not apply.
For VI002:
- If a child has reached 17 or 18 months of age when registering with the practice and had not had an MMR vaccination, then the automatic PCA will be applied. However, if the child is 16 months or younger and does not receive one dose of MMR vaccination before they turn 18 months, then the practice would not achieve this indicator for the specific child.
For VI003:
- If a child registered with a practice on or after 4 years and 11 months of age and had either (1) two MMR vaccinations but no booster DTap/IPV or (2) only one MMR and the booster DTap/IPV, then the automatic PCA will be applied as there is insufficient time.
- If a child registered with a practice on or after 4 years and 10 months of age and had either (1) only had one MMR and no booster DTap/IPV or (2) no MMR but had the booster DTap/IPV, then the automatic PCA will be applied as there is insufficient time.
- If a child registered with a practice on or after 4 years and 9 months of age and had no MMR vaccinations and no booster DTap/IPV, then the automatic PCA will be applied as there is insufficient time.
- If a child registered with a practice younger than 4 years and 9 months of age and does not receive both MMR vaccinations and the booster DTap/IPV then the practice would not achieve the indicator for this specific child.
VI001 (NICE 2020 menu ID: NM197)
VI001 Rationale
Diphtheria, tetanus and pertussis (whooping cough) are acute infectious diseases that can have severe complications. The routine immunisation schedule states that the hexavalent (6-in-1) vaccine is due at 8, 12 and 16 weeks old for immunisation to diphtheria, tetanus and pertussis (DTaP) as well as poliomyelitis (IPV), haemophilus influenzae type B (Hib) and hepatitis B (Public Health England 2020).
The indicator supports early vaccination according to the routine immunisation schedule. Measurement by 8 months old allows for vaccination deferral due to febrile illness but aims to achieve immunisation against the named acute infectious diseases as early as possible.
The lower threshold for this indicator has been lowered to 89% and the upper threshold has been raised to 96% to extend the payment thresholds for this indicator.
VI001 Reporting and verification
See indicator wording for requirement criteria.
The only personalised care adjustment applicable is where the intervention described in the indicator is contraindicated for the patient.
VI002 (NICE 2020 menu ID: NM198)
VI002 Rationale
MMR is the combined vaccine that protects against measles, mumps and rubella. These are highly infectious conditions that can have serious complications such as meningitis and encephalitis. The first MMR vaccine (MMR1) is due as part of the routine vaccination schedule for England within a month of the child’s first birthday (Public Health England 2020).
The indicator supports early vaccination with the first dose of the MMR vaccine according to the routine immunisation schedule. Measurement by 18 months old allows for vaccination deferral due to febrile illness but aims to achieve vaccination as early as possible.
The lower threshold for this indicator has been lowered to 86% and the upper threshold has been raised to 96% to extend the payment thresholds for this indicator.
VI002 Reporting and verification
See indicator wording for requirement criteria.
The only personalised care adjustment applicable is where the intervention described in the indicator is contraindicated for the patient.
VI003 (NICE 2020 menu ID: NM199)
VI003 Rationale
The indicator supports immunisation according to the routine immunisation schedule. Measurement by 5 years old aims to achieve full immunisation against these infectious diseases before children start school.
The lower threshold for this indicator has been lowered to 81% and the upper threshold has been raised to 96% to extend the payment thresholds for this indicator.
VI003 Reporting and verification
See indicator wording for requirement criteria.
The only personalised care adjustment applicable is where the intervention described in the indicator is contraindicated for the patient.
VI004 (based on NM201)
VI004 Rationale
Shingles is caused by the reactivation of a latent varicella zoster virus infection. Incidence and severity of disease are associated with increasing age. The routine immunisation schedule states that the shingles vaccine is due at 70 years old (Public Health England 2020). Patients remain eligible for the vaccination until their 80th birthday.
The indicator supports vaccination against shingles for patients 70 years old and over. The effectiveness of the shingles vaccine decreases with increasing age so earlier vaccination is encouraged to ensure optimal protection against shingles.
VI004 Reporting and verification
See indicator wording for requirement criteria. Patients should have received a complete course to be included in the numerator for this indicator. Practices may use a personalised care adjustment if the vaccine is contraindicated or if the patient has declined vaccination.
Public health domain – additional services
For contractors providing additional services the following indicators apply.
Cervical screening (CS)
| Indicator | Points | Thresholds |
| CS005. The proportion of women eligible for screening aged 25-49 years at end of period reported whose notes record that an adequate cervical screening test has been performed in the previous 3 years and 6 months | 7 | 45-80% |
| CS006. The proportion of women eligible for screening and aged 50-64 years at end of period reported whose notes record that an adequate cervical screening test has been performed in the previous 5 years and 6 months | 4 | 45-80% |
CS indicator 005 (NICE 2017 menu ID: NM154) and CS indicator 006 (NICE 2017 menu ID: NM155)
CS005 and CS006 Rationale
These indicators are designed to encourage and incentivise contractors to offer age appropriate cervical screening in line with the recommendations of the NHS Screening Programme and to continue to achieve high levels of uptake of this.
Specific requirements apply to these indicators in relation to the Personalised Care Adjustment. These are detailed in Section 6.
CS005 and CS006 Reporting and verification
See indicator wording for requirement criteria.
Commissioners may require that the contractor can provide a computer print-out showing the number of eligible women on the contractor list, the number with a personalised care adjustment and the number who have had a cervical screening test performed at the appropriate time interval.
Women need to be sent a minimum of three invitations before the personalised care adjustment of not responding to invitations for care can be applied as described in Section 6 of this guidance. Since 2019, there is a discrete SNOMED code to record that women have not responded to three invitations for cervical screening.
Section 5: Quality Improvement domain
Workforce and Wellbeing
| Indicator | Points | Thresholds |
| QI013. The contractor can demonstrate continuous quality improvement activity focused upon workforce and wellbeing as specified in current QOF guidance. | 27 | N/A |
| QI014. The contractor has participated in network activity to regularly share and discuss learning from quality improvement activity focused on workforce and wellbeing as specified in current QOF guidance. This would entail attending two primary care network meetings, at the start and towards the end of QI activity. If a practice is not within a PCN, the expectation is that two meetings would be held locally with other practices. | 10 | N/A |
Optimising demand and capacity in general practice
| Indicator | Points | Thresholds |
| Part 1: Optimise use of staff capacity QI016. The contractor can demonstrate that it has in place a recognised and validated approach to understanding demand/activity, capacity and appointment data and has made improvements to data quality to better reflect practice work. | 10 | N/A |
| QI017. The contractor can demonstrate that it has utilised demand and capacity data to inform operational decisions and plan for demand and capacity matching | 6 | N/A |
| QI018. The contractor has participated in network activity to review the smart cards of all staff employed under the Additional Roles Reimbursement Scheme (ARRS) | 6 | N/A |
| Part 2: Reducing avoidable appointments 40% of General Practice appointments are from the top 10% of patient users. Focused improvement work to reduce avoidable appointments will support the contribution of GP practices to a system-wide approach to address high intensity service users, support practices to embed their care navigation processes and build a shared understanding across the system about the impact on general practice workload. It will enable clinical time to be spent on managing more appropriate appointments, enabling more time with complex patients and contribute to more manageable workloads and GP retention. The starting point for addressing avoidable appointments is understanding current activity. QI019. The contractor can demonstrate improvement in reducing avoidable appointments. A suggested approach is outlined below: 1. Using BI tools, if available and practice collected data where not, to understand the practice activity including variations over the days of the week, time of day and time of year. 2. Developing an understanding of the telephone queue either by extracting data from their cloud- based telephony system or asking staff to collect data over a period. 3. Using that data to understand their peaks of activity and better matching their capacity to their demand by, for instance, reviewing rotas. 4. Using improvement techniques described in the Primary Care Transformation Team’s webinar series on Demand and Capacity which provides practical advice and guidance. 5. Referencing the Royal College of General Practitioner’s 6 steps to start to improve delivering continuity of care from their Continuity Toolkit for those who need it and adapting to suit the needs of the practice. | 15 | N/A |
Rationale for inclusion of a Quality Improvement domain
The aim of this domain is to provide support for contractors and their staff to recognise areas of care which require improvement, and take steps to address this through the development and implementation of a quality improvement plan and sharing of learning across their network. Being skilled in quality improvement has been recognised as a key role for healthcare professionals in the Shared View of Quality.
Practices are also encouraged to consider how their activity may support research and innovation within primary care. Covid-19 has highlighted that when front line nurses, doctors and other health professionals work with research teams to embed research in care and treatment pathways, we can give more people an opportunity to get involved in and benefit from research. The evidence generated by research improves patient care and quality of life. Covid-19 has also demonstrated the value of innovation in tackling and responding to key health challenges. NHSE is committed to supporting more practices, staff and patients to get involved in research and proven innovation.
This guidance sets specific objectives for each topic which contractors are expected to work towards and provides advice on potential quality improvement actions.
Within the parameters set out in this guidance, contractors are encouraged to understand where they have the potential to make quality improvements and then to design and implement bespoke quality improvement plans, including improvement targets to address these. There are no deadlines given for the completion of the diagnostic activities, the subsequent plan or the network meetings. However, contractors are advised that they are expected to be working on these improvement activities throughout the QOF year.
The two topic areas for 2023/2024 are workforce and wellbeing and Optimising demand and capacity in general practice.
Through practice engagement with these and future modules, we expect to see measurable improvement in the quality of care and patient experience at a national level against the areas of focus described in the individual modules – though we recognise that in some instances these improvements may only be realised with a lag, i.e. after the end of 2023/2024. Furthermore, while we expect to see measurable improvements at a national level, we also recognise that not all quality improvement activity at a practice level will be successful in terms of its impact upon patient care.
Where an individual practice undertakes a QI project but does not observe measurable improvements, this should not necessarily be taken as a sign of failure, as the learning from this project may be used to inform future improvement activities.
The focus of the indicators and associated points is on contractor engagement and participation in the quality improvement activity both in the practice and through sharing of learning across their network. This is to recognise that not all quality improvement activity will be successful in terms of its immediate impact upon patient care. If a contractor does not achieve the targets which they have set themselves this would not in itself be a reason to withhold QOF points and associated payments, unless they have also failed to participate in the activities described in the guidance.
All the supporting information and resources referred to in this guidance will be made available on NHS England’s website by the end of March 2023. Further information as to how to undertake quality improvement activities is available from a number of sources including:
General Practice Improvement Programme – this is a national resource to support quality improvement activity in primary care and includes training, practical advice and support from quality improvement specialists.
RCGP QI resources – resources including the RCGP QI Guide for General Practice and other quick guides to the use of quality improvement tools and techniques. These are available to both members and non-members.
Health Foundation – an easy to read and practical guide to undertaking QI
NICE Practical Steps – online guide to putting NICE guidance into practice and tools to support this.
Institute for Health Improvement – a US site with a range of resources to support QI activity.
NHS England resources to support working with people and communities.
For further information on how practices can get more involved in the primary care research community and receive support in adopting proven innovation is available from a number of sources:
Health Innovation Networks – There are 15 Health Innovation Networks across England, established by NHS England in 2013 to spread innovation at pace and scale. Their site includes tools, resources and case studies for healthcare innovation as well as contact details for each network to request further information or support.
NIHR – The National Institute for Health Research (NIHR) is the research arm of the NHS. They provide funding for research studies as well as academic training, facilities, career development and research capability development. See about us or contact signposting@nihr.ac.uk for more information on NIHR’s support offer.
NIHR Learn – “The Research and Quality Improvement Learning Community for Primary Care” through NIHR Learn including account registration can be accessed at here: learn.nihr.ac.uk
Workforce and wellbeing rationale
Rationale
General Practice teams are facing extreme pressures and staff morale is low. The Government states that “it is imperative that we look after the NHS workforce and continue to prioritise their safety, health and wellbeing” (Department of Health and Social Care, 2022). It is recognized that the NHS can only achieve the extraordinary things for patients that it does if the safety, health and wellbeing of our people is recognised as a key priority (NHS England, 2023).
As part of the Fuller Stocktake report (May 2022), Dr Fuller warned that “left as it is, primary care as we know it will become unsustainable in a relatively short period of time”. The Eleventh National GP Worklife Survey 2021 found that more than eight out of ten GPs reported experiencing considerable or high pressure from increasing workloads and increased demands from patients. Over a third (33.4%) of GPs said there was a considerable or high likelihood of them leaving ‘direct patient care’ within five years. The 25 July 2022 Expert Panel: evaluation of Government’s commitments in the area of the health and social care workforce in England stressed that “it is more urgent than ever” to ensure that the wellbeing the workforce is properly addressed.
There is a clear need to focus on supporting and improving the wellbeing of the general practice workforce. The pandemic put a significant additional strain on the workforce and the demands on general practice remain high. This module aims to provide practices with the means and resources to evaluate how they currently support their staff and aims to enable the introduction of processes and initiatives that will provide structure and support to staff on a long-term basis.
The 2020 NHS People Plan highlights staff health and wellbeing, support for flexible working, improved culture and leadership and tackling inequalities as a focus in retaining NHS staff (NHS England, 2020). The NHS Operational Guidance in 2021/22 and 2022/23 instructs leaders across the NHS to consider the health and wellbeing of staff as a strategic priority (Department of Health and Social Care, 2022). The 2022 Fuller Stocktake report recommended the need to improve the experience of working in primary care for everyone by making the employment culture more compassionate and inclusive, and listening much more effectively to primary care staff. The Fuller Stocktake also noted that support is needed for the development of training and supervision, recruitment and retention and increased participation of the primary care workforce, including GPs.
A 2022 Kings Fund report explored integrating additional roles into primary care networks. One of the strongest themes that emerged from their work was one of isolation and loneliness for people working in these roles. The report also found that the fundamentals of effective teams within primary were not present, stating that it was “rare” for a PCN to have an effective team approach in place. The report noted the importance of peer support, for several reasons including promoting teamworking, belonging and to help prevent isolation and attrition.
As part of the 2022 NICE Guideline NG212 Mental wellbeing at work, NICE strongly advise organisational-level approaches as the best starting point when considering strategies to improve mental wellbeing at work. This approach demonstrates a commitment to mental wellbeing at work from employers, which is essential for encouraging employees to take up interventions. NICE recommended that individual- level approaches should be used alongside organisational-level approaches because these are less likely to be effective on their own. NICE noted that because of the variation in the size and structure of workplaces, many interventions might need to be tailored to match the specific workplace in which they were going to be delivered.
Overview of the QI module
The overarching aim of this QI module is to lead to improvements in relation to the following aspects of workforce and wellbeing:
- Improving wellbeing, resilience, and risk of burnout for the GP workforce
- Creating a compassionate and inclusive culture in GP
- Supporting the onboarding of new staff
- Supporting the training and development of the whole team in GP Establishing peer support networks.
Practices will need to undertake the following steps:
Step 1
Undertake an exercise to evaluate current workforce wellbeing factors and identify areas for improvement – this should include a review of:
- how to develop and support your workforce eg, access to flexible working opportunities
- reasons reported for absences eg, sickness menopause
- the support different staff groups have access to
- the support currently provided by the practice for new starters.
Step 2
Create an improvement plan with clear aims, improvement measures and change ideas iterative PDSA cycles – using data from cycle 1 to inform what happens in cycle 2.
Step 3
Implement the improvement plan.
Step 4
Participate in a minimum of 2 local Primary Care Network peer review meetings.
Step 5
Complete the QI monitoring template.
The following section includes further detail on the types of things practices could do to deliver this module. These are suggestions and recommendations only and the decision about what to include in the QI plan and which QI methodologies to use should be made by practices and shared with their peers through the network meetings. Ideally the improvement plan will contain work that will support improvement in all three parts of objectives listed above; better understand the skills available across your team and PCN, understand views around access issues and, consider access beyond the practice by reviewing links/pathways with parts of the system beyond the practice and PCN.
Practices are expected to undertake quality improvement activity in both evaluating the current wellbeing of their practice team and in undertaking actions in partnership with their PCN to improve the wellbeing of their practice team.
We know that practices will be at different points in relation to supporting the well- being of their workforce therefore these resources are designed to be used in a flexible way to meet individual organisational needs.
Detailed contractor guidance
Identifying areas for improvement
All practices should start with an assessment of factors that currently impact on workforce wellbeing which should focus on a review of workforce planning including access to flexible working, the reasons reported for sickness absence, the support different staff groups have access to and the support currently provided by the practice for new starters.
It is anticipated that practice QI activity will dovetail with both local priorities and wider wellbeing activities. Practices may also find it useful to undertake a reflective group meeting and complete a ‘SWOT’ (strengths, weaknesses, opportunities and threats) analysis. Guidance can be found in the RCGP publication: How to get started in QI.
Creating an improvement plan
Following the diagnostic phase above, practices should focus their QI activities on outcomes that could include the following (noting that these are suggestions and the decision about what to plan will be made by practices):
Improving wellbeing, resilience, and risk of burnout
- Establish organisational processes that ensure that a stress risk assessment is carried out for each role as required by the Health and Safety at Work Act 1974, for example, using the Health and Safety Executive’s risk assessment template.
- Ensure that systems are in place to provide (or access) support for employees for whom external factors are influencing their mental wellbeing e.g. establish a mental health champion/wellbeing guardian that staff can access for support.
- Monitor and evaluate the support practices provide (or have access to) at least on an annual basis using a relevant evaluation tool. Public Health England’s evaluation in health and wellbeing provides a list of resources and summarises what they are used for.
- Encourage all staff to engage with staff support programmes such as the free, confidential Looking After You coaching offers.
- Encourage patient facing staff members to complete the Handling difficult situations – Caring for yourself and others with compassion e-learning module.
- Raise awareness of Practitioner Health, a free, confidential NHS primary care mental health and addiction service with expertise in treating health and care professionals.
Creating a compassionate and inclusive culture
- Create or update staff lists on a regular basis to include names, job titles and emails for the PCN as good practice and to support the roll out of the national staff survey in general practice.
- Support and encourage all team members to complete the primary care staff survey when launched in Autumn 2023.
- Establish how team members can access a Freedom to Speak Up guardian
- Actively recognise and take action to prevent discrimination eg., practice inclusive recruitment.
- Support staff to attend and implement learnings from training on inclusion and/or inequalities such as the Core managers: Inclusive leadership in health and care e-learning.
- Establish or support team members to access staff peer support networks (this may potentially be provided across a PCN or system).
- Ensure practices and processes are in place to support meeting the equality standards outlined in the NHS Workforce Race Equality Standard and the Workforce Disability Equality Standard.
- Consider establishing a Workforce Equity Champion or EDI lead to support creating a compassionate and inclusive culture (this may potentially be provided across a PCN or system).
- Consider all flexible working requests as noted in NHS flexible working guidance.
- All staff in a leadership role to access training on compassionate leadership and how to lead a team (22,23).
- Encourage all leaders, managers and anyone who supports or organises a team of people to access free, confidential Looking after your team coaching support with creating and maintaining a positive culture if appropriate.
Supporting the onboarding of new staff
- Review current support provided for new starters in the first six months of working in general practice.
- Create or review existing induction processes for new starters including links with a named ‘buddy’.
Supporting the training and development of the whole team in GP
- Establish organisational processes to ensure team members have appropriate line management and access to clinical and educational supervision where required (this may potentially be provided across a PCN or system).
- Establish organisational processes that facilitate all general practice staff to have an annual appraisal including a personal development review that includes:
- setting of meaningful objectives
- provision of clear responsibilities
- discussion of training and development needs in relation to individual aspirations and the service/organisation’s need24.
- Link with the local training hub to identify relevant training and development opportunities for all general practice staff to attend.
- Establish organisational processes that facilitate all team members to be supported and encouraged to take regular protected learning time.
- Encourage team members to access the free, confidential Looking after your career coaching offer to reflect on career development and ambitions, where
- Reflect on how practices are working as a multidisciplinary team at network level during one of the PCN meetings.
Establishing peer support networks
- Practices to discuss how support networks and peer mentoring can be facilitated at a PCN level (at one of the two required meetings).
- Encourage use of or create a shared space that team members can use to take a break and communicate informally with colleagues, either in person or virtually where possible.
Once practices have identified their area/s for improvement they should be clear about:
- The aims of the project – what will be achieved and by when. These aims should be SMART (specific, measurable, achievable, relevant, and time-bound).
- The measures – what data will be collected to know if the aims have been met. Measurements to assess the effectiveness of changes made should be straightforward to collect regularly.
- The changes – what different ways of doing things will be tested e.g. new models of contact, shift in tasks to other clinicians and/or staff.
Practices should choose their own quality improvement activities and set their own targets for improvement based on their baseline evaluation. These should be challenging but realistic.
Targets/ambitions should be validated by network peers as part of the initial network review meeting. Incremental measures of improvement are recommended. Practices should aim to find a way to ensure improvement is continuous and that quality improvement around improving workforce wellbeing in general practice becomes routine. This should include a review and evaluation of the measures implemented as a result of the initial plan.
Implementing the plan
Practices should implement the improvement plan they have developed to support the objectives they have identified. It is recommended that these plans and associated improvement activities should involve the whole practice team and practices are encouraged to engage with colleagues outside the practice, where practicable, for example specialist third sector organisations.
Primary Care network peer review meetings
A key objective of the network peer review meetings is to enable shared learning across the network. Contractors should participate in a minimum of two network peer review discussions unless there are exceptional and unforeseen circumstances which impact on a contractor’s ability to participate. Whilst these meetings would usually be face to face, networks are able to explore other mechanisms to facilitate real time peer learning and sharing including virtual meetings.
The peer review group will usually be the Primary Care Network of which the practice is a member. Where the practice is not part of a network their peer review group should be agreed with the commissioner.
The Network Clinical Lead should choose a suitable facilitator to support sharing of learning and quality improvement. For example, this could be the Network Clinical Lead, another network clinician, a practice manager, or alternatively someone external. A record of attendance should be kept, and it is recommended that the Network Clinical Lead and Network Health Inequalities Lead attend, irrespective of who facilitates the meetings.
It is for the network to determine the timing of these meetings, but it is recommended that the first meeting takes place early in the QI activity at the stage of deciding on what quality improvement activities to undertake and the second towards the end to share outcomes and learning from these activities.
Possible discussion points for PCN peer review meetings include:
- How are practices working as a multidisciplinary team at network level? How can this be improved?
- How are practices ensuring the whole practice team are involved?
- How can clinical supervision and peer support groups be supported at a network level?
- Sharing updates of work undertaken to understand workforce wellbeing. What ideas for change are practices considering? and how does this fit in to wider PCN objectives?
Reporting and verification
The contractor must complete the QI monitoring template in relation to this module and self-declare that they have completed the activity described in their QI plan. The contractor will also self-declare that they have attended a minimum of two peer review meetings (either in person, where appropriate, or virtually) as described above, unless there are exceptional and unforeseen circumstances which impact on a contractor’s ability to participate. In these circumstances contractors are expected to make efforts to ensure alternative participation in peer review.
Verification – Commissioners may require contractors to provide a copy of the QI monitoring template as written evidence that the quality improvement activity has been undertaken. Commissioners may require a written record of attendance at the peer review meetings. If a contractor has been unable to attend a meeting due to exceptional circumstances, then they will need to demonstrate other active engagement in network peer learning and review.
If practices would like to share your template with the national primary care culture and wellbeing team to help inform future health and wellbeing initiatives, please send to england.primarycareworkforce@nhs.net. Please note there is no obligation to share the template with the team, this would be entirely voluntary.
We recommend practices include their QI work in any inspections by the Care Quality Commission to demonstrate a visible and consistent approach to quality improvement.
Resources are available from our GP contract web pages
Quality improvement reporting template
Download a Word version of the following QI reporting template.
It is anticipated that the responses noted here should total a maximum of 2 A4 sides in Arial font size 11.
Practice name and ODS code |
|---|
| What area(s) of workforce wellbeing did the practice identify for quality improvement? |
| What was the defined “Smart Aim” of your quality improvement work |
| What were the changes that you tested? |
| What changes have been adopted? |
| How will these changes be sustained in the future? |
| What measures/indicators did you use to track your improvement? |
| Did you observe improvements in relation to these measures/indicators? Please provide details of any improvements achieved. |
| How did the network peer support meetings and patient participation influence the practice’s QI plans on improving workforce wellbeing? |
Optimising access to general practice
Rationale
General Practice is under significant pressure due to high demand, challenges with recruitment and related capacity issues, increasing complexity of health needs and changing patient expectations.
With increased demand for general practice services, it is more important than ever that practices can access and understand relevant data and use this to effectively match capacity to demand, optimise use of the multi-disciplinary team and wider primary care services, and use care navigation and triage to support equitable access to care for patients.
This module aims to support practices to access and use data to be able to assess their demand and capacity, and to make changes based on proven improvement approaches.
Overview of the QI module
The overarching aim of this QI module is to support practices in relation to the following:
- Understanding data in relation to practice demand and
- Raising understanding of quality improvement techniques
- Making changes that enable more effective use of capacity and therefore better meet demand (e.g. reducing the number of avoidable appointments)
- Improving staff well-being
- Improving patient experience
Practices will need to undertake the following steps:
Step 1
Access and make improvements to data relevant to demand and capacity in the practice
Step 2
Understand patterns of and variation in both demand and capacity, analysing relevant data and trends.
Step 3
Use data analysis to discuss, agree and plan improvements to processes and procedures
Step 4
Carry out improvements including communication and /or training needed to make the changes (s)
Step 5
Monitor and review the impact of changes
Detailed contractor guidance
The following section includes further detail on the types of things practices could do to deliver this module. These are suggestions and recommendations only and the decision about what to include in the QI plan and which QI methodologies to use should be made by practices and shared with their peers through the network meetings.
We know that practices will be at different points in relation to understanding and matching their capacity to demand, therefore these resources are designed to be used in a flexible way to meet individual organisational needs.
Understanding demand and capacity
All practices should start with understanding their data.
The key steps are as follows:
- Bring together and analyse demand information – This includes total requests for a service via phone, via all online channels and in-person (demand), appointments provided plus urgent extra work (activity), people asked to ring back and ‘dropped calls’ (unmet demand).
Is demand fairly predictable? What patterns and trends are there across days, weeks, different periods of the year? - Count daily available capacity across all appointment modes (face to face, video, telephone, home visits, group clinics, triage capacity etc) and across all staff dealing directly with patients or patient requests/issues
- Plot on a run chart (examples: AQUA and ELFT) to view variation and assess how demand is distributed in comparison to capacity across different times of day, days of the week and months of the year.
Where practices have access to a Business Intelligence tool, this should assist the practice in understanding their demand and activity by day and time of day. Where possible, ICBs should support practices to access effective tools for providing business information and intelligence to practices and PCNs, to enable data on demand and capacity to be more easily accessed and visualised. Where BI tools are available, practices may find it helpful to have training or support in how to most effectively use the tool.
Practices may have access to an ‘avoidable appointments’ tool to monitor the use of appointments slots across a period of time and then to assess the suitability of use of this capacity, and where changes could be made. Clinicians working together to define which appointments may be avoidable can enable a practice to review its care navigation and triage processes to give patients more appropriate services.
Where necessary, a practice may have to undertake manual counts using tally charts to collect demand and queue information (e.g., the number of patients asked to call back tomorrow).
Smart cards are used to ensure that staff have the access permissions appropriate to their role on clinical systems. Through this they also support a better understanding of the contribution that staff are making to primary care. Many national data sources (e.g. Appointments in General Practice and the General Practice Extraction Service) will use Smartcards to identify which type of health care professional is delivering an appointment or consultation.
Roles recruited to the Additional Roles Reimbursement Scheme (ARRS) were added to NHS Smartcards in October 2021, but smart card uptake is still low. Practices should work at network level to ensure that all ARRS staff have smart cards and, once the appropriate access rights have been added to the cards, that staff use them when undertaking activity.
In order to get a smartcard and access a practice should contact their local Registration Authority who will be able to advise on the specifics of the local process, but will include:
- Verification of Identity – either via a face to face meeting when original documents are produced (2 Photo ID, 1 proof of address OR 1 photo ID, 2 proof of address), a video appointment (which is temporarily allowed to support pandemic recovery but likely to end in next 2/3 months or using our online verification service ‘Apply for Care ID’ – more information at Apply for Care ID – NHS Digital
- The issuance of a smartcard
- Their local Registration Authority assigning relevant access rights to the smartcard.
A list of primary care Registration Authority providers available at: Primary care service provider contact details – NHS Digital
Utilise demand and capacity data to plan improvements in operational processes
Based on the above data and analysis, plan and agree specific improvements to be made that are expected to better match demand and capacity. To identify areas for improvement, when looking at data trends and variation, consider the following:
- Where in the day, week, year do you need the greatest number of urgent appointments?
- When are the best times to schedule appointments in the day, week, year for non-urgent care that can be booked in advance?
- Reduce avoidable appointments where possible
- Signpost patients effectively
- Smooth capacity variation
- Plan ahead – manage holiday and training across the year
- Plan for staff sickness – when is this likely?
- What types of capacity are needed at different times of the day?
- What is the variation in ‘historic practices’ in the team?
Improvements could also focus on understanding the current volume and types of non-patient facing activity being managed across the practice and putting in place measures to reduce and streamline these tasks, thereby reducing workload pressure for staff and providing more capacity for other activity.
There are examples of QI activities that could be taken forward below (noting that these are suggestions and the decision about what to take forward will be made by practices).
Implementing the plan
Once practices have identified their area/s for improvement they should be clear about:
- The aims of the project – what will be achieved and by These aims should be SMART (specific, measurable, achievable, relevant, and time-bound).
- The measures – what data will be collected to know if the aims have been Measurements to assess the effectiveness of changes made should be straightforward to collect regularly.
- The changes – what different ways of doing things will be tested g. new models of contact, shift in tasks to other clinicians and/or staff
Practices should choose their own quality improvement activities and set their own targets for improvement based on their baseline evaluation. These should be challenging but realistic.
Practices should implement the improvement plan they have developed to support the objectives they have identified. It is recommended that these plans and associated improvement activities should involve the whole practice team and practices are also encouraged to engage with colleagues outside the practice, where possible, for further support and input.
Targets/ambitions should ideally be shared and discussed with network peers as part of the initial network review meeting, to provide additional insight and constructive challenge.
Incremental measures of improvement are recommended.
Practices should aim to find a way to embed continuous improvement cycles, so that quality improvement becomes routine.
Examples of SMART goals
Area for improvement (example 1): A practice has limited understanding of their demand and capacity and how it varies throughout the week and year and has a high level of unmet demand.
SMART aim: To reduce the amount of unmet demand in the practice from a baseline of [x to y] by [date]
Actions:
- Reception/Admin teams count ‘unmet need’ every day for 2 weeks
- This data is used alongside other appointment data (i.e. scheduled work and booked appointments) and any extra appointments that get ‘squeezed in’ or added onto daily scheduled work to create a picture of unmet (expressed) demand.
- This data is then used to help predict future demand and make changes to how capacity is planned to help meet this demand.
Area for improvement (example 2): A practice is not consistently coding data on appointment/consultation type/mode and so is not able to describe how care encounters are taking place or to distinguish consultations from other work (such as administrative work) to help with understanding of demand and capacity.
SMART aim: To increase the percentage of appointments that are coded correctly from a baseline of [x% to y%] by [date]
Actions:
- Team reviews appointment/consultation (encounter) type coding currently in use and variation across team members and gets a baseline of the percentage of appointments correctly coded from an initial 2-week sample.
- Practice discusses reasons for variation and issues.
- Practice agrees standard way of capturing appointment/consultation modes.
- Practice carries out ongoing tests of change to improve the coding with repeated measurements after each test to see where improvements can be made.
Area for improvement (example 3): Following a discussion at a practice team meeting about re-attendance, the practice wants a better understanding of appropriateness and variation in re-attendance rates to see if there is an opportunity to review how capacity is being used.
SMART aim: To reduce the number of patients reattending for the same problem within two weeks of initial contact from [x to y] by [date]
Actions:
- A data search is carried out to see how many patients have reattended within 14 days each month for the previous 6 months.
- Each clinician searches for 10 patients who have re-attended within 14 days of an initial contact.
- In a team meeting, the practice undertakes a focused review of the reason for re- attendance.
- The findings are used to identify the proportion of attendances that may be avoidable e.g. routine follow-up, normal test result or administrative issue.
- The practice then agrees changes to reduce avoidable re-attendance and monitors the impact of the changes.
Area for improvement (example 4): the practice wants to increase understanding of how appointment slots are used and whether demand could be distributed more appropriately across different members of the team.
SMART aim: To reduce the number of avoidable appointments booked in to see a GP/nurse/other clinician (to be chosen by the practice) from a baseline of [x%] of appointments to [y%] of appointments by [date]
Actions:
- Undertake a period of data collection over a defined period eg 2 weeks with clinicians tallying whether the appointments booked with them would have been better managed another If available, an online tool can be used for this data collection.
- Review the results of the audit, involving clinical and non-clinical staff from across the practice, to assess where demand could be differently allocated.
- Based on the review, agree new or amended protocols for receptionists and care navigators to enable them to direct patients to the right clinicians or services.
- Train staff in the new/amended protocols.
- Monitor and evaluate the impact of changes, which is likely to include rerunning the audit, and make further adjustments to protocols as needed.
Area for improvement (example 5): The practice has received complaints from patients who were unable to access appointments, and therefore wants to improve the process for making/booking/allocating appointments.
SMART aim: To improve patient satisfaction with booking processes from a baseline of [x%] reporting as good/very good to [y%] by [date]
Actions:
- Undertake a short patient survey or use data from an existing patient survey to obtain a baseline of patient satisfaction with booking processes and to understand the challenges that are being faced.
- The practice team map all processes for patients to request care and either directly book appointments or have an appointment offered to Involve a selection of patients and carers in this mapping process to help identify where issues arise.
- The practice team identify issues and inconsistencies with these processes.
- The team work collaboratively to generate and test potential solutions to some of these issues.
- Rerun the patient survey to see if patient satisfaction has improved.
NB: This process mapping exercise is even more impactful if it is done with patients/carers as well as the practice team. Seeing the process from the patient’s point of view often reveals the most important problems. An extra qualitative dimension can be added by using an emotional journey map to associate an indication of the emotional status of the user at each stage in the process.
Area for improvement (example 6): At a practice team meeting, GPs raised that a large amount of time was being taken up with non-clinical work e.g. completing follow-up administrative tasks.
SMART aim: To reduce how much time GPs are spending on non-patient facing work from a baseline of x hours/week to y hours/week by [date].
Actions:
- Audit to take place of how much time GPs are spending on non-patient facing work over a week.
- Discussion across the team as to what opportunities are available to reduce this workload e.g. through administrative staff buddying with GPs (see buddying guidance here), reviewing administrative processes, peer to peer learning on how to reduce time spent. Consider using tools of idea generation and prioritisation to help decide on opportunities for improvement.
- Undertake small tests of change with ongoing measurements of each test to decide what improvements can be implemented to reduce GP time spent on administrative tasks.
Area for improvement (example 7): A practice wants to improve continuity of care for specific patients.
SMART aim: For an identified cohort of patients identified as benefitting from continuity of care, to increase the proportion of contacts with their regular GP from an average of x to an average of y by March 2024.
Actions:
- In a team meeting decide which cohort(s) of patients would benefit from continuity of care with a regular GP g. multimorbidity, patients with dementia.
- Retrospectively review their appointment data to obtain a baseline of the proportion of GP contacts that are with their regular GP.
- Agree changes to improve continuity such as: clearly communicating the days of the week different clinicians work, flagging the records using alerts, using micro teams – with GP, nurse, administrators and other team members g. pharmacist, social prescribing link workers, to provide team continuity for groups of patients.
- Continue to measure with each test of change to see if this is improving the continuity of care for the identified cohort(s).
Improvement support
The Primary Care Transformation Team at NHS England will provide webinars (including on Demand and Capacity), resources, guidance and intensive ‘hands on’ support where needed to help practices to make improvements as well as work with ICBs to provide relevant support within systems. This improvement support will have a particular focus on:
- Demand and capacity management
- Triage and navigation processes
- Workload management (non-patient facing work)
- Enabling effective contact journeys for patients via telephone
- Enabling effective contact routes for patients online
Practices are encouraged to consider their own support needs and to access support as appropriate to assist them in making improvements in these areas.
Reporting and verification
The contractor must complete the QI monitoring template in relation to this module and self-declare that they have completed the activity described in their QI plan. The contractor will also self-declare that they have attended a minimum of two peer review meetings (either in person, where appropriate, or virtually), unless there are exceptional and unforeseen circumstances which impact on a contractor’s ability to participate. In these circumstances contractors are expected to make efforts to ensure alternative participation in peer review.
Verification – Commissioners may require contractors to provide a copy of the QI monitoring template as written evidence that the quality improvement activity has been undertaken. Commissioners may require a written record of attendance at the peer review meetings. If a contractor has been unable to attend a meeting due to exceptional circumstances, then they will need to demonstrate other active engagement in network peer learning and review.
If practices are happy to share completed templates with the national Primary Care Transformation team to help inform development of the general practice improvement programme, please send these to []. Please note there is no obligation to share the template with the team, this would be entirely voluntary.
We recommend practices include their QI work in any inspections by the Care Quality Commission to demonstrate a visible and consistent approach to quality improvement.
Resources are available from our GP contract web pages
Reporting template
Download a Word version of the QI reporting template.
It is anticipated that the responses noted here should total a maximum of 2 A4 sides in Arial font size 11.
| Practice name and ODS code |
|---|
| What area of demand and capacity did the practice identify for quality improvement? |
| What was the defined “Smart Aim” of your quality improvement work? |
| What were the changes you tested? |
| What changes have been adopted? |
| How will these changes be sustained in the future? |
| What measures/indicators did you use to track your improvement? |
| Did you observe improvements in relation to these measures/indicators? Please provide details of any improvements achieved. |
| How did the network peer support meetings and patient participation influence the practice’s QI plans on optimising access? |
| Optional: We would be very grateful if you would consider sharing your improvement project as an example of good practice. If you would be willing to do this, please upload it to the QOF QI case studies – Primary Care Improvement CONNECT – FutureNHS Collaboration Platform |
Section 6: Personalised care adjustment
As of 1 April 2019, exception reporting is being replaced with a Personalised Care Adjustment (PCA). This will allow practices to differentiate between the following reasons for adjusting care and removing a patient from the indicator denominator:
- Unsuitability for the patient, eg because of medicine intolerance or allergy, or contra-indicated polypharmacy;
- Patient choice, following a shared-decision making conversation;
- The patient did not respond to offers of care – recording of this will change to capture actual invitations sent to patients;
- The specific service is not available (in relation to a limited number of indicators only); or
- Newly diagnosed or newly registered patients, as per existing rules.
As with exception reporting applying a PCA to the patient record will remove that patient from an indicator denominator if the QOF defined intervention has not been delivered. It will not result in patients being removed from the disease register or other target population.
This mechanism differs from ‘exclusions’ which refer to patients on a particular clinical register who are not included in an indicator denominator for definitional reasons. For example, an indicator (and therefore the denominator) may refer only to patients of a specific age group, patients with a specific status (e.g. those who smoke), or patients with a specific length of diagnosis, within the register for that clinical area.
Principles
When considering whether a PCA applies to an individual patient practices are reminded that:
- The duty of care remains for all patients,
- The decision to apply a personalised care adjustment should be based on clinical judgement, informed by patient preferences and underpinned by shared decision-making principles, with clear and auditable reasons coded or entered in free text on the patient record
- There should be no blanket personalised care adjustments: the relevant issues with each patient should be considered by the clinician at each level of the clinical indicator set and this decision reviewed on a regular basis.
In each case where a personalised care adjustment is applied then in addition to what needs to be reported for payment purposes (in accordance with the Business Rules), the contractor should also ensure that the reason for the adjustment is fully recorded in a way that can facilitate both safe and effective patient care and audit of the patient record. For example, where a patient has not tolerated medication, the nature of the contraindication should be recorded in the patient’s record as well as a code to indicate intolerance.
Criteria for the personalised care adjustment
Personalisation of care can occur for the following reasons which are listed in the order in which they will be extracted in the Business Rules:
- The investigative service or secondary care service is unavailable (where relevant to the indicator)
- Intervention described in the indicator is clinically unsuitable
- The patient has chosen not to receive the intervention described in the indicator
- The patient has not responded to invitations for the intervention described in the indicator (a minimum of two invitations for the intervention in the preceding 12 months, except for the cervical screening indicators where women should receive a total of three invitations for screening)
- The patient has registered with the practice or has been newly diagnosed with the condition of interest in the preceding 3 months and has not received the defined clinical measurements e.g. blood pressure measurement
- The patient has registered with the practice or has been newly diagnosed with the condition of interest in the preceding 9 months and has not achieved the defined clinical standards e.g. blood pressure control within target levels.
It is recognised that patients may meet more than one of these criteria and in these circumstances all reasons for personalisation should be recorded in the patient’s record to facilitate safe and effective patient care. However, as a patient can only be acknowledged as having a personalised care adjustment once within the Business Rules for a given indicator, they will be allocated to the first criterion they meet in the hierarchy listed above. For example, where a patient is recorded as having registered with the practice in the preceding 3 months and has also chosen not to receive the intervention described in the indicator they would be identified in the Business Rules as having chosen not to receive the care.
The hierarchy listed above seeks to prioritise clinical judgement and patient choice over other criteria. Applying this hierarchy consistently in the Business Rules in conjunction with the recording changes described below will support better attribution of the reason for care being personalised, allowing for more meaningful conversations between clinicians, commissioners and regulators.
Interpretation and recording of the personalised care adjustment
The interpretation of these categories and how they should be recorded is detailed further below.
The investigative service or secondary care service is unavailable
This care adjustment will apply only to the following indicators: AST011, COPD0014 and DM014.
Where one of these services is unavailable this should be recorded using specific codes which state that the service is unavailable. The contractor is expected to explore fully with their ICB, if a suitable investigative or secondary service could be commissioned for the patient prior to entering a service unavailable code in the patient record.
The frequency with which service unavailable codes should be added to the patient record is noted below and may vary between indicators. Some codes may need to be entered annually whereas others may only need to be entered once in the relevant timeframe stated in the indicator.
Table 2: Frequency of data entry
| Indicator ID | Service unavailable may be recorded |
| AST011 | Within 6 months of diagnosis of asthma |
| COPD014 | Required each year the patient becomes eligible for pulmonary rehabilitation |
| DM014 | Within 279 days of diagnosis of diabetes |
Intervention described in the indicator is clinically unsuitable
We envisage this being the main reason for personalisation of care, recognising the importance of clinical judgement in determining the applicability of guideline recommendations to individual patients.
This category encapsulates the historical exception reporting criteria of 1) patients for whom it is not appropriate to review their chronic disease parameters due to particular circumstances e.g. receiving end of life care, 2) those who are on maximal tolerated doses of medication, 3) those who have an allergy, contraindication or adverse reaction to medication, 4) those who have not tolerated medications and 5) where the patient has a supervening condition which would make treatment of their condition inappropriate.
This criterion will be supported by both generic ‘patient unsuitable’ codes which will apply to all indicators in the clinical area except for indicators VI001, VI002 and VI003) and more specific codes which can be attributed to single indicators.
Indicators in the Vaccination and Immunisation domain will be supported by specific codes for clinical unsuitability for a vaccination. Over time, more specific codes will be introduced which define the clinical reasons which might make the intervention clinically unsuitable for an individual patient.
Codes which indicate ongoing and permanent reasons for personalisation of care such as allergies to specified medication may be entered once in the medical record.
Other codes will need to be recorded on an annual basis following an individual patient review of the applicability of the intervention described in the indicator.
It is not acceptable to exclude all patients who are under the care of a consultant. Each case needs to be carefully considered and all reasonable efforts made to provide optimal care.
Even when a patient is under the care of a consultant only, the contractor should ensure it has evidence that all the requirements of the contract have been carried out. If this evidence is not available, the contractor should assume that the action has not been carried out and either fulfil the requirements of the relevant indicator(s) or obtain evidence from secondary care that the particular test/check has been carried out. Where the secondary care clinician, in agreement with the primary care clinician, has exercised clinical judgement and decided further action or testing is inappropriate, this should be noted in the patient record. A personalised care adjustment may them be applied.
The patient has chosen not to receive the intervention described in the indicator
This criterion requires that there has been a personal contact or a discussion recorded in the patient record which ideally notes the reasons for the intervention being declined. This contact may be face-to-face, video conferencing or telephone contact between a health professional and the patient.
This criterion will be supported by both generic ‘informed dissent’ codes which will apply to all indicators in the clinical area and more specific codes which can be attributed to single indicators. Practices are encouraged to use more specific codes where they are available.
The decision to decline a QOF intervention should be reviewed with the patient on an annual basis and recorded annually if necessary. The exceptions to this are indicators CS005 and CS006 where the choice not to receive the intervention need only be entered once during the time-period stated in the indicator. However, as noted in the underpinning principles, good practice would be to revisit this decision on a regular basis. Women who choose to withdraw from the cervical screening call/recall will receive no further offers of screening from the central screening service.
The patient has not responded to invitations for the intervention described in the indicator
To be removed from an indicator denominator using this criterion patients must have been sent a minimum of two invitations for QOF care at two unique time points in the QOF year i.e. 1 April to 31 March separated by a minimum of seven calendar days.
The exceptions to this are indicators CS005 and CS006 where the patient should have been sent a minimum of three invitations at three unique time points during the timeframe stipulated in the indicator. However, care should continue to be offered on an opportunistic basis where appropriate.
General standards and recording requirements for invitations
Many different methods of communication are already available to invite patients for QOF care and these are likely to expand with the ongoing development of digital technology. The NHS also has a legal duty to ensure that patients who have a disability, impairment or sensory loss get information that they can access and understand as set out in the Accessible Information Standard. The first step to making an effective invitation for care therefore is that it is made in a manner which is accessible to the patient. Therefore, practices should prospectively and opportunistically record individual patients preferred methods of communication, for example at the time of the next patient contact. Where a preferred contact method is recorded this would be used to make the first invitation for care. The second invitation may be via any method.
All invitations should be personalised to the patient i.e. use their name and specify what they are being invited for. Where invitations are being sent via letter or email these should also include information for the patient as to why this care is being offered and its importance for their health care.
Invitations should be coded at the time they are sent to the patient. For data extraction purposes, there should be a minimum of seven calendar days between each invitation, but practices should use their judgement in determining the optimal spacing between invitations for their practice population. A longer period may be more appropriate. Codes currently exist to indicate the communication method used to make the invitation and that the patients preferred method was used. Both will be acceptable for QOF purposes.
Patients should be sent a minimum of two invitations for care within the QOF year ie 1 April – 31 March. If these invitations are correctly coded then they will be identified through the business rules and there will be no need to add additional codes at year-end to indicate that a patient has not responded to these invitations.
As at present, generic invitations such as messages added to the right-hand side of prescriptions or notices in the waiting room inviting groups of patients to attend clinics or make appointments will not be acceptable.
Invitations for cervical screening
As noted above, the requirement for women to be invited on three separate occasions will continue in line with national screening programme requirements. Therefore:
- In those areas where the first two invitations are sent via the central screening service, then contractors are responsible for offering the third invitation, or
- Where the central screening service sends out only one letter, then contractors are responsible for offering the second and third invitation.
- Where contractors have opted to run their own call/recall system then they are responsible for making all three invitations.
Where a woman does not respond to these three invitations then contractors will need to code that this has been the case. Each invitation should be recorded in the patient record as evidence of these may be required for assessment and audit purposes.
Women may choose to withdraw from the national screening programme. This should be undertaken with caution as women who withdraw from cervical screening call/recall will receive no further offers of screening from the central service. Where women actively decline cervical screening, this should be recorded as such.
The patient has registered with the practice or been newly diagnosed with the condition in the last 3 months of the QOF year and has not received defined clinical measurements
Where a patient newly registers with a practice or is newly diagnosed with a clinical condition in the last three months of the QOF year (1 January – 31 March) this criterion applies automatically, unless the contractor has recorded the defined clinical measurements within the timeframe for the indicator. This is because achievement automatically over-rides any PCA.
The patient has registered with the practice or has been newly diagnosed with the condition in the last 9 months of the QOF year and has not achieved defined clinical standards
Where a patient newly registers with a practice or is newly diagnosed with a clinical condition in the last nine months of the QOF year (1 July – 31 March) this criterion applies automatically, unless the contractor has achieved the defined clinical standards within the timeframe for the indicator. This is because achievement automatically over-rides any PCA.
Section 7: Indicators no longer in QOF (INLIQ)
The indicators included in INLIQ in 2023/2024 are detailed below.
| Indicator ID | Indicator description |
| CHD003 | The percentage of patients with coronary heart disease whose last measured cholesterol (measured in the preceding 12 months) is 5 mmol/l or less |
| CKD002 | The percentage of patients on the CKD register in whom the last blood pressure reading (measured in the preceding 12 months) is 140/85 mmHg or less |
| CKD004 | The percentage of patients on the CKD register whose notes have a record of a urine albumin:creatinine ratio (or protein:creatinine ratio) test in the preceding 12 months |
| NM84 | The percentage of patients on the CKD register with hypertension and proteinuria who are currently treated with renin-angiotensin system antagonists |
| CVD- PP002 | The percentage of patients diagnosed with hypertension (diagnosed after or on 1 April 2009) who are given lifestyle advice in the preceding 12 months for: smoking cessation, safe alcohol consumption and healthy diet |
| DM005 | The percentage of patients with diabetes, on the register, who have a record of an albumin:creatinine ratio test in the preceding 12 months |
| DM011 | The percentage of patients with diabetes, on the register, who have a record of retinal screening in the preceding 12 months |
| EP002 | The percentage of patients 18 or over on drug treatment for epilepsy who have been seizure free for the last 12 months recorded in the preceding 12 months |
| EP003 | The percentage of women aged 18 or over and who have not attained the age of 55 who are taking antiepileptic drugs who have a record of information and counselling about contraception, conception and pregnancy in the preceding 12 months |
| LD002 | The percentage of patients on the learning disability register with Down’s syndrome aged 18 or over who have a record of blood TSH in the preceding 12 months |
| MH004 | The percentage of patients aged 40 or over with schizophrenia, bipolar affective disorder and other psychoses who have a record of total cholesterol:hdl ratio in the preceding 12 months |
| MH007 | The percentage of patients with schizophrenia, bipolar affective disorder and other psychoses who have a record of alcohol consumption in the preceding 12 months |
| MH008 | The percentage of women aged 25 or over and who have not attained the age of 65 with schizophrenia, bipolar affective disorder and other psychoses whose notes record that a cervical screening test has been performed in the preceding 5 years. |
| PAD002 | The percentage of patients with peripheral arterial disease in whom the last blood pressure reading (measured in the preceding 12 months) is 150/90 mmHg or less. |
| PAD003 | The percentage of patients with peripheral arterial disease in whom the last measured total cholesterol (measured in the preceding 12 months) I 5 mmol/l or less |
| PAD004 | The percentage of patients with peripheral arterial disease with a record in the preceding 12 months that aspirin or an alternative anti- platelet is being taken. |
| RA002 | The percentage of patients with rheumatoid arthritis, on the register, who have had a face-to-face review in the preceding 12 months) |
| RA003 | The percentage of patients with rheumatoid arthritis aged 30 or over and who have not attained the age of 85 who have had a cardiovascular risk assessment using a CVD risk assessment tool adjusted for RA in the preceding 12 months |
| RA004 | The percentage of patients aged 50 or over and who have not attained the age of 91 with rheumatoid arthritis who have had an assessment of fracture risk using a risk assessment toll adjusted for RA in the preceding 24 months |
| SMOK001 | The percentage of patients aged 15 or over whose notes record smoking status in the preceding 24 months |
| STIA005 | The percentage of patients with a stroke shown to be non- haemorrhagic, or a history of TIA whose last measured total cholesterol (measured in the preceding 12 months) is 5 mmol/l or less |
| THY001 | The contractor establishes and maintains a register of patients with hypothyroidism who are currently treated with levothyroxine |
| THY002 | The percentage of patients with hypothyroidism, on the register, with thyroid function tests recorded in the preceding 12 months |
Section 8: Glossary of acronyms
A&E – Accident and Emergency
ABPM – Ambulatory Blood Pressure Monitoring
ACE-Inhibitor or ACE-I – Angiotensin Converting Enzyme Inhibitor
ACR – Albumin Creatinine Ratio
AF – Atrial Fibrillation
APDF – Adjusted Practice Disease Factor
ARB – Angiotensin Receptor Blocker
AST – Asthma
ATS/ERS – American Thoracic Society/European Respiratory Society
BMD – Bone Mass Density
BMI – Body Mass Index
BMA – British Medical Association
BMJ – British Medical Journal
BNF – British National Formulary
BP – Blood Pressure
BTS – British Thoracic Society
CABG – Coronary Artery Bypass Grafting
CAN – Cancer
CAT – COPD Assessment Test
CG – Clinical guideline (NICE)
CHD – Coronary Heart Disease
CHADS2 – Congestive (HF) Hypertension Age (75 or over) Diabetes Stroke
CHA2DS2- VASc – Congestive (HF) Hypertension Age (75 or over) Diabetes Stroke (prior stroke) Vascular Disease (peripheral artery disease) Age (65– 74 years) Sex Category (i.e. female)
CKD – Chronic Kidney Disease
CMO – Chief Medical Officer
COPD – Chronic Obstructive Pulmonary Disease
CPA – Care Programme Approach
CQRS – Calculating Quality Reporting Service
CRP – C-Reactive Protein
CS – Cervical Screening
CVD – Cardiovascular Disease
CVD-PP – CVD Primary Prevention
DEM – Dementia
DEP – Depression
DM – Diabetes Mellitus
DMARD – Disease Modifying Anti-Rheumatic Drugs
DXA – Dual-Energy X-ray Absorptiometry
ED – Erectile Dysfunction
eGFR – Estimated Glomerular Filtration Rate
EOLC – End of Life Care
EP – Epilepsy
ES – Enhanced Service
ESR – Erythrocyte Sedimentation Rate
FEV1 – Forced Expiratory Volume in One Second
FVC – Forced Vital Capacity
GFR – Glomerular Filtration Rate
GMC – General Medical Council
GMS – General Medical Services
GOLD – The Global Initiative for Chronic Obstructive Lung Disease
GP – General Practitioner
GPC England – General Practitioners Committee England
GPES – General Practice Extraction Service
GSF – Gold Standards Framework
HbA1c – Glycated Haemoglobin
HBPM – Home Blood Pressure Monitoring
HF – Heart Failure
HYP – Hypertension
IFCC – International Federation of Clinical Chemistry and Laboratory Medicine
INLIQ – Indicators no longer in in QOF
IQ – Intelligence Quotient
JCVI – Joint Committee on Vaccination and Immunisation
LD – Learning Disabilities
LDL – Low Density Lipoprotein
LVSD – Left Ventricular Systolic Dysfunction
MDT – Multi-disciplinary team
MH – Mental Health
MI – Myocardial Infarction
mmHg – Millimetres of Mercury
mmol/l – Millimoles per Litre
MRC – Medical Research Council
NCSI – National Cancer Survivorship Initiative
NDH – Non-Diabetic Hyperglycaemia
NG – NICE guideline
NHS – National Health Service
NICE – National Institute for Health and Care Excellence
NRT – Nicotine Replacement Therapy
NSF – National Service Framework
OB – Obesity
OGTT – Oral Glucose Tolerance Test
ONS – Office for National Statistics
OST – Osteoporosis
PAD – Peripheral Arterial Disease
PC – Palliative Care
PCA – Personalised Care Adjustment
PCRJ – Primary Care Respiratory Journal
PEF – Peak Expiratory Flow
PH – Public health
PPI – Proton pump inhibitor
PVD – Peripheral Vascular Disease
QI – Quality Improvement
QOF – Quality and Outcomes Framework
QS – Quality standard (NICE)
RA – Rheumatoid Arthritis
RCGP – Royal College of General Practitioners
RCP – Royal College of Physicians
RCN – Royal College of Nurses
SFE – Statement of Financial Entitlements
SMOK – Smoking
SPICT – Supportive and Palliative Care Indicators Tool
SWOT analysis – Strengths, weaknesses, opportunities and threats analysis
STIA – Stroke or Transient Ischemic Attack
TA – Technology appraisal (NICE)
TIA – Transient Ischemic Attack
TSH – Thyroid Stimulating Hormone
UK – United Kingdom
US – United States
WHO – World Health Organisation
Section 9: Queries
Queries fall into three main categories:
- Those which can be resolved by referring to guidance and/or FAQs
- Those requiring interpretation of the guidance or Business Rules
- Those not anticipated in guidance.
Queries may incorporate one or more of the following areas: Business Rules, coding, payment, CQRS, GPES, and clinical or policy issues. The recipient of the query will liaise with other relevant parties in order to respond and where necessary the query will be redirected.
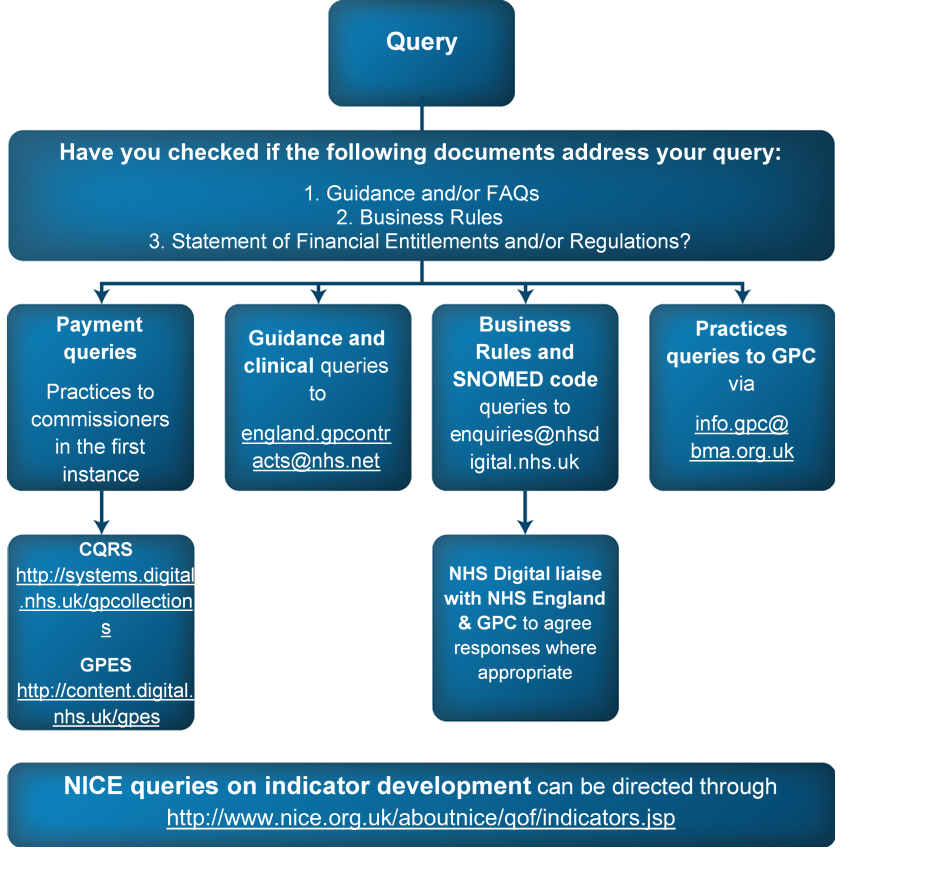
Image text:
Query
Have you the checked if the following documents address your query?
- Guidance and/or frequently asked questions
- Business rules
- Statement of financial requirements and/or regulations
Payment queries – practices to commissioners in the first instance | CQRS – CQRS Local for Primary Care Providers and GP Practices GPES – General Practice Extraction Service (GPES) – NHS Digital
Guidance and clinical request – queries to england.gpcontracts@nhs.net
Business rules and SNOMED codes – queries to enquiries@nhsdigital.nhs.net | NHS Digital liaise with NHS England and GPC to agree response where appropriate
Practices queries to GPC via info.gpc@bma.org.uk
NICE queries on indicator development can be directed through Indicators | Standards and Indicators | NICE
Publication reference: PRN00917

